Abstract
Jumping spiders are known to possess ultraviolet (UV) receptors in the retinas of their large-principal eyes. The existence of UV visual cells, however, does not prove that jumping spiders can see into the UV part of spectrum (300–400 nm) or whether such an ability plays any role in salticid intra-specific interactions. In the study reported herein, we performed behavioural experiments to test whether a UV−reflecting jumping spider, Cosmophasis umbratica, is sensitive to UV wavelengths and whether UV cues are important in intra-specific communication. The absence of UV cues not only affected intra-specific behaviour by significantly reducing the frequency of agonistic displays, but also elicited unprecedented courtship displays in males towards their own mirror images and conspecific opponents. Furthermore, C. umbratica males were able to respond rapidly to changes in UV cues of conspecific mirror images by switching between agonistic and courtship displays. These findings clearly demonstrate that C. umbratica males are capable of seeing UV wavelengths and that UV cues are necessary and sufficient for this species to enable the agonistic displays. Hence, UV light may have an important role to play in intra-specific communication in jumping spiders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jumping spiders (Salticidae) are renowned for their unique and complex eyes that support spatial resolution (≈0.04°), which are unparalleled among animals of comparable size (Land and Fernald 1992; Land and Nilsson 2002). The highest spatial resolution ever recorded in insects is about 0.4° (Labhart and Nilsson 1995). The acuity of the human eyes is around 0.007° (Kirschfeld 1976), which is about five times better than that of salticids.
Like most spiders, salticids have four pairs of eyes in three rows, including a single pair of principal (anterior median) eyes with movable retinas, and three pairs of secondary eyes (anterior lateral, posterior median and posterior lateral eyes) that have simple, single-layered retinas incapable of movement. The smaller secondary eyes, positioned along the sides of the carapace, are used to detect movement in the environment (Homann 1928; Land 1971). The larger, forward-facing principal eyes are primarily responsible for acute vision (see Blest 1985; Forster 1985; Land 1985; Blest et al. 1990) and allow salticids to identify rivals, mates, prey and predators (Jackson and Blest 1982) and to discriminate colours (Homann 1928; Kästner 1950; Nakamura and Yamashita 2000).
Land (1969) examined the retinas of the principal eyes of two jumping spider species, Phidippus johnsoni and Metaphidippus aeneolus, and found that they have four layers of receptor cells (1–4 from the deepest layer forwards). He also predicted that layers 1–4 contain red, blue–green, violet-UV and UV−sensitive visual cells, respectively. Using techniques of intracellular recordings, DeVoe (1975) reported UV cells (maximum sensitivity at 360 nm), green cells (532 nm) and UV−green cells (both 370 and 525 nm) in the principal eyes of a large salticid Phidippus regius, but found no evidence for red-sensitive cells. Subsequently, Blest et al. (1981) examined the retinas of the principal eyes of Plexippus validus and found only two cell classes: UV (maximum sensitivity at 360 nm) and green (520 nm) cells. They then concluded that the dual-peaked (UV−green) cells reported in the principal eyes of P. regius by DeVoe (1975) were experimental artefacts due to small sample size. However, Yamashita and Tateda (1976) examined the intracellular receptor potentials of anterior median eyes of Menemerus confuses and reported four cell classes: UV (maximum sensitivity at 360 nm), blue (480–500 nm), green (520–540 nm) and yellow cells (580 nm). These data support the hypothesis proposed by Land (1969) that each layer of the retina contains different receptor cells. More recently, Peaslee and Wilson (1989) psycho-physically examined the retinas of the principal eyes of Maevia inclemens, based on an “off-saccadic” oculomotor reflex, and reported that this salticid has a broad spectral sensitivity extending from UV (330 nm) to deep red (700 nm), with maximum sensitivities in the UV and green regions.
