Abstract
A morpho-functional investigation of the sex pheromone-producing area was correlated with the pheromone release mechanism in the female gypsy moth Lymantria dispar. As assessed by male electroantennograms (EAG) and morphological observations, the pheromone gland consists of a single-layered epithelium both in the dorsal and ventral halves of the intersegmental membrane between the 8th and 9th abdominal segments. By using the male EAG as a biosensor of real-time release of sex pheromone from whole calling females, we found this process time coupled with extension movements of the ovipositor. Nevertheless, in females in which normal calling behavior was prevented, pheromone release was detected neither in absence nor in presence of electrical stimulation of the ventral nerve cord/terminal abdominal ganglion (TAG) complex. Tetramethylrhodamine-conjugated dextran amine stainings also confirm the lack of any innervation of the gland from nerves IV to VI emerging from the TAG. These findings indicate that the release of sex pheromone from the glands in female gypsy moths is independent of any neural control exerted by the TAG on the glands, at least by way of its three most caudally located pairs of nerves, and appears as a consequence of a squeezing mechanism in the pheromone-producing area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The nervous system of moths consists of a brain and a nerve cord comprising a series of intersegmental ganglia. Some of these, mainly involved in locomotion and flight control, are located in the thorax, while the abdominal ones are implicated in several other functions such as digestion, excretion and reproduction.
In particular, the caudalmost terminal abdominal ganglion (TAG) is thought to play a major role in “calling behavior” and the production and/or release of species-specific sex pheromones from specialized gland cells (Crnjar et al. 1988; Itagaki and Conner 1987, 1988; Christensen et al. 1991, 1994; Thyagaraja and Raina 1994; Christensen and Hildebrand 1995). For instance, in the Arctiid female Utetheisa ornatrix a neural control exerted by the TAG appears to exist in the rhythmic exposure of the sex pheromone glands during calling (Itagaki and Conner 1987). Similarly, in the gypsy moth Lymantria dispar, alternate cyclic movements of extension and retraction of the ovipositor observed during calling are under the control of the TAG, at least by way of its three most caudally located nerve pairs (IV, V and VI) (Crnjar et al. 1988). Moreover, Tang et al. (1987) indicate that calling is disrupted in L. dispar with transected ventral nerve cord (VNC) and Crnjar et al. (1988) suggest that the TAG has a high degree of autonomy in controlling calling behavior. However, the direct involvement of the TAG in pheromone biosynthesis and/or release is not unequivocally demonstrated in Lepidopterans, where more than one mechanism–hormonal, neural or a combination of both—may modulate pheromone gland activity, even in the same species. Moreover, these mechanisms may be differentially activated at different times during the life of the insect (Raina 1993; for a review see Christensen and Hildebrand 1995).
Studies carried on the Noctuiid moths Helicoverpa zea and Heliothis virescens and on the sphinx moth Manduca sexta show that pheromone glands receive neural connections from the TAG, and that electrical stimulation of the terminal nerves elicits an increase in both pheromone biosynthesis and release (Christensen et al. 1991, 1994). Since these responses are abolished by transection of the terminal nerves, innervation appears to provide an alternate pathway for gland activation for which blood-borne factors are not necessary. In the gypsy moth instead, pheromone production appears to be primarily regulated by pheromone biosynthesis-activating neuropeptides (PBANs) (Thyagaraja and Raina 1994; Golubeva et al. 1997), although transection of the VNC anterior to the TAG or removal of the ganglion strongly depresses pheromone production (Hollander and Yin 1982; Tang et al. 1987; Thyagaraja and Raina 1994). In this, the possibility that pheromonotropic hormones may be released from neurosecretory cells located in the abdominal ganglia has been proposed (Golubeva et al. 1997). However, the release mechanism of pheromone in the gypsy moth has up to now been elusive.
Based on these observations, in the present study we investigated, using both electrophysiological and morphological approaches, whether or not sex pheromone release is controlled by the TAG by way of nerve pairs IV to VI, generally regarded as the neural pathways primarily involved in the control of reproductive functions as well as calling behavior in the female gypsy moth L. dispar (Crnjar et al. 1988).
To this end, we first acquired morpho-functional data on the abdominal portions accounting for pheromone production and release, i.e., the pheromone glands. To ascertain any functional interconnection between glands and the TAG we then stained the projections of nerves IV to VI toward the caudalmost abdominal districts such as the ovipositor and glands and verified whether electrical stimulation of the TAG/VNC complex was related to pheromone release.
Materials and methods
Insects
All experiments were performed on 2- or 3-day-old gypsy moths (day 1 being the day of emergence), obtained as pupae from the Gypsy Moth Rearing Unit at the Otis Pest Survey Detection and Exclusion Laboratory (U.S. Department of Agriculture, Otis ANGB, MA, USA). They were kept in an environmental growth incubator (24-25°C, 70% R.H., 16 h light/8 h dark photoperiodic regime) and checked daily until adult emergence. Males and females were kept separate to avoid any exposure of males to female sex pheromone.
