Abstract
Rod and cone visual pigments of 11 marine carnivores were evaluated. Rod, middle/long-wavelength sensitive (M/L) cone, and short-wavelength sensitive (S) cone opsin (if present) sequences were obtained from retinal mRNA. Spectral sensitivity was inferred through evaluation of known spectral tuning residues. The rod pigments of all but one of the pinnipeds were similar to those of the sea otter, polar bear, and most other terrestrial carnivores with spectral peak sensitivities (λmax) of 499 or 501 nm. Similarly, the M/L cone pigments of the pinnipeds, polar bear, and otter had inferred λmax of 545 to 560 nm. Only the rod opsin sequence of the elephant seal had sensitivity characteristic of adaptation for vision in the marine environment, with an inferred λmax of 487 nm. No evidence of S cones was found for any of the pinnipeds. The polar bear and otter had S cones with inferred λmax of ∼440 nm. Flicker-photometric ERG was additionally used to examine the in situ sensitivities of three species of pinniped. Despite the use of conditions previously shown to evoke cone responses in other mammals, no cone responses could be elicited from any of these pinnipeds. Rod photoreceptor responses for all three species were as predicted by the genetic data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pinnipeds, a sub-group of the order Carnivora, are unique among mammals in that they are the only large monophyletic lineage that is distinctly amphibious. This makes them particularly interesting from an evolutionary standpoint in that they represent an intermediate stage between terrestrial and aquatic adaptation. Vision is important for pinnipeds, both on land and in water (Schusterman et al. 2000), and there is clear anatomical evidence (e.g., round lens, flattened cornea) that their visual systems are markedly evolved from those of their terrestrial ancestors to enable them to function more effectively in these two diverse media (Walls 1942; Lavigne et al. 1977). Among the features of the visual system that may provide a good index for evaluating the nature and extent of the amphibious adaptation of pinnipeds are the visual pigments. These are often closely correlated with the visual environment, particularly in marine habitats, where visual conditions may vary dramatically (Bowmaker 1995; Hunt et al. 1996; Fasick and Robinson 2000).
Three classes of visual pigments are typically present in mammalian retinas, each characterized by an underlying opsin protein: rod opsin found in the rod photoreceptors, and two opsin types found in the cone photoreceptors, the short wavelength (S) and middle/long wavelength (M/L) sensitive cone opsins (Yokoyama and Yokoyama 1996). While only three opsin classes exist, alterations in the primary structures of individual opsins within these classes can tune the spectral sensitivity of the corresponding visual pigments over a wide range of wavelengths. The effects of these changes are increasingly better understood allowing one to infer the spectral sensitivity of a visual pigment from the translated genetic sequence of the opsin (Fasick and Robinson 2000; Yokoyama and Radlwimmer 2001; Shi and Yokoyama 2003).
Evidence suggests that pinniped visual pigments are different from those of terrestrial carnivores. Although some of these animals have rod pigments similar to those of terrestrial mammals (Crescitelli 1958; Lavigne and Ronald 1975), some deep-diving species have been shown to have modified rod pigments that provide increased sensitivity to the blue-green wavelengths of light that predominate in deep ocean water, λ∼475 nm (Lythgoe and Dartnall 1970; Kirk 1994; Southall et al. 2002). There is also evidence that the cone visual pigments of some pinnipeds have been modified for use in the marine environment. Although most mammals possess two distinct cone pigment types (Jacobs 1993; Yokoyama and Yokoyama 1996), several pinniped species have been found to possess only M/L cones (Peichl and Moutairou 1998; Peichl et al. 2001; Griebel and Peichl 2003). At present, the only large group of mammals known to uniformly lack S cones is the exclusively aquatic cetaceans (Levenson and Dizon 2003). Available information on the M/L cones of pinnipeds is limited, and equivocal. In ERG experiments, the harbor seal (Phoca vitulina) had peak spectral sensitivity near 510 nm (Crognale et al. 1998). A behavioral evaluation of the harp seal (Pagophilus groenlandicus) also reported a peak in spectral sensitivity between 500 and 525 nm, as well as a second peak near 550 nm (Lavigne and Ronald 1972; Lavigne et al. 1977).