Many salticids possess pigments and structural characteristics that generate garish displays of colours independently or in concert with each other (Oxford and Gillespie 1998). To the human observers, salticid colouration appears to be sexually dimorphic, with male commonly being more colourful and attractive than females. Although it has been suggested that sexual selection has driven the evolution of the elaborate colouration in male jumping spiders (Peckham and Peckham 1889, 1890, 1894), there have been no studies of salticid visual signalling from their point of view (i.e. what the salticid actually sees). Of particular interest are some salticids with body parts that reflect UV light (J.J. Li, M.L.M. Lim and D. Li, unpublished data). Our recent study demonstrated that a highly active and ornate iridescent jumping spider, Cosmophasis umbratica, not only exhibits UV colourations, but that it also shows an extremity in UV sexual dimorphism (Lim and Li in press). All of the body parts of adult males that are involved in agonistic and courtship interactions (Lim and Li 2004) are capable of reflecting UV light. Females do not reflect any UV light in the corresponding body parts.
Despite several differences and disagreements about the actual number of receptor classes, the corresponding peak sensitivities and the nature of salticids’ colour vision (i.e. dichromatic, trichromatic or tetrachromatic), there is a consensus: jumping spiders generally have UV−sensitive cells in the retinas of their principal eyes that are maximally sensitive at 330–380 nm. Considering the early studies on salticid UV visual receptors, as well as the recent report of UV colours in jumping spiders, UV vision and UV colorations seem to be intimately involved in salticid communication. However, the mere existence of UV−sensitive cells and UV colours does not prove that salticids can see into UV wavelengths or that they are capable of discriminating UV from human-visible wavelengths (i.e. 400–700 nm). We also cannot assume that the UV colours reported in jumping spiders are involved in salticid visual interactions. In this study, we investigated whether both the UV vision and UV colours of jumping spiders are involved in intra-specific communication. In a series of behavioural experiments, we aimed to show that the UV−reflecting salticid, C. umbratica, is not only capable of UV vision, but also highly dependent on UV wavelengths for intra-specific communication.
Materials and methods
Study subjects and maintenance
All experiments were performed using the salticid species, C. umbratica, a small jumping spider (body length: adult male 5–7 mm; adult female ca. 5 mm), which exhibits strong sexual colour dimorphism in UV light (Lim and Li in press). This species is often found on leaves and flowers of ‘‘sun-loving’’ shrubs or plants that are fully exposed to sunlight. The male has complex iridescent markings on several body parts, particularly on the dorsal and lateral cephalothorax, and on the lateral femora of each leg. The abdomen is predominantly black in colour, with silvery-white lines that run in an anterior–posterior direction on the dorsal and lateral abdomen. The female is generally green on the dorsal and lateral cephalothorax with a mixture of brown, white and black coloration on the abdomen. Morphologically, adult males have slimmer abdomens and longer legs than adult females (Fig. 1a–c). No known electro-physiological experiments have been performed to investigate the visual spectral sensitivity of this species, but the results from anatomical and electro-physiological experiments on other genera can probably also be applied to this species. The size and complexity of the display repertoire of C. umbratica resemble that of other salticids’ intra-specific (i.e. male–male and male–female) interactions that have been studied in detail (e.g. Jackson 1980, 1986a, b; Jackson and Macnab 1989; Jackson and Whitehouse 1989; Li et al. 2002). The agonistic and courtship behaviour of C. umbratica males are manifested by distinctive hunched (Fig. 1b) and arched postures, respectively (Fig. 1c) (Lim and Li 2004).