Electrophysiology
EAG recording technique
A standard male electroantennogram (EAG) bioassay was used to evaluate either the pheromone content of dissected abdominal segments accounting for pheromone production or the real-time course of pheromone release from whole calling females.
Recordings were performed on isolated antennae from male moths, one per moth, positioned in such a way as to expose the largest surface to stimulation, that is, with the ventral side of the antenna facing upwind. The recording electrode, a glass micropipette (20 μm in average tip diameter) filled with physiological saline solution (potassium phosphate buffer 20 mM, NaCl 12 mM, KCl 6.4 mM, MgCl2 12 mM, CaCl2 1 mM, glucose 354 mM, KOH 9.6 mM, final pH 6.60; Kaissling 1995), containing an Ag/AgCl wire, was gently pressed against the cut tip of the antenna. A similar Ag/AgCl wire, inserted into the base of the antennal shaft, acted as the reference electrode. EAGs were recorded with a high input impedance electrometer (WPI M707), digitized by means of the Axon Digidata 1200B A/D converter (10,000 Hz) and stored in a computer for further analyses (Axoscope 8.1 software). Student’s “t” test with a 95% confidence level (P ≤ 0.05) was used for statistical analyses.
Odor delivery system
An air stimulus controller (model CS-55, Syntech) was used for air and odor delivery with a constant flow (500 ml/min) of charcoal-filtered and humidified air passing over the antennal preparation through the open end of a steel tube (15 mm diameter, 15 cm in length), positioned 15 mm from the antenna. During odor stimulation, 100 ml/min of air was switched for 2 s through a Pasteur pipette (15 cm in length) containing the stimulus, inserted by about 3 mm into a small hole in the side of the steel tube. The air containing the stimulus was removed from the experimental arena by means of a suction pump operating at a flow rate slightly higher than the rate of stimulation. When not in use, stimuli were stored at −20°C.
Stimuli
To ascertain which parts of the female abdomen mainly account for pheromone production and/or release, male antennae were stimulated with the three caudalmost abdominal segments—the 7th, the 8th [including the intersegmental membrane (IM) between the 8th and the 9th], and the 9th (ovipositor)—obtained from ten females. Hereafter they will be referred to as S7, S8-IM and S9 respectively (Fig. 1).
The abdomens were forcibly extruded and quickly frozen for precise slicing of the different segments (at rest, S8-IM and S9 are retracted within S7). Then, after thawing, they were immediately placed within Pasteur pipettes and then puffed onto the preparation with the odor delivery system described above. In a subsequent batch of experiments, S8-IM was further divided into the dorsal and ventral halves (S8-IMd and S8-IMv). Stimuli were presented in a randomized sequence, separated by intervals between stimuli of at least 2 min. If possible, each stimulus was tested more than once to verify the reproducibility of responses.
In a second group of experiments, antennae were stimulated with whole calling females in accordance with the experimental procedure reported by Christensen et al. (1994) to see if pheromone release was TAG-controlled. Briefly, the insect was pinned, ventral side up, to a wax platform and a patch of cuticle along the midline of segment S7 was cut out to expose the TAG, the emergence of its six nerve pairs and the VNC. The preparation was then placed at approximately 5 mm from the antenna under a continuous air stream generated by the odor delivery system described above. The TAG was continuously perfused with saline to prevent drying.
The EAG of each antenna was continuously monitored with the following sequence of stimuli: clean air, (+)-disparlure, free calling females, females with pinned ovipositors, VNC stimulation in unpinned females before and after progressive resection of nerve pairs IV, V and VI, and finally (+)-disparlure and clean air again. Thus each male antenna was tested at the beginning and the end of each experiment, with clean air and (+)-disparlure, which were our controls of choice. Concurrent with the EAG recordings, ovipositor movements of extension and retraction were visually monitored and recorded as real time comments on Axoscope 8.1 or voice tags.
The length of ovipositor extension and retraction, as well as their relative EAG values, were calculated on a maximum of five complete cycles (one cycle comprises one ovipositor extension and the following retraction) evoked by each of the ten calling females tested in this group of experiments.
Electrical stimulation of the VNC anterior to the TAG was performed in accordance with Christensen et al. (1994), with a pair of fine silver-wire bipolar electrodes. Trains of 2-25 electric pulses were delivered by means of an electronic stimulator at a frequency of 20 Hz and the voltage amplitude was adjusted until slight contractions of abdominal muscles could be detected, typically 1–5 V.