Although these data provide some evidence suggestive of adaptation for aquatic vision in the photopigments of pinnipeds, further evaluation of additional species is necessary to better understand the nature of their amphibious adaptation. Towards this end, we have used molecular genetic methods to examine the opsin proteins underlying the rod, S cone, and M/L cone photoreceptor pigments of numerous species representing all three pinniped families. For comparative purposes, opsins of the sea otter (Enhydra lutris) and the polar bear (Ursus maritimus) were also examined. Finally, flicker-photometric electroretinography (ERG) was used to compare in situ spectral sensitivity with that predicted from the genetic data.
Methods
Genetic evaluation of rod and cone visual pigments
To evaluate the visual pigments expressed in the retinas of some marine carnivores, rod and cone opsin sequence data was obtained from retinal messenger RNA (mRNA). The polar bear, the sea otter, and seven pinniped species were examined (Table 1). Retinae from an additional species, P. vitulina, were also examined for the presence of S cones and published M/L sequences for P. vitulina and P. groenlandicus were used to infer their spectral sensitivity.
Eyes used for molecular analysis were obtained from animals that had died of natural causes or that had been euthanized in captivity or wildlife rehabilitation facilities as a consequence of terminal illness/injury. Samples were collected as soon as possible after death and subsequently kept frozen at −20°C or colder. To obtain retinal mRNA, each eye was thawed, the excised retinal tissue was homogenized in Trizol (Gibco BRL, Rockville, MD; ∼1 ml/100 mg of tissue) and RNA was extracted and precipitated with chloroform and isopropanol. Extracted samples were incubated in DNase I (Ambion, Austin, TX, USA) at 37°C for 45 min to eliminate any DNA contamination. The purified mRNA was then converted to cDNA by incubating ∼50 ng with MMLV reverse-transcriptase (Clontech/BD Biosciences, Palo Alto, CA, USA), oligonucleotide (dt)18 primer, and excess dNTPs for 60 min at 42°C. Nonretinal, nuclear DNA was obtained for P. vitulina from skin using a lithium–chloride extraction and ethanol precipitation procedure (Hillis et al. 1996).
Rod and cone opsin gene sequences were amplified and sequenced from the cDNA using polymerase chain reaction (PCR) and dideoxy-terminator cycle sequencing (Sanger et al. 1977). An attempt was made to amplify and sequence rod, M/L, and S cone opsins from the retinal cDNA of every individual examined. Oligonucleotide primers for PCR and sequencing were 15–23 base pairs (bp) in length and were designed from homologous gene sequences obtained from Genbank of cow, dog, cat, and harbor seal (see Table 1, Fig. 1). Some PCR primers for the S cone opsin were additionally optimized using the pinniped sequence obtained from nuclear DNA. For each opsin gene, two pairs of forward and reverse primers were used to obtain two overlapping 400–650 bp sequence fragments. Primer annealing locations were chosen so that the consensus of the two fragments would provide sequence data for nearly the entire gene, including all of the currently described spectral tuning sites for each opsin. Studies indicate that variability in the distal portions of the N and C-terminal regions of the opsin protein are unlikely to affect its spectral properties and so they were not evaluated (Hargrave 1982; Hunt et al. 2001; Shi and Yokoyama 2003).
Parsimonious phylogenetic tree of rod, M/L, and S cone opsin sequences created using bootstrapping (1,000 replicates). Species are listed by the abbreviated forms of their binomial designation, (see Table 1), followed by the opsin type. Bootstrapping values above 50 were retained and are shown at their respective nodes. Individual rod, S, and M/L cone gene trees are identified. Additional sequences were obtained from Genbank for the cow (Bos taurus, U92557, AB062417), dog (Canis familiaris, X71380), cat (Felis catus, AJ417432, AF132040), goat (Capra hircus, H006594), beaked whale (Mesoplodon bidens, AF055316), dolphin (Tursiops truncatus, AF055457), and human (Homo sapiens, AH005296, AH005298, U53874)
Each 50 μl PCR reaction consisted of 5 μl of cDNA (∼100 ng), 36 μl of water, 5 μl of 10×PCR buffer with MgCl2 (2.0 mM), 1 μl of each oligonucleotide primer (10 μM), and 1 μl of Taq DNA polymerase. For amplification, reactions were initially denatured for 5 min at 94°C and then cycled 35 times through the following conditions: denature for 45 s (94°C), anneal for 1 min (55°C), and extend for 1.5 min (72°C). Cycling was followed by a final 5 min extension at 72°C. PCR products were analyzed qualitatively and by size using ethidium–bromide stained agarose-gel electrophoresis. If a reaction failed to produce a desired product, the annealing temperature of the PCR was adjusted to between 37 and 65°C to improve primer performance. This procedure was repeated as necessary until the product was obtained or until the entire range of temperatures (in 1° increments) had been attempted. Once a desired fragment was amplified, the PCR product was purified using Qiaquick columns (Qiagen, Valencia, CA, USA), cycle-sequenced using Applied Biosystems (ABI) Big Dye Version 3 chemistry (Applied Biosystems, La Jolla, CA, USA), and analyzed with an ABI 3100 automated capillary sequencer. The obtained sequences were aligned with homologous sequences of other select mammals to evaluate their identity and the condition of known spectral tuning sites. Alignments were performed with ClustalW (Thompson et al. 1994) and confirmed by eye. Phylogenetic trees and nucleotide identity proportions were determined using PAUP software (Swofford 2001). Spectral sensitivities were inferred for each opsin using available information. As described below, these procedures varied for each opsin type.