All spiders were collected from Singapore as sub-adults (one more moult before becoming mature adults) and maintained in conditions described in previous salticid studies (see Lim and Li 2004; in press). The cage design followed that of earlier salticid studies (see Jackson and Hallas 1986; Lim and Li 2004), and only the essential details are given here. All of the spiders were kept individually in opaque cylindrical cages (diameter × height: 6.5×8.5 cm) to prevent contact amongst individuals, and maintained in a laboratory under controlled environmental conditions (relative humidity: 80–85%; temperature: 25±1°C; light regime: 12:12 h; lights on at 08:00 h). Additional lights (Arcadia Natural Sunlight Lamp, Croydon, UK) were used to illuminate the cages for 4 h daily (09:00–11:00 h; 16:00–18:00 h) to provide a light spectrum that simulates natural sunlight, as the spiders are frequently spotted on plants that were exposed to sunlight during late morning and early afternoon (M.L.M. Lim and D. Li, personal observations). Water and sugar water were provided ad libitum through dental rolls. The spiders were fed twice a week on a diet of houseflies (Musca domestica), fruit flies (Drosophila melanogaster) and small instars of crickets (Acheta domestica) twice a week (see Lim and Li 2004).
Experimental set up
All behavioural experiments were conducted from 09:00 to 17:00 h under full spectrum illumination (see Fig. 2) provided by ten equi-spaced (10-cm interval) 1.8-m, 110-W fluorescent tubes (Voltarc Ultra Light, USA) that were suspended 1 m above the test apparatus. The testing apparatus was surrounded by black curtains, which did not reflect any light and the curtain could also minimize the interference from observer movements during the experiments. A video camera (Panasonic NV-MX500, NJ, USA) with a lens protruding through the curtains was used for recording all behaviour with minimal disturbance to the spiders. All lights were turned off for 5 min before each trial to facilitate a “quiescent” period to discourage movements and remove directional preference of test spiders prior to commencement of experiments (i.e. when the lights were turned on).
Mirror image response test
Uniquely among spiders, salticid males are well known to respond by displaying species-specific behavioural postures when exposed to mirrors (mirror-image response). Males, in particular, often engage in prolonged bouts of agonistic displays towards their own mirror images (Homann 1928; Forster 1982). In preliminary trials, we established that C. umbratica males respond to their own mirror images by exhibiting the same display behaviour as they do when confronted with a conspecific male under light conditions similar to the solar spectrum. We then tested the mirror-image responsiveness of C. umbratica males under two light conditions manipulated by filters (Fig. 2), full spectrum light (i.e. human-visible and UV light: UV+; 300–700 nm) and visible light only (i.e. no UV light: UV−; 400–700 nm). Light transmissions through the filters were measured and corresponding spectra recorded using a USB2000 UV/VIS miniature fibre-optic spectrometer (Ocean Optic Inc., Dunedin, USA). We used a horizontal stage (18×7 cm) with a mirror (10 cm wide × 8 cm high) that reflected UV+ wavelengths. To reflect images without UV, we used the same mirror but inverted it as half of the mirror was coated with a transparent film-like UV blocking filter (Photonitech Pte. Ltd., Singapore) that allowed visible but not UV light to pass through and did not drastically reduce other wavelengths (Fig. 2), and positioned it vertically at one end of the stage. Before a trial was started, we connected a vial (1.5 cm diameter × 7 cm high) near the other end of the stage (14 cm mark; Fig. 3) in which the male to be tested was placed.
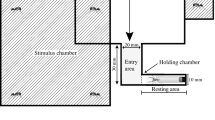
Horizontal stage used in the mirror image response test. Not to scale. The stage, made of plastic, was covered by a black matted surface that minimized any reflection of light that might interfere with the experiment. A mirror, vertically positioned at one end of the stage, had one half of the mirror that permitted UV−transmission (300–700 nm) and the other half that blocked UV wavelengths using a UV−blocking (400–700 nm) filter. Inverting of mirror facilitated either image of males with or without UV cues. A vial (diameter 1.5 cm, height 5 cm) connected to the other end of the stage, 14 cm away from the mirror, held the test spider (C. umbratica male) before each trial
A trial started when the spider left the vial and moved towards the mirror. The trial ended when the spider made physical contact with the mirror or when 5 min had elapsed, whichever came first. Between trials, the stage and mirror were wiped with 75% ethanol, and then allowed to dry for at least 30 min to remove pheromones and draglines left by previous males that could interfere with the behaviour of subsequent test subjects. Successful trials were recorded when males looked at their images in the mirror, and either responded (i.e. displayed hunched or arched legs) or ignored their own images. Trials were aborted whenever 5 min elapsed without the individual approaching the mirror at all, or when spiders leapt off the stage without looking at their own images. A total of 30 successful trials using different individuals were obtained. The spiders were randomly assigned to one of the two groups. Individuals from one group viewed their mirror images under UV+ first and then under UV− conditions a week later, and the other group interacted with their images in the reverse order.