Male antennae, female abdominal segments and whole calling females were tested between 7 and 10 h after photophase onset to reduce discrepancies due to diel periodicity either in male antennal sensitivity or in female pheromone release (Giebultowicz et al. 1992; Tang et al. 1992; Thyagaraja and Raina 1994).
A 5 μg (non-saturating) amount of (+)-disparlure (Sigma-Aldrich, code 510769, 95% pure) was dissolved in 25 μl paraffin oil, applied to a pleated filter paper strip (80 mm × 5 mm), and used in all EAG experiments as a control. In each experiment, before each stimulation the response to air was tested and its value subtracted from the EAG value obtained in response to the test stimulation that ensued.
Morphology
Histology of pheromone gland
Fully extruded abdominal segments S7–S9 from calling females were fixed in alcoholic Bouin fixative (Humason 1967), dehydrated, embedded in paraffin and serially sectioned. Cross-sections, 6 μm thick, were stained with the hematoxylin-eosin staining procedure (Humason 1967).
Electron microscopy
Samples were obtained from the pheromone gland area, located on the dorsal and ventral sides of the ovipositor. Specimens were fixed with 1.25% glutaraldehyde and 1% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.2, embedded in epoxy resin for transmission electron microscopy (TEM) and stained with bismuth subnitrate as reported by Riva (1974). Observations of ultrathin sections, 90 nm thick, were made with a JEOL 100S TEM.
Axonal fills and laser scanning confocal microscopy
To identify and localize the projection neurons of the nerve pairs IV–VI toward the caudalmost peripheral districts (pheromone glands and ovipositor), axonal fills with a tetramethylrhodamine-conjugated dextran amine (TMR-DA) combined with laser scanning confocal microscopy (LSCM) were performed in several specimens (seven for nerves of pair IV, six of pair V and six of pair VI) on nerve stumps projecting distally. The staining procedure was directly performed on the whole abdomen. Insects were first anaesthetized on ice and, after removing head and thorax, the abdominal segments were dissected along the ventral midline and pinned down on a Sylgard-coated Petri dish in saline. The stump of the selected cut nerve emerging from the TAG was isolated from the surrounding saline in a small depression made in petroleum jelly and filled with distilled water. The nerve was then cut again to a shorter stump in this hypotonic bath to allow infusion of a 1% (w/v) aqueous solution of TMR-DA (Molecular Probes, D3308, MW 3,000). After dye infusion for a maximum of 2 days at 4°C, the preparations were fixed overnight at 4°C in 4% (w/v) paraformaldehyde in sodium phosphate buffer (0.1 M, pH 7.4), subsequently dehydrated in 10 min steps with graded ethanol of ascending concentrations, and cleared in methyl salicylate.
Laser scanning confocal microscopy analysis was performed 24–36 h after staining using a Leica 4D LSCM with an Argon-Krypton laser. Confocal images were generated using 10×, 40× oil and 100× oil objectives in fluorescence mode (568 nm of excitation wavelength). Each frame (512 lines and 512 columns) was acquired eight times and then averaged to obtain noise-free images. Confocal images (Tredici et al. 1993) were obtained from the maximum number of scans allowed by specimen thickness. Optical sections, usually at consecutive intervals of 0.5–1 μm, were imaged through the depth of the labeled neurons and saved as image stacks. All confocal images were white-labeled on a black background, in a gray scale ranging from 0 (black) to 255 (white) and processed in gray scale value with Leica Scanware 4.2a. Total or partial three-dimensional (3D) reconstructions were performed by means of Confocal Assistant 4.00 software and processed with Paint Shop Pro 7.0 and Power Point programs; where needed, the digitized images were modified only to enhance contrast and to add false colors.
Results
Male EAG responses to odor from female three caudalmost abdominal segments
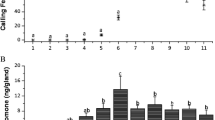
As shown by samples of EAG recordings in Fig. 2a, stimulation of the male antennal chemoreceptors with the S7, S8-IM and S9 female abdominal segments produced different EAG responses. In detail, mean EAG values obtained from S8-IM, S9 and S7 were stimulatory in decreasing order, with 1.48 ± 0.15 mV, 0.94 ± 0.11 mV and 0.27 ± 0.05 mV respectively (Fig. 2b). EAG values from female abdominal segments were in any case significantly lower than those elicited by (+)-disparlure used as a control.