Electroretinogram measurements
Visual pigments were evaluated in situ using electroretinograms (ERGs) for a subset of the pinniped species. For this purpose, juvenile pinnipeds were obtained from the marine mammal rehabilitation program at SeaWorld, San Diego, CA. Included in this group were one female and three male Zalophus californianus, one male P. vitulina, and two female Mirounga angustirostris. The animals were maintained in seawater tanks at the Center for Marine Biotechnology and Biomedicine, Scripps Institution of Oceanography, La Jolla, CA, prior to recording and until their eventual release to the wild. All animal husbandry and experimental procedures were conducted according to protocols approved by the National Marine Fisheries Service (NMFS) Office of Protected Resources (permit 732-1487) and the University of California, San Diego Institutional Animal Care and Use Committee (IACUC; permit S00092).
Spectral sensitivity measurements were made using ERG flicker-photometry. Animals were masked and initially anesthetized with 5% isoflurane or halothane. After induction of anesthesia they were intubated and maintained on 0.75–2.0% isoflurane or halothane for the remainder of the experiments. The electrocardiogram and heart rate were monitored throughout testing and during subsequent recovery. As in earlier measurements made on marine mammals (Crognale et al. 1998), anesthetized animals were placed on a slant board and the head position was stabilized through the use of padded restraints. The cornea was anesthetized with topical application of proparacaine hydrochloride (0.5%). Atropine (0.04%) and ophthalmic neosynepherine (10%) were then applied topically to achieve pupillary dilation. ERGs were sensed with bipolar contact lens electrodes of the Burian-Allen design.
The general flicker photometric technique of Jacobs et al. (1996a) was used. In this application, the eye was stimulated with a train of light pulses that originated from two sources: a high-intensity grating monochromator having a 10 nm passband (test light) and an achromatic reference light drawn from a tungsten-halogen lamp (reference light). The two lights were each modulated with a 25% duty cycle. The fundamental components of the ERGs elicited by the two lights were extracted by filtering, averaged over 50 presentations, and then compared. The intensity of the test light was adjusted until the response it elicited exactly matched that produced by the reference light (Jacobs et al. 1996a). The intensity of the test light at this point of equation was taken as a measure of the relative sensitivity of the eye to that test light. During the course of any experiment, each equation point was determined on at least two occasions and these values were subsequently averaged. For these experiments, deviations between matches made under the same test conditions typically did not exceed 0.04 log units. The frequency of the flickering lights, the wavelengths of the test lights, and the intensity of the reference light were varied across experiments. These are noted below as they are appropriate.
Results
Genetic sequencing of rod and cone opsins
Putative rod and M/L cone opsin sequences were obtained from retinal mRNA/cDNA for all species examined (Table 1). Both exhibited genetic distances of less than 0.1 from homologous sequences of their terrestrial relatives. Putative S cone sequences obtained for the sea otter and polar bear showed similarly high levels of identity with other mammalian homologues. No introns or missense mutations were apparent in any of these sequences. No S cone opsin sequences could be obtained from the retinal cDNA of any of the pinnipeds examined. All eight species, including P. vitulina, were tested over a broad range of annealing temperatures, 37 to 65°C, using the same primers that worked effectively on the polar bear and sea otter. Mg2+ conditions were also varied from 1.5 to 3.0 mM in a further attempt to obtain S cone opsin product without success. As a positive control of the oligonucleotide primers, one of the S cone primer sets was used to amplify a fragment of S cone opsin from the nuclear DNA of P. vitulina (Accession number DQ287333). This sequence was readily obtained and the coding region of the nuclear DNA sequence shows a high level of identity with other S cone opsins (Table 1). However, the P. vitulina nuclear DNA S cone opsin sequence was easily distinguished from those obtained from the retinal cDNA of the polar bear and sea otter by the presence of large internal intron sequences.