Conspecific response test
A second experiment was carried out to examine the interactions between a pair of UV cue manipulated adult males. The procedure in this test was similar to that in the mirror image response test except that we used a pair of size-matched adult males to replace mirror images, and we replaced the horizontal stage with a Plexiglas arena (length × width × height: 20×5×3.5 cm) that had a UV+ (300–700 nm) or a UV− (400–700 nm) glass filter positioned across the centre that created two equal-sized chambers (Fig. 4). The sidewalls were made of transparent Plexiglas to facilitate video recording of interactions; the walls facing the glass filter and the floor were made of Plexiglas coated with a layer of UV−absorbing black matted material (ORACL® 651 Intermediate black, Oracl USA, Jacksonville, USA) that provided maximum background colour contrast. The lids were UV−transmitting glass that allowed full spectrum light (300–700 nm) to illuminate the arena and the test spiders.
Testing arena used in the conspecific response test. The testing arena measured 20×5×3.5-cm high, divided equally into two areas by a removable glass that is either UV−transmitting (UV+, 300–700 nm) or has a UV−blocking (UV–, 400–700 nm) transparent and colourless filter. The plastic floor and the walls facing the removable glass are covered with a black matte material that is UV−absorbing, and the transparent sidewalls are of UV−transmitting material that facilitates clear video recording of salticid interactions. UV−transmitting glass material covering the arena permitted transmission of full spectrum light (300–700 nm) from the above to illuminate the test spiders
Before each trial, the lights were turned off and a pair of size-matched adult males each was transferred from its home cage to one of the two chambers of the testing arena. A UV+ or UV– glass and a separate piece of opaque cardboard were placed between the chambers. As in the first experiment, individuals were left in darkness for 5 min before visual interactions. A trial began when full spectrum lights were turned on and the opaque board removed to allow visual interactions between the two males. All subsequent behaviour was video-recorded. Each trial lasted for 15 min. Between trials, the testing arena and the glass were all wiped off with 75% ethanol and allowed to dry for at least 30 min. A total of 22 pairs were used, and no pair was used in more than one trial.
Rapid response test
A third experiment was performed to determine whether males responded rapidly to sudden changes in UV light, or more specifically, to the UV cues of conspecific images. The experimental set up was similar to the first experiment except that only a UV+ mirror was used and a removable UV−filter sheet (positioned between full spectrum lights and the horizontal stage) replaced the UV− mirror. These filter sheets were placed about 10 cm away from the light source, some distance from the stage so that the action of withdrawal and introduction would not startle the test subjects. When the full spectrum lights were switched on, an individual was allowed to orient towards the mirror and reacted to its own image. It was allowed 1 s to display an appropriate form of behaviour (see the next section for appropriate forms of behaviour), and UV filters were either quickly introduced (UV+ to UV−) or withdrawn (UV− to UV+) (time taken ca. 2 s), such that the next behavioural response of individuals, due to the introduced or removed UV filters, were video recorded. Immediately after the male displayed the expected behaviour (i.e. hunched posture initially under UV+ or arched posture initially under UV−), a group of adult males (n=30) were randomly divided into two groups, where one group was exposed to sudden removal of UV light (i.e. UV+ to UV−) and then a week later these same individuals were subjected to the sudden introduction of UV light (i.e. UV− to UV+). The opposite sequence was used for the other group.