Sample of EAG recordings (a) and mean amplitude values ± SE (b) elicited by male antennal preparations following stimulation with segments S7, S8-IM and S9, as compared to clean air and (+)-disparlure (5 μg stimulus load). The line under the EAG traces represents the duration of the stimulus. c Mean amplitude values ± SE elicited by male antennal preparations following stimulation with segment S8-IM divided into its dorsal (S8-IMd) and ventral (S8-IMv) halves. The asterisk indicates significant differences (P ≤ 0.05). Recordings from ten specimens
Since S8-IM appeared to be the most effective region in eliciting the male antennal response, further analyses were restricted to its dorsal (S8-IMd) and ventral (S8-IMv) sides; however, no differences were found between EAG values elicited by dorsal (0.78 ± 0.12 mV) or ventral sides (0.83 ± 0.13 mV) (Fig. 2c). Based on these results, the pheromone gland in the gypsy moth L. dispar is mainly located in segment S8-IM and occupies both the dorsal and ventral regions.
Male antennal detection of pheromone release from females under physiological conditions vs. electrically evoked calling behavior
Upon placing whole calling females near the antenna under a constant air stream, fluctuations in male EAG response were in all cases detected as a consequence of a discontinuous pheromone release and appeared to be synchronized with the ovipositor movements of extension and retraction, typically observed during “calling behavior”. Calling was at first irregular, probably due to the stress brought about by manipulation prior to testing for females; EAG fluctuations greatly varied in shape, amplitude and time-course, but usually settled within two to three minutes from the beginning of experiments. As summarized in Fig. 3a, d, the peaks in EAG response (0.32 ± 0.04 mV) were closely associated with ovipositor extensions, which lasted on average 5.35 ± 0.4 s. The EAG responses associated with the ovipositor extensions are comparable in sign and time-course to those evoked in the same specimens by stimulation with (+)-disparlure, although to a lesser amplitude (Fig. 4a, b). The ovipositor retraction lasting 1.71 ± 0.11 s corresponded instead to a repolarization. No EAG was obtained after removing the female from the experimental arena; placing it back again produced total recovery of the response.
Sample of EAG recordings from the same male antenna in response to sex pheromone released by females during normal “calling behavior” (a) or with the ovipositor pinned in the fully extruded position, before (b) and after (c) electrical stimulation of the VNC (frequency: 20 Hz). Numbers in c (lower trace) represent impulse counts of each electrical stimulation. Black bars and white bars in a denote extension and retraction of the ovipositor, respectively. d Mean time values (histograms) ± SE from five ovipositor extensions and five retractions for each of the ten female gypsy moths tested (n = 50) and EAG amplitude values ± SE (inset) elicited from five EAG responses for each of the ten males tested (n = 50). The asterisk indicates significant differences (P ≤ 0.05)
When the ovipositor was pinned in a fully extruded position (Fig. 3b), the EAG response completely decayed within a short time (5–10 s), this delay probably due to residual pheromone molecules blown by the air stream from the surface of the glands.
Similarly, when we electrically stimulated the VNC anterior to the TAG in females with pinned extruded ovipositors, no EAG response was detected in any case (Fig. 3c). This lack of pheromone release occurred both when all nerves from the TAG were kept connected, and when the nerves were selectively transected from the ganglion, leaving intact only the IV, V or VI pair respectively.
Histology of pheromone gland
According to electrophysiological observations, segment S8-IM is the region of the female abdomen most effective in eliciting the strongest stimulating effect on male antennae. Thus an in-depth morphological study of the same segment was performed by means of the hematoxylin-eosin staining protocol.
As shown in the light micrographs of Fig. 5a–c, the examination of cross-sections at different magnifications of the extended dorsal IM confirms the presence of a single layer of a modified epithelium with glandular appearance, directly beneath the cuticle. It appears best developed along both the dorsal and the ventral regions of the cuticle, where folds and extensive convolutions are present, and also extends, although to a much lesser extent, to the lateral part (results not shown). This epithelium consists of large hypertrophic columnar- to cone-shaped cells, 30–40 μm in height and 15–20 μm in width, with prominent nuclei. Dark granules of secretory material are distributed in the cytoplasm, as well as in isolated cytoplasmic compartments nearby, thus confirming the glandular feature of this epithelium. The cuticle covering this glandular region is thicker than in other regions of the body, and appears divided into two different layers, an inner and thick hyaline zone of endocuticle and a darker outer zone of thin epicuticle, with a roughness due to button-like protrusions enhancing the external surface. However, no cuticular pore channels or connections of the glandular epithelium to the outside are visible in the light microscopy.
Ultrastructure of pheromone gland
Transmission electron microscopy of ultrathin cross-sections of the glandular tissue in the ventral half of IM is shown in Fig. 6. The cuticle covering the glandular region consists of a dense uniform outer epicuticle which overlies the lamellated endocuticle. The cells are not attached to the endocuticle with their apical side, because there is a narrow hypocuticular space filled by numerous microvilli just beneath the latter (Fig. 6a, b). A few electron-lucent small vacuoles with some lamellar membrane profiles are visible inside the cytoplasm; nuclei are enlarged with sparse zones of dense chromatin (Fig. 6a). Sometimes, inside the 1st–4th endocuticle lamellae, there are small dense black ovoidal bodies which are located in the layer between the lamellae (Fig. 6b); some of these bodies are also found in the hypocuticular space.