Figure 1 shows the results of a parsimonious bootstrap analysis of the opsin sequences obtained from retinal mRNA. Also included are several sequences from Genbank (see caption for details). In support of the putative identities, all obtained rod, M/L, and S cone opsin genes grouped exclusively with their respective homologues. Moreover, the rod and M/L cone opsin gene trees were nearly identical in structure, and represent the species examined in accordance with current phylogenetic hypotheses for this group (Rice 1998; Bininda-Emonds 2000).
Aligned translations of all the obtained rod opsin sequences, as well as those of two previously examined pinnipeds, dog, cat, cow, and a deep-diving beaked whale are provided in electronic supplementary material S1. Table 2 summarizes the 23 amino acid differences existing between the rod opsins of the pinnipeds, polar bear, and sea otter-in comparison with those of two terrestrial carnivores, dog and cat. Of these, all residues that could potentially affect the spectral sensitivity of the rod opsins were identified according to Hunt et al. (2001). As indicated, nonsynonymous variations were found at only three spectrally significant sites, residues 83, 292, and 299 (numbered from bovine rhodopsin). Listed at the right side of Table 2 are the inferred spectral sensitivities for each rod opsin, as determined from the mutagenesis data of Fasick and Robinson (2000) for these three sites. M. angustirostris was the only species examined which exhibited any substantial deviation in inferred rod opsin sensitivity from the dog and cat; it possessed a rod opsin with maximum sensitivity (λmax) of 487 nm, at least 12 nm shorter than any of the other pinnipeds. Two of the phocid pinnipeds, as well as the polar bear and sea otter had inferred λmax of 501 nm, which is similar to that of a previously examined phocid, P. vitulina (Fasick and Robinson 2000). The remaining pinnipeds had rod opsins with inferred λmax of 499 nm. In all cases where the sensitivity had previously been determined, inferred sensitivities were within a few nanometers of measured values.
Aligned translations of the obtained M/L cone opsin sequences, as well as previously reported sequences for cat and goat are provided in electronic supplementary material S2. M/L cone sequences of P. groenlandicus and P. vitulina from Genbank were also included for the evaluation of inferred sensitivity. A total of 27 variable sites were identified in comparison with the cat and goat. From these, two separate methods were used to infer M/L cone sensitivity. First, the five-sites rule (Yokoyama and Radlwimmer 1998, 1999, 2001) was used to predict M/L cone sensitivity from variability at residues 180, 197, 277, 285, and 308 (numbered from the human M/L cone opsin). Table 3 shows the inferred sensitivities for each, as well as the effects of substitutions at each of these sites as calculated from the human L cone pigment (λmax 560 nm; Oprian et al. 1991). Both otariid pinnipeds (Z. californianus and Odobenus rosmarus) had all five residues identical to the human L cone, and thus the same inferred spectra. All the phocids had one substitution, Y277F (substitution of Y with F at residue 277), shifting their inferred M/L cone λmax to 552 nm. The M/L cone of the polar bear is the same as that of the cat with the same five critical residues (inferred λmax of 553 nm). The sea otter M/L cone has slightly shorter peak sensitivity than the polar bear and cat with an inferred λmax of 545 nm as a consequence of the substitution, T285A.
A second evaluation of inferred M/L cone sensitivity was attempted using the neural network model of Robinson et al. (1999). The model is restricted to M/L cone opsins with the same residues as either the human “red” or “green” M/L cone opsin at residues 116, 180, 230, 233, 277, 285, and 309. For this method, human “red” and “green” cones have λmax of 561 nm and 533 nm, respectively (Asenjo et al. 1994; Robinson et al. 1999). For four pinnipeds, Erignathus barbatus, M. angustirostris, Leptonychotes weddellii, and Hydrurga leptonyx, it was impossible to evaluate sensitivity with this model as a consequence of the nonsynonymous substitution Y/S116H, the effects of which have not been evaluated. The remaining three phocid pinnipeds, P. groenlandicus, P. vitulina, and Pusa hispida, had another novel substitution G233S. However, as glycine and serine are both hydrophilic polar amino acids of similar size, we proceeded to estimate sensitivity for these species under the assumption that G233S was a synonymous substitution. Assuming this, the inferred λmax of the M/L cones of these species was 550 nm, just 2 nm from that predicted by the five-sites rule. Inferred M/L cone sensitivities for Z. californianus and O. rosmarus were 558 nm, 554 nm for the polar bear, and 543 nm for the sea otter, all of which were similarly close to those obtained with the five-sites model.