Data analysis
The intra-specific behavioural elements of C. umbratica (Lim and Li 2004) were used as guidelines for behavioural analysis via video playbacks. The following variables in the first and second experiments were noted: the number of males that first displayed agonistic or courtship behaviour (between the two males in the conspecific response test) and the duration (to the nearest 0.1 s) of the display. McNemar tests for significance of changes were used when analysing data on the number of males responding to their mirror image in the first experiment or the conspecific males in the second experiment. We also performed paired Wilcoxon signed-rank tests for the first two experiments to compare the duration of displaying a particular behavioural element under UV+ and UV− light conditions (Sokal and Rohlf 1995).
In the third experiment that tested for rapid response to sudden changes in UV cues, the spiders were observed for behavioural changes when UV light was changed. In the UV+ to UV− sequence, the initial behavioural element was agonistic, and a change was recorded if the resultant behaviour was non-agonistic (neutral or courtship behaviour). In the UV− to UV+ sequence, a change in behaviour was recorded when individuals started with either neutral or courtship behaviour ended with agonistic behaviour, or a courting behaviour that ended with either neutral or agonistic behaviour. In both the UV+ to UV− and UV− to UV+ sequences, a total of 30 successful trials were completed. We performed Fisher’s exact tests to compare the number of males that changed their display behaviour when light conditions were switched between UV+ and UV− light.
Two-tailed tests were used and the significance level was set at 0.05 (Zar 1996). All statistical tests were analysed using SPSS 13.0, Chicago, IL, USA.
Results
Adult C. umbratica males displayed different behaviour towards their own mirror image in the presence or absence of UV cues. A significantly higher proportion of males exhibited agonistic display behaviour to their mirror image in the presence of UV light (i.e. UV+; Table 1). In addition, the duration of agonistic display in the presence of UV light was significantly reduced when UV light was removed (Wilcoxon signed rank test: n=30, Z = −3.315, P<0.001; Fig. 5a). More males courted their mirror image when UV cues were absent, but this difference was not statistically significant (Table 1). Similarly, the duration of courtship displays increased when UV light was blocked but this difference was not significant (Wilcoxon signed rank test: n=30, Z = −1.601, P=0.109; Fig. 5b).
Results from the mirror image (a, b) and conspecific (c, d) response tests. Mean (±SE) proportion of duration during which a male C. umbratica displayed to its own image (n=30 individuals) or another size-matched male (n=22 pairs) with agonistic (hunched posture) or courtship (arched posture) behaviour under UV+ (filled bars) or UV– (open bars) light conditions. Asterisks denotes P<0.001 (Wilcoxon signed rank test)
The results from the conspecific response test showed that the removal of UV light significantly reduced the duration of agonistic displays (Wilcoxon signed rank test: n=22, Z = −3.712, P<0.001) (Fig. 5c) and significantly more males showed agonistic displays to conspecifics in the presence of UV light (UV+) (Table 1). When UV part of spectrum was blocked (UV-), a significant increase was observed in both the number of males exhibiting courtship displays (Table 1) and in the duration of courtship displays (Wilcoxon signed rank test: n=22, Z = −3.421, P<0.001; Fig. 5d).
In the third experiment, the rapid response of males to changes in UV light was evident. In the UV+ to UV- sequence, a significant number of males switched from agonistic to non-agonistic or courtship displays (20 of 30; Fisher’s exact test: P<0.05) in response to the sudden removal of UV wavelengths. However, the UV− to UV+ sequence produced opposite outcomes: a significant number of males that initially performed non-agonistic or courtship displays immediately switched to agonistic displays upon introduction of UV cues (23 of 30; Fisher’s exact test: P<0.01).