TEM micrographs of the pheromone glands at the ventral intersegmental membrane level. a Nuclei are enlarged (N) and small vacuoles (arrow) with membrane profiles are visible. A narrow hypocuticular space filled by numerous microvilli (MV) is present beneath the endocuticle (Edc). b Small dense black ovoidal bodies (arrowhead) are located both in the microvilli (MV)-containing hypocuticular space (asterisk) and between the endocuticular lamellae (Edc). Scale bar = 2 μm (a) and 0.5 μm (b)
TMR-DA fills from the TAG to the caudalmost abdominal segments
Tetramethylrhodamine-conjugated dextran amine stainings of nerve V (Fig. 7a) distal stump resulted in labeling of somata and processes of bipolar neurons within the ovipositor. In this, afferences from mechano- or chemoreceptors scattered over the ovipositor surface travel via nerve V toward the TAG. The same procedure also labels efferent projections innervating the muscles in the ovipositor (Fig. 7b). Likewise, a number of bipolar cells were clearly detected on the ovipositor surface when the same staining protocol was applied to the nerve VI distal stump (arrows in the Fig. 7c, d). At least 30–40% of the bipolar neurons associated with the ovipositor sensilla were successfully stained in each of seven to ten specimens (unstained sensilla can also be seen in Fig. 7a, c).
Partial depth reconstructions of three-dimensional confocal images showing tetramethylrhodamine-dextran amine axonal stainings of distal stumps of nerves V (a) and (b) and VI (c) and (d) toward the ovipositor. a Longitudinal sections (ventral up, posterior left) with somata and processes of bipolar neurons (arrows) scattered over the ovipositor surface. Dashed area indicates the enlargement in (b). b The ovipositor shows a bipolar neuron (arrows) and nervous fibers (nf) to muscular tissue (M). c and d Longitudinal sections of the ovipositor (ventral right, posterior up) showing a number of bipolar neurons (arrows). Dashed area in c indicates the enlargement in d. Scale bar = 100 μm (a) and (c), 25 μm (b) and (d)
No labeled innervations of the pheromone gland were instead obtained by TMR-DA stainings through the IV–VI nerve distal stumps (data not shown).
Discussion
Localization of the pheromone gland
The first step in the present study was to better characterize the abdominal portions accounting for pheromone production and release, i.e., the pheromone glands, in the female gypsy moth L. dispar.
Our electrophysiological observations showed that of the caudalmost abdominal segments, S8-IM—the 8th one—with the IM between the 8th and the 9th (S9, the ovipositor)—clearly appears to be the most stimulating portion for the male olfactory apparatus, with both the dorsal (S8-IMd) and ventral (S8-IMv) halves equally effective. These data are in good agreement with those previously reported by Hollander et al. (1982), who located the sex pheromone-producing gland of the female gypsy moth at the IM level by means of their behavioral trials based on a wing fanning bioassay. Conversely, S9 and especially S7, the 7th segment, evoked some unexpected EAG activity, possibly due to the fact that pheromone, once produced at the IM level, may reach the other two segments tested by mechanical spreading through the cyclic telescopic movements of extensions and retractions of the ovipositor during calling behavior occurring before electrophysiological tests started.
Our histological and ultrastructural analyses of the IM support these findings, as it is lined with a single-layered epithelium of glandular appearance mainly developed in both the dorsal and the ventral region and, to a much lesser extent, in the lateral areas (data not shown). IM also bears a morphological resemblance to the glandular tissue shown by Hollander et al. (1982), and possesses the general cytological features generally encountered in the sex pheromone-producing gland cells of other Lepidopterans (Percy-Cunningham and MacDonald 1987; Raina et al. 2000; Ma and Roelofs 2002; Raspotnig et al. 2003). These features include the presence of vacuoles both in the cell and the overlying cuticle, and also development of apical plasma membrane folds. Such characteristics are either absent or not well defined in epidermal cells occurring elsewhere in the IM and in other parts of the terminal abdominal segments.