In addition to the residues evaluated above, more than 20 other variable sites were found among the obtained M/L cone opsin sequences (see electronic supplementary material S2). Of these, however, only three were nonsynonymous substitutions that could potentially affect the electrochemical nature of the residue (Nathans 1990). One substitution, A58T, occurred in the N-terminal region of the opsin and is thus unlikely to affect sensitivity. The substitution I225T is also unlikely to affect tuning as it does not occur within the chromophore binding region of the opsin, as indicated by evaluation of homologous cite 208 of the rod opsin (Hunt et al. 2001). It is possible that the final substitution, P187R, which occurred in P. groenlandicus, may affect sensitivity, based on its location at the edge of the chromophore binding pocket, although changes at this site have not previously been attributed to any change in spectral sensitivity.
As discussed, no S cone opsin sequences could be found in the retinal mRNA/cDNA of any of the pinnipeds. However, S cone data was obtained from the retinal cDNA of the sea otter and polar bear. Table 4 summarizes the residues of both species, as well as that from cow and human for the nine sites thought to be critical in determining the spectral properties of S cone opsins (Fasick et al. 2002; Shi and Yokoyama 2003). Both the sea otter and polar bear have the same residues as the cow at seven of the nine critical sites. In addition, at one of the two variable sites, residue 118 (numbered according to the bovine S cone opsin), polar bear, sea otter, and cow all have hydrophilic polar residues, i.e., nonsynonymous substitutions. Finally, at residue 93, both the sea otter and polar bear have a nonsynonymous substitution previously shown to cause a 3 nm long-wavelength shift in sensitivity (Cowing et al. 2002). As this is the only apparent functional difference between the two carnivores and the cow, the inferred λmax for the S cones of both the sea otter and the polar bear is 441 nm.
ERG measurements
In earlier ERG measurements made on a pair of P. vitulina, we found evidence for a spectral mechanism having a λmax value of about 510 nm and an absorption spectrum with a shape appropriate for a single photopigment (Crognale et al. 1998). Since this mechanism was detected under test conditions that typically yield indications of photopic activity in many other types of mammal (e.g., light adapted eye, flickering stimulus lights), it seemed to represent a contribution to the ERG from an M/L cone photopigment. The estimated peak of that mechanism, however, is very different from that predicted from the opsin sequence data (Table 3). That striking disparity stimulated us to undertake additional measurements on P. vitulina and on two other species of pinniped.
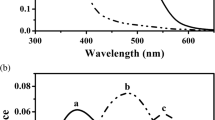
To attempt to provide stimulus conditions even more favorable for eliciting photopic signals, the ambient illumination in the laboratory was increased from that of the previous experiment (from 100 to 495 lux) and the intensity of the reference light was similarly increased by about a factor of four. With these changes in place, spectral sensitivity of a P. vitulina was measured at two wavelengths, 500 and 550 nm. These measurements were made at four different flicker rates, ranging from 10 to 25 Hz. Each pair of the sensitivity values obtained from this procedure was best fitted using standard photopigment absorption curves (Govardovskii et al. 2001) with the λmax values determined to the nearest 0.1 nm. The logic of the experiment was that if the 510 nm peak value obtained earlier reflected mostly contributions from rods, then those signals should become progressively attenuated at higher flicker rates, i.e., one should see a Purkinje shift. Values obtained from six separate measurements on P. vitulina are shown in Fig. 2. The straight line in that plot is the best-fit linear regression. The slope of this line does not differ significantly from zero ( r 2=0.001, ns) indicating that over this range of pulse frequencies there is no significant shift in the spectral peak of the mechanism. The average peak value so measured across all stimulus frequencies was 502.5 nm. That value is very close to the peak predicted and observed in vitro for the rod pigment in this species (λmax 501 nm; Fasick and Robinson 2000) although somewhat shorter than that was measured in two P. vitulina previously tested, λmax 508 nm and 512 nm (Crognale et al. 1998). Finally, measurements were also attempted at frequencies beyond those represented in Fig. 2, but it proved impossible to record any reliable ERG signal from the eye of P. vitulina at rates in excess of about 25 Hz.