Discussion
The findings from the first two experiments clearly demonstrate that the visual sensitivity of C. umbratica is extended into the UV wave range (300–400 nm), as the presence of UV cues in both the mirror images and conspecific males elicits agonistic displays. The complete removal of UV cues from both mirror images and conspecifics, resulting in the lack of agonistic behaviour, clearly shows that UV cues are necessary and sufficient for C. umbratica to enable the agonistic displays. Interestingly, the absence of the UV cues not only removed the aggressive behavioural element, but also induced courting behaviour. Males seem to perceive, or rather misidentify, mirror images or conspecifics that lack UV cues as potential mates instead of competing males. The data indicate that UV wavelengths reflected from the male’s body may serve as sex-recognition cues because males reacted agonistically to conspecifics and images with UV cues, but courted them in the absence of UV wavelength, and this may explain the occurrence of UV sexual dimorphism of this species where only male but not female body parts are capable of reflecting UV light (Lim and Li in press).
No statistically significant increase was found in the duration and number of males that displayed courtship behaviour when a UV- mirror replaced a UV+ mirror in the mirror-image response test. This may probably be attributed to the confusion that might arise for a male courting its own image because a female does not court a male or respond to male courtship in the same manner. This unfamiliarity with the image’s courting posture may perhaps discourage the male from proceeding in its courtship as a female normally either reacts agonistically, ignores the male or runs away upon seeing a courting male (Lim and Li 2004). In the conspecific response test, males under UV– conditions either responded to conspecific males by performing agonistic displays or by ignoring them when they were courted; this behaviour was typical of females during courtship. Therefore, significant increases in the number of males performing courtship displays, and in the duration of courtship displays in males, were observed in the absence of UV cues, as courting males could have easily mistaken aggressive males for females.
The third experiment demonstrated that C. umbratica adult males could rapidly respond to changes in UV light. UV light in a microhabitat is never constant (Endler 1993), and in the preferred open habitats of C. umbratica (Lim and Li 2004; in press), males may mistake other conspecific males for females in habitats with UV reduced light (e.g. under leaves or branches). It may be vital that C. umbratica males respond rapidly to changes in the perceived hues of conspecific males by switching their behaviour appropriately (i.e. from courting to agonistic or to avoiding stronger males) to avoid injuries or the loss of UV-reflecting scales from confrontations. Our findings suggest that for C. umbratica males, there are visual signals of which UV cues are an integral part, and thus appear to act like a behavioural switch: when a C. umbratica male sees a conspecific adult carrying this signal, it responds with hunched legs (agonistic display); when it sees a conspecific adult without this signal, it responds with arched legs (courtship display).
For the past few decades jumping spiders have been known to possess UV receptors, and it has often been postulated that UV-based signals are important in salticid visual communication (Crane 1949a,b; DeVoe 1975; Yamashita and Tateda 1976; Blest et al. 1981; Land 1985; Peaslee and Wilson 1989; Blest et al. 1990). Our study is the first behavioural demonstration of the ability of jumping spiders to see into the UV part of the spectrum and highlight the importance of UV cues during intra-specific interactions among salticids. Specifically, UV wavelengths appear to have a significant influence on the male–male interactions of salticids that can reflect UV light. This differs from previous studies that investigated the significance of UV cues in female mate selection (see Cuthill et al. 2000a,b) and other functions (for a review, see Tovée 1995). These studies pointed out that females rely on body-reflected UV hues to judge the relative attractiveness and status of competing males, and prefer UV brighter males. Here, we have shown that the removal of UV cues interferes significantly in male–male interactions. It not only reduces agonistic displays but also induces courtship displays by males in response to their own mirror image and their conspecific opponents. C. umbratica males exhibit inter-male variations in UV reflectance (Lim and Li in press), but whether the quality of males can be accurately assessed by competing males and selective females is unknown. More experiments are required to ascertain the reliability of UV cues as reliable indicators of male quality or physical strength. The roles of UV reflectance and UV-visual ability in male–male interactions of C. umbratica have strong implications for future studies on the sexual selection and coloration of jumping spiders and other animals. Any observation of animal intra-specific interactions under light devoid of, or lacking in UV wavelengths, may result in an incomplete assessment of agonistic or courtship behavioural elements. Therefore, the potential visual sensitivity to UV wavelengths should be taken into account in the future behavioural studies of UV-reflective salticids.