Pheromone release is closely associated with ovipositor movements
Once characterized, we then investigated the possibility of the gypsy moth pheromone gland being somewhat neurally controlled, i.e., directly controlled by the TAG, as this ganglion is thought to play a role, to different extents in different moth species, in the control of “calling behavior” and pheromone production and/or release. In Lepidopterans, gland activity has previously been described as a species-dependent mechanism that may involve neural or hormonal factors or both, possibly activated at different times during the life of the insect. Evidence from several species indicates that blood-borne factors are not necessary in inducing pheromone biosynthesis and release, since the pheromone gland is innervated and regulated by neural activity arising from the TAG, thus providing an alternate route for gland activation. This is the case of the Noctuiid moths H. zea and Heliothis virescens, where neural connections between the TAG and pheromone gland exist and mediate gland activity, as well as in the sphinx moth M. sexta, where electrical stimulation of terminal nerves evokes an increase in both pheromone production and release (Christensen et al. 1991, 1994; see, for a review, Christensen and Hildebrand 1995 and cited literature). By contrast, our electrophysiological observations obtained using a male EAG as a biosensor of real-time female pheromone release revealed that in gypsy moth calling females, puffs of pheromone occurred only when cyclic movements of ovipositor extension and retraction typical of normal “calling behavior” were allowed. Moreover, puffs were closely synchronized with the extension phase of the ovipositor, as demonstrated by the simultaneous depolarization of male antennal receptors, whereas ovipositor retraction always led to the repolarization phase. Conversely, when the ovipositor was experimentally pinned in a fully extruded position, no EAG response was detected in any case. This lack of pheromone release also occurred following electrical stimulations of the VNC/TAG complex—the same procedure used in M. sexta and Heliothine moths (Christensen et al. 1991, 1994), both when all nerves emerging from the TAG were kept connected and when the nerves were selectively transected from the ganglion, leaving intact only the IV, V or VI pair respectively. Therefore, in female gypsy moths, unlike what has been observed in the aforementioned Lepidopteran species, the release of pheromone appears primarily to be a consequence of ovipositor movements associated with “calling behavior”, rather than of any neural control directly exerted by the TAG on the glands. At the same time, it appears unlikely that upper projections arising from the VNC control reproductive districts topologically confined in the most caudal abdominal segments.
This hypothesis is also strengthened by morphological observations obtained with TMR-DAs as bi-directional tracers (Richmond et al. 1994; Kaneko et al. 1996), by staining the projections of nerves IV–VI emerging from the TAG, that is, those most reasonably involved in the control of reproductive functions. In none of the specimens examined did we find evidence of fibers descending from these nerves and contacting the pheromone gland cells, despite the fact that the dye we used passed through the IM containing the pheromone gland in order to reach a more caudal district such as the ovipositor (Fig. 7).
Three-dimensional confocal image reconstructions were made just to show a partial depth reconstruction of the preparations. Cell bodies are distributed in many different planes and not all of them are visible in the figures. Although only a portion of the bipolar neurons in the ovipositor sensilla were successfully stained (30–40%), this may be taken as an estimate of the backfilling success for the whole innervation of the ovipositor region. Under these circumstances, we confide that any existing gland innervation would have been found. The lack of staining of gland cells is no proof of a lack of innervation, but rather supports the idea that innervation may be lacking.
All things considered, pheromone release appears to be primarily due to ovipositor movements associated with “calling behavior”. In this respect, the involvement of the TAG in the control of calling behavior has been extensively proved in a number of moth species as well as in the gypsy moth, where alternate cyclic movements of extension and retraction of the ovipositor observed during calling were shown to be under the control of the TAG, at least by way of the three most caudally located nerve pairs, IV, V and VI (Crnjar et al. 1988). Moreover, Tang et al. (1987) indicated that disruption of calling occurs in gypsy moths following transection of the VNC. Also in the Arctiid female U. ornatrix, a TAG-controlled mechanism was found to regulate the rhythmic exposure of the sex pheromone glands during calling (Itagaki and Conner 1987).
On this basis, and due to the fact that pheromone is released only during the phase of ovipositor extension, we hypothesize for the gypsy moth the same mechanism proposed by Raina et al. (2000) in H. zea, by which the active pheromone, once produced in the glandular cell of the IM, is transferred through the cuticle onto its external side. This mechanism is corroborated by the presence of numerous small dense black ovoidal bodies located between the endocuticular lamellae (Fig. 6). Although we cannot refer to these as pheromonal material with certainty, the presence of numerous microvilli projecting from the apical side of gland cells into the hypocuticular space just beneath the endocuticle, their presence in the same space and in the innermost endocuticular lamellae indicate a secretion of material—possibly pheromonal—from the inside to the outside of the cuticle.
During calling the ovipositor is extruded and the area bearing the pheromone molecules is exposed to air: the presence of extensive convolutions and cuticular roughness apparently increases the surface area to facilitate evaporation of the pheromone. Once the sex attractant is dissipated, the ovipositor is retracted and during this phase a new aliquot of pheromone is squeezed onto the external surface of the cuticle and exposed to air during the ensuing ovipositor extension; this sequence is repeated over and over throughout calling activity.