Spectral sensitivity as a function of flicker rate measured for two species of pinniped. Plotted for Phoca vitulina and Zalophus californianus are the peaks (λmax) of the photo pigment absorption function obtained from best fits to a series of two-point spectral sensitivity functions (see text). The dashed lines are best-fit linear regressions; neither of these has a slope significantly different from zero (see text)
Complete spectral sensitivity functions were measured using flicker-photometry for four Z. californianus. Figure 3 (top curve) shows the averaged results (±1 SD) obtained from three animals using 12.5 Hz flicker. The best-fitting photopigment absorption curve (continuous line) has a peak value of 501.3 nm. For all four animals complete spectral sensitivity curves at 12.5 Hz were also obtained with a considerably brighter reference light. Those data (Fig. 3, middle curve) are well fit with a pigment having a peak value of 499.2 nm. The peaks of these curves are the same as that predicted for the Z. californianus rod pigment (Table 2) and there is no hint that the brighter lights caused any shift toward the longer wavelengths as would be expected if there were both rod and cone contributions. In a still further attempt to detect cone signals, an experiment was conducted on Z. californianus similar to that described above for P. vitulina, i.e., measuring spectral sensitivity across a range of stimulus frequencies. Sensitivity was measured at 500 and 560 nm at five stimulus frequencies ranging from 5 to 30 Hz and the resulting data were best fit with photopigment absorption curves. The results are shown in the top panel of Fig. 2. As in the case of P. vitulina there is no hint of a shift in spectral sensitivity as a function of flicker frequency ( r 2=0.087, ns). Across seven separate tests, the average spectral peak was 497.8 nm with the individual values covering a total range of only 3.2 nm.
Spectral sensitivity functions for Zalophus californianus (open and filled circles) and Mirounga angustirostris (triangles) as measured with ERG flicker photometry. The data points are mean values for three animals (top), four animals with brighter reference light (middle) and two animals (bottom), respectively (see text). Error bars are ±1 standard deviation. The lines are best-fitting photopigment absorption functions having peaks of 501.3 and 499.2 nm for Z. californianus and 491.7 nm for M. angustirostris
It proved a challenging task to gain good ERG recording from the two Mirounga angustirostris as the recorded signals were generally quite small. Nevertheless, it was eventually possible to make spectral sensitivity measurements for a single condition (12.5 Hz) for both animals. These data are shown in the bottom curve of Fig. 3. Note that despite the difficulty in the recording the results from the two animals are virtually identical. The averaged values for the M. angustirostris tightly fit a photopigment absorption curve having a λmax of 491.7 nm. This short wavelength-shifted sensitivity is reasonably close to the inferred λmax of 487 nm and the observed λmax of 483 nm for this species (Southall et al. 2002). It was impossible to obtain any reliable ERG signals for flicker rates of 20 Hz and higher in this species.
Discussion
The high degree of nucleotide identity seen between the genes sequenced here and homologous rod, M/L, and S cone opsins (Table 1) strongly suggests that these genes are members of the classes to which they have been ascribed, as does their phylogenetic organization into appropriate gene groupings (Fig. 1). In addition, the presence of the obtained opsin genes within the retinal mRNA of these various species is an indication that these visual pigment genes are expressed in their respective retinae. The absence of any introns or missense mutations also suggests that they underlie functional pigment proteins. Although behavioral observations are required to confirm this, we conclude that the presence of these mRNA sequences reflects the existence of functional rod and M/L cone photoreceptors.
Similarly, our inability to amplify any S cone opsin sequences from the retinae of any of the pinnipeds implies these species do not produce S cone visual pigment and thus lack S cone photoreceptors. Of course, negative results must be interpreted cautiously. It is possible that there are so few S cones that amplification procedures were somehow inadequate for their detection. However, the relative ease with which these primers amplified sea otter and polar bear S cone sequences from mRNA and pinniped S cone sequence from nuclear DNA suggests that the lack of S cone mRNA in the retina reflects its absence rather than just our inability to amplify it. This conclusion is supported by previous reports of the absence of S cones in P. vitulina, P. hispida, and E. barbatus obtained from opsin immunolabeling (Peichl et al. 2001; Griebel and Peichl 2003). In addition to a reduction in photopic visual sensitivity, these results also indicate that these pinnipeds are incapable of making cone-based color discriminations. The discrepancy between these findings and previous reports of color vision in some pinnipeds are difficult to reconcile. One possibility is that these animals possess some atypical form of rod and cone based color vision (see Crognale et al. 1998; Peichl et al. 2001 for discussions).