References
Blest AD (1985) The fine structure of spider photoreceptors in relation to function. In: Barth FG (ed) Neurobiology of arachnids. Springer, Berlin Heidelberg New York, pp 79–102
Blest AD, Hardie RC, McIntyre P, Williams DS (1981) The spectral sensitivities of identified receptors and the function of retinal tiering in the principal eyes of a jumping spider. J Comp Physiol 145:227–239
Blest AD, O’Carroll DC, Carter M (1990) Comparative ultrastructure of layer 1 receptor mosaics in principal eyes of jumping spiders: the evolution of regular arrays of light guides. Cell Tissue Res 262:445–460
Crane J (1949a) Comparative biology of salticid spiders at Rancho Grande, Venezuela. Part III. Systematics and behavior in representative species. Zoologica 34:31–52
Crane J (1949b) Comparative biology of salticid spiders at Rancho Grande, Venezuela. Part IV. An analysis of display. Zoologica 34:159–215
Cuthill IC, Partridge JC, Bennett ATD (2000a) Avian UV vision and sexual selection. In: Espmark Y, Amundsen T, Rosenqvist G (eds) Animal signals: signalling and signal design in animal communication. Tapir Academic Press, Trondheim, pp61–82
Cuthill IC, Partridge JC, Bennett ATD, Church SC, Hart NS, Hunt S (2000b) Ultraviolet vision in birds. Adv Stud Behav 29:159–214
DeVoe RD. (1975) Ultraviolet and green receptors in principal eyes of jumping spiders. J Gen Physiol 66:193–207
Endler JA (1993) The colour of light in forests and its implications. Ecol Monogr 63:1–27
Forster LM (1982) Visual communication in jumping spiders (Salticidae). In: Witt PN, Rovner JS (eds) Spider communication: mechanisms and ecological significance. Princeton University Press, Princeton, NJ, pp 161–212
Forster LM (1985) Target discrimination in jumping spiders (Araneae: Salticidae). In: Barth FG (ed) Neurobiology of arachnids. Springer, Berlin Heidelberg New York, pp 249–274
Homann H (1928) Beiträge zur Physiologie der Spinnenaugen. I. Untersuchungsmethoden. II. Das Sehvermögen der Salticiden. J Comp Physiol 7:201–268
Jackson RR (1980) The mating strategy of Phidippus johnsoni (Araneae, Salticidae). Behav Ecol Sociobiol 6:257–263
Jackson RR (1986a) The display behaviour of Cyllobelus rufopictus (Simon) (Araneae, Salticidae), a jumping spider from Kenya. N Z J Zool 13:27–43
Jackson RR (1986b) The display behaviour of Cosmophasis micariodes (L. Koch) (Araneae, Salticidae), a jumping spider from Queensland. N Z J Zool 13:1–12
Jackson RR, Blest AD (1982) The distances at which a primitive jumping spider makes visual discriminations. J Exp Biol 97:441–445
Jackson RR, Hallas SEA (1986) Comparative biology of Portia africana, P. albimana, P. fimbriata, P. labiata and P. schultzi, araneophagic web-building jumping spiders (Araneae: Salticidae) utilization of webs, predatory versality and intraspecific interactions. N Z J Zool 13:423–489
Jackson RR, Macnab AM (1989) Display behaviour of Corythalia canosa, an ant-eating jumping spider (Araneae: Salticidae) from Florida. N Z J Zool 16:169–183
Jackson RR, Whitehouse MEA (1989) Display and mating behaviour of Thorellia ensifera, a jumping spider (Araneae: Salticidae) from Singapore. N Z J Zool 16:1–16
Kästner A (1950) Reaktion der Hüpfspinnen (Salticidae) auf unbewegte farblose und farbige Gesichtsreize. Zoo Beitr NS 1:12–50
Kirschfeld K (1976) The resolution of lens and compound eyes. In: Zettler F, Weiler R (eds) Neural principles of vision. Springer, Berlin, pp 354–370