There exists the possibility that gland activity may be affected by a neuromodulatory rather than a neural control, both for pheromone production and release. Previous reports demonstrate that the neurotransmitter octopamine elicits a photoperiodic- and age-dependent increase in pheromone production in Heliothine moths (Christensen et al. 1991, 1992), although its exact role and site of action are unclear (Rafaeli and Gileadi 1995; Ramaswamy et al. 1995). In the gypsy moth the TAG displays a remarkable octopaminergic activity through specific receptors positively coupled to a Ca2+ ion-inhibitable adenylyl cyclase isoform by way of a stimulatory G protein (Olianas et al. 2006). In the latter study octopamine was shown to increase the spike activity of emerging nerves IV, V and VI, thus suggesting a role in the control of calling. In the present work, preliminary experiments suggest that octopamine injections close to the TAG elicit an appreciable increase in pheromone release only when females are able to display normal calling behavior by increasing the frequency of cyclic ovipositor movements (personal observation). Although this aspect requires further investigation, it appears to support our hypothesis concerning pheromone release.
In conclusion, in the female gypsy moth L. dispar, sex pheromone (+)-disparlure is biosynthesized in the final active molecule by the glandular tissue located in both the dorsal and ventral epithelium of the IM between the 8th and 9th segments. Pheromone is then released by a “squeezing” mechanism due to the TAG-controlled rhythmic activity of calling, in order to reach the cuticular surface outside the IM in discrete amounts during ovipositor retraction and be alternatively exposed to free air during ovipositor extension.
This process appears to be independent of any neural control exerted by the TAG on the glands, at least by way of its three most caudally located pairs of nerves. However, we cannot completely exclude a glandular modulation exerted by neuromodulators or neural inputs arising from upper nervous centers above the TAG.
Abbreviations
- EAG:
-
Electroantennogram
- LSCM:
-
Laser scanning confocal microscopy
- S7:
-
The 7th abdominal segment
- S8-IM:
-
The 8th abdominal segment (including the intersegmental membrane)
- S8-IMd:
-
Dorsal half of the 8th abdominal segment
- S8-IMv:
-
Ventral half of the 8th abdominal segment
- S9:
-
The 9th abdominal segment (ovipositor)
- TAG:
-
Terminal abdominal ganglion
- TMR-DA:
-
Tetramethylrhodamine-conjugated dextran amine
- VNC:
-
Ventral nerve cord
References
Christensen TA, Hildebrand JG (1995) Neural regulation of sex pheromone glands in Lepidoptera. Invert Neurosci 1:97–103
Christensen TA, Itagaki H, Teal PEA, Jasensky RD, Tumlinson JH, Hildebrand JG (1991) Innervation and neural regulation of the sex pheromone gland in female Heliothis moths. Proc Natl Acad Sci USA 88:4971-4975
Christensen TA, Lehman HK, Teal PEA, Itagaki H, Tumlinson JH, Hildebrand JG (1992) Diel changes in the presence and physiological actions of octopamine in the female sex-pheromone glands of heliothine moths. Insect Biochem Mol Biol 22(8):841–849
Christensen TA, Lashbrook JM, Hildebrand JG (1994) Neural activation of the sex-pheromone gland in the moth Manduca sexta: real-time measurement of pheromone release. Physiol Entomol 19:265–270
Crnjar R, Angioy AM, Pietra P, Yin C-M, Liscia A, Tomassini Barbarossa I (1988) Control mechanisms of calling behaviour in Lymantria dispar: an electrophysiological investigation on the role of the terminal abdominal ganglion. J Insect Physiol 34:1087–1091
Giebultowicz JM, Webb RE, Raina AK, Ridgway RL (1992) Effects of temperature and age on daily changes in pheromone titer in laboratory reared and wild gypsy moth (Lepidoptera: Lymantriidae). Environ Entomol 21:821–826
Golubeva E, Kingan TG, Blackburn MB, Maser EP (1997) The distribution of PBAN (Pheromone Biosynthesis Activating Neuropeptide)-like immunoreactivity in the nervous system of the gypsy moth, Lymantria dispar. Arch Insect Biochem Physiol 34:391–408
Hollander AL, Yin C-M (1982) Neurological influences on pheromone release and calling behaviour in the gypsy moth Lymantria dispar. Physiol Entomol 7:163–166
Hollander AL, Yin C-M, Schwalbe CP (1982) Location, morphology and histology of sex pheromone glands of the female gypsy moth, Lymantria dispar (L.). J Insect Physiol 28:513–518
Humason GL (1967) Animal tissue techniques, 2 edn. WH Freeman and Co., San Francisco
Itagaki H, Conner WE (1987) Neural control of rhythmic pheromone gland exposure in Utetheisa ornatrix (Lepidoptera: Arctiidae). J Insect Physiol 33(3):177–181