The inclusion of an additional five species to the list of pinnipeds without S cones lends support to the hypothesis that the entire pinniped group lacks this cone type (Peichl et al. 2001). The absence of S cones has been documented in nearly half of all pinnipeds, now including numerous species from the phocid and otariid families, as well as the only living member of the odobenid family. The widespread loss of S cones among the pinnipeds probably reflects the occurrence of a mutational event(s) at a basal point in the pinniped lineage. A similar phenomenon has been shown to underlie the loss of S cones among cetaceans (Levenson and Dizon 2003). The absence of S cones in pinnipeds is remarkable as the vast majority of terrestrial mammals studied have been found to possess both S and M/L cone visual pigments (Jacobs 1993; Jacobs et al. 1993; Yokoyama and Yokoyama 1996) with a loss of S cones limited to isolated occurrences at the most distal branches of the primate, carnivore, and rodent lineages (e.g., Jacobs et al. 1996b; Peichl and Moutairou 1998). The convergent widespread loss of S cones in these two divergent marine mammal groups suggests that the absence of this cone type may be linked to adaptation for aquatic vision; for example, to produce a reduction in sensitivity to the short wavelengths which are highly scattered in aquatic environments and hence potentially not conducive to good spatial vision (see Peichl et al. 2001, Cowing et al. 2002 for discussions).
In contrast, our results predict that the M/L cone pigments of pinnipeds have maximum sensitivities of 550–560 nm, values much more similar to those of terrestrial carnivores than to the short-wavelength shifted M/L cones of the bottlenose dolphin (Tursiops truncatus; Fasick and Robinson 1998). If one assumes that the sensitivity of the dolphin’s M/L cones is shifted from that of its terrestrial relatives as a consequence of adaptation to the relatively short wavelengths of light present under water, then clearly the M/L pigments of pinnipeds have not undergone a similar adaptation for aquatic life. Pinnipeds have similar spectral sensitivities to other terrestrial carnivores, yet, their M/L cone opsins have different residues at several of the identified spectral tuning sites (see Table 3). A parsimonious interpretation of this suggests that the maintenance of the long-wavelength sensitivity of pinniped M/L cones reflects an active selective process, rather than being solely a consequence of phylogenetic inertia and the maintenance of the ancestral terrestrial condition.
Like the M/L cone pigments, the rod visual pigments of most pinnipeds do not appear to be specifically adapted for the aquatic environment. With the addition of the present data roughly half of the pinniped group has now been examined including most of the deeper-diving species, as well as species from a wide range of habitats. It appears that the rod visual pigments of most pinnipeds are quite similar to those of terrestrial mammals, with λmax very near 500 nm. Despite the examination of a number of other deep diving species, such as E. barbatus and L. weddellii, the short-wavelength shifted rod pigments of M. leonina and M. angustirostris have been found to be exceptional among the pinnipeds. Only they have rod pigments with λmax below 495 nm, similar to the rod opsins of “blue-shifted” cetaceans (McFarland 1971; Fasick and Robinson 2000) and many deep-sea fish (Hunt et al. 1996; Bowmaker 1995, 1998).