Labhart T, Nilsson DE (1995) The dorsal eye of the dragonfly Sympetrum: specializations for prey detection against the blue sky. J Comp Physiol A 176:437–453
Land MF (1969) Structure of the retinae of the eyes of jumping spiders (Salticidae: Dendryphantinae) in relation to visual optics. J Exp Biol 51:443–470
Land MF (1971) Orientation by jumping spiders in the absence of visual feedback. J Exp Biol 54:119–139
Land MF (1985) The morphology and optics of spider eyes. In: Barth FG (eds) Neurobiology of arachnids. Springer, Berlin Heidelberg New York, pp 53–78
Land MF, Fernald RD (1992) The evolution of eyes. Ann Rev Neurosci 15:1–29
Land MF, Nilsson DE (2002) Animal eyes. Oxford University Press, Oxford
Li D, Yik SH, Seah WK (2002) Rivet-like nest-building and agonistic behaviour of Thiania bhamoensis, an iridescent jumping spider (Araneae: Salticidae) from Singapore. Raffles B Zool 50:143–151
Lim MLM, Li D (2004) Courtship and male–male agonistic behaviour of Cosmophasis umbratica Simon, an ornate jumping spider (Araneae: Salticidae) from Singapore. Raffles B Zool 52:435–448
Lim MLM, Li D. Extreme ultraviolet sexual dimorphism in jumping spiders (Araneae: Salticidae). Biol J Linn Soc (in press)
Nakamura T, Yamashita N (2000) Learning and discrimination of coloured papers in jumping spiders (Araneae, Salticidae). J Comp Physiol A 186:897–901
Oxford GS, Gillespie RG (1998) Evolution and ecology of spider coloration. Annu Rev Entomol 43:619–643
Peaslee AG, Wilson G (1989) Spectral sensitivity in jumping spiders (Araneae, Salticidae). J Comp Physiol A 164:359–363
Peckham GW, Peckham EG (1889) Observations on sexual selection in spiders of the family Attidae. Occ Pap Wisconsin Nat Hist Soc 1:3–60
Peckham GW, Peckham EG (1890) Additional observations in sexual selection in spider of the family Attidae. Occ Pap Wisconsin Nat Hist Soc 1:117–151
Peckham GW, Peckham EG (1894) The sense of sight in spiders with some observations of the color sense. Trans Wisconsin Acad Sci Arts Lett 10:231–261
Sokal RR, Rohlf FJ (1995) Biometry: the principles of statistics in biological research, 3rd edn. W. H. Freeman, New York
Tovée MJ (1995) Ultraviolet photoreceptors in the animal kingdom: their distribution and function. Trends Ecol Evol 10:455–460
Yamashita S, Tateda H (1976) Spectral sensitivities of jumping spider eyes. J Comp Physiol A 105:29–41
Zar JH (1996) Biostatistical analysis, 3rd edn. Prentice-Hall, NJ
Acknowledgments
We thank Poh Moi Goh for rearing the houseflies. Comments and suggestions from Lian Pin Koh, Robert R. Jackson, Tien Ming Lee, Kathy, F. Y. Su, Eunice J. M. Tan, Jeremy R. W. Woon and Reuben C. Gopalasamy greatly helped to improve the manuscript. This work was supported by grants to D. Li from the National University of Singapore ARC (R−154-000-140-112) and National Natural Science Foundation of China (30470229). The experiments comply with the ‘‘Principles of animal care’’, publication no. 86–23, revised 1985 of the National Institute of Health and also with the current laws of Singapore.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, M.L.M., Li, D. Behavioural evidence of UV sensitivity in jumping spiders (Araneae: Salticidae). J Comp Physiol A 192, 871–878 (2006). https://doi.org/10.1007/s00359-006-0126-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-006-0126-5