Itagaki H, Conner WE (1988) Calling behavior of Manduca sexta (L) (Lepidoptera: Sphingidae) with notes on the morphology of the female sex pheromone gland. Ann Entomol Soc Am 81:798–807
Kaissling KE (1995) Single unit and electroantennogram recordings in insect olfactory organs. In: Spielman AI, Brand JG (eds) Experimental cell biology of taste and olfaction, current techniques and protocols. CRC, Boca Raton, pp 361–377
Kaneko T, Saeki K, Lee T, Mizuno N (1996) Improved retrograde axonal transport and subsequent visualization of tetramethylrhodamine (TMR)-dextran amine by means of an acidic injection vehicle and antibodies against TMR. J Neurosci Methods 65:157–165
Ma PWK, Roelofs WL (2002) Sex pheromone gland of the female European corn borer moth, Ostrinia nubilalis (Lepidoptera, Pyralidae): ultrastructural and biochemical evidences. Zool Sci 19:501–511
Olianas MC, Solari P, Garau L, Liscia A, Crnjar R, Onali P (2006) Stimulation of cyclic AMP formation and nerve electrical activity by octopamine in the terminal abdominal ganglion of the female gypsy moth Lymantria dispar. Brain Res 1071:63–74
Percy-Cunningham JE, MacDonald JA (1987) Biology and ultrastructure of sex pheromone-producing glands. In: Prestwich GD, Blomquist GJ (eds) Pheromone biochemistry. Academic, New York, pp 27–75
Rafaeli A, Gileadi C (1995) Modulation of the PBAN-stimulated pheromonotropic activity in Helicoverpa armigera. Insect Biochem Mol Biol 25:827–834
Raina AK (1993) Neuroendocrine control of sex pheromone biosynthesis in Lepidoptera. Annu Rev Entomol 38:329–349
Raina AK, Wergin WP, Murphy CA, Erbe EF (2000) Structural organization of the sex pheromone gland in Helicoverpa zea in relation to pheromone production and release. Arthropod Struct Dev 29:343–353
Ramaswamy SB, Jurenka RA, Linn CE, Roelofs WL (1995) Evidence for the presence of a pheromonotropic factor in hemolymph and regulation of sex pheromone production in Helicoverpa zea. J Insect Physiol 41:501–508
Raspotnig G, Schicho R, Stabentheiner E, Magnes C, Stelzl M (2003) Morphology of female sex pheromone gland in the horse chestnut leafminer Cameraria ohridella (Lep., Gracillariidae). J Appl Entomol 127:121–126
Richmond FJ, Gladdy R, Creasy JL, Kitamura S, Smits E, Thomson DB (1994) Efficacy of seven retrograde tracers, compared in multiple-labelling studies of feline motoneurones. J Neurosci Methods 53:35–46
Riva A (1974) A simple and rapid staining method for enhancing the contrast of tissues previously treated with uranyl-acetate. J Microsc 19:105–108
Tang JD, Charlton RE, Cardé RT, Yin C-M (1987) Effect of allatectomy and ventral nerve cord transection on calling, pheromone emission and pheromone production in Lymantria dispar. J Insect Physiol 33:469–476
Tang JD, Charlton RE, Cardè RT, Yin C-M (1992) Diel periodicity and influence of age and mating on sex pheromone titer in gypsy moth Lymantria dispar (L.). J Chem Ecol 18:749–760
Thyagaraja BS, Raina AK (1994) Regulation of pheromone production in the gypsy moth, Lymantria dispar, and development of an in vivo bioassay. J Insect Physiol 40(11):969–974
Tredici G, Di Francesco A, Miani A, Pizzini G (1993) Real complete three-dimensional reconstruction of Golgi-impregnated neurons by means of a confocal laser scanning microscope. NeuroImage 1(2):87–93
Acknowledgments
We are grateful to John Tanner from the Otis Pest Survey Detection and Exclusion Laboratory (U.S. Department of Agriculture, Otis ANGB, MA, USA) for supplying gypsy moth specimens. We also thank Felice Loffredo for technical assistance during ultrastructural analyses, Alessandro Riva for the use of the HRSEM Hitachi S4000 electron microscope, Giuliana P. Serra for the use of Leica 4D LSCM, Piera Angioni for technical support during electrophysiological experiments and David Nilson for improving the English. We also thank anonymous reviewers for comments that improved this manuscript. This work was partly supported by the “Stazione Sperimentale del Sughero, Regione Autonoma della Sardegna” (Sardinia, Italy) and by the Italian MURST (FISR). Experiments comply with the ‘‘Principles of animal care’’, publication No. 86–23, revised 1985 of the National Institute of Health, and also with the current laws of the EC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solari, P., Crnjar, R., Spiga, S. et al. Release mechanism of sex pheromone in the female gypsy moth Lymantria dispar: a morpho-functional approach. J Comp Physiol A 193, 775–785 (2007). https://doi.org/10.1007/s00359-007-0232-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-007-0232-z