The similarity between pinniped and terrestrial mammals’ rod opsins does not appear to be solely a consequence of phylogenetic inertia. As is the case for the M/L cones, although pinnipeds have maintained rod opsins with λmax near 500 nm, many species have different combinations of spectrally significant residues than their terrestrial relatives. In fact, only three species, P. vitulina, P. hispida, and E. barbatus, in addition to the polar bear and the sea otter, possess the same combination of critical amino acids as the terrestrial carnivores. The similarity between the sensitivities of visual pigments of pinnipeds and terrestrial mammals suggests that these animals have a continued need for effective terrestrial vision. Unlike cetaceans, pinnipeds still spend significant portions of their lives out of water. They give birth on land or ice, and most species remain ashore for substantial periods of time to rest, breed, and molt. During this time they are involved in potentially complex intra-species interactions and may face the threat of terrestrial predators where the tele-receptive properties of the visual sense would be quite valuable. It is true that relatively long-wavelength sensitivity may provide some benefit to species inhabiting shallow coastal waters where maximum light transmission occurs at wavelengths near or above 500 nm (Kirk 1994). However, the similarity of pigments among nearly every species examined from such a wide range of aquatic habitats supports the hypothesis that pinniped visual pigments continue to be primarily selected for good visual sensitivity out of the water. These findings emphasize that, in addition to vision, the well-developed nonvisual sensory modalities (e.g. hearing, tactile-vibrissae sensitivity) are probably also of great importance to pinnipeds under water (Schusterman et al. 2000; Dehnhardt et al. 2001).
ERG measurements
The peaks predicted for pinniped rod pigments based on amino acid sequence (Table 2) agree with inferences drawn from the ERG measurements. The average of the several estimates made for P. vitulina tested at different stimulus frequencies is 502.5 nm, which compares well with the prediction of 501 nm from the genetic data. The several different estimates made for Z. californianus from both full spectra and two-point spectral sensitivity functions average 499.4 nm, essentially identical to that inferred from the sequence information. Finally, the spectra measured for two M. angustirostris had an average peak of 491.7 nm which also compares well with the 487 nm peak predicted from the genetic data. The in situ measurements seem to yield slightly longer peaks than the genetic predictions, especially for the elephant seal. However, deviations of this sort are not unique to pinnipeds. For example, ERG measurements of rod signals in the dog (Jacobs et al. 1993) suggest a spectral position that is also slightly longer than that predicted by tuning models. Reconciliation of these differences may well be unique for each case, but it is worth remembering that in situ measurements like these ERG recordings are influenced not only by the pigment spectrum, but also by any intraocular filtering and reflectivity. In general all these factors will tend to displace spectral sensitivity curves toward the longer wavelengths. That being said, the agreement between the direct measurements and tuning model predictions are remarkably close for the pinniped rod pigments here examined.
Whereas there is good agreement between the predictions from rod opsin sequences and ERG spectral sensitivity for these pinnipeds, this was not the case for cones. Indeed, there was no clear evidence in the ERG signals of any contribution from cone pigments that were predicted from the molecular genetic results. This is particularly surprising, because these very same ERG procedures have been successfully employed to access cone-generated signals in a very wide range of other mammals (Jacobs 1993). It has been known for well more than half a century that rod and cone contributions to the ERG can be separated by using flickering lights with rod contributions being superceded by cone contributions as flicker rate is increased (Adrian 1946). The current clinical standard for measuring human cone-based vision with the ERG specifies 30 Hz flicker (Marmor and Zrenner 1999), but cone spectra emerge from ERG flicker measurements at much lower rates than that. For example, flicker rates as slow as 12.5 Hz yield clear cone-based spectral sensitivity functions in rodents like the mouse and rat (Jacobs et al. 1991, 2001) and carnivores such as the ferret (Calderone and Jacobs 2003). The two-point spectral sensitivity functions (Fig. 2) obtained from Z. californianus and P. vitulina are particularly compelling in this regard. In these animals there is no hint of an intrusion of any cone contribution up to rates as high as 30 Hz. The absence of any cone contribution can be made explicit by calculating what would happen to measured spectral sensitivity in Z. californianus if there were joint rod and cone contributions. Given the estimated rod and cone peaks in this species (499 and 560 nm), combining the two in a proportion of 5:1 would shift peak spectral sensitivity from the rod peak of 499 nm out to 510 nm; even with the rod:cone ratio set at 9:1 the sensitivity peak would shift to about 504 nm, a value which is a full 7 nm longer than was measured at a stimulus frequency of 30 Hz and 5 nm displaced from what was measured at any flicker rate. In short, no hint of cone signals was detected in the ERGs of these pinnipeds with test conditions that routinely elicit such signals in a range of terrestrial mammals.
Is there something unique to pinniped eyes that render particularly difficult the recording of cone signals with the ERG? For instance, could it be that the cone population is simply too small to yield reliable signals? Although there is no information regarding either Z. californianus or M. angustirostris, opsin antibody labeling has been used to assess photoreceptor numbers in numerous pinnipeds, including P. vitulina and several other phocid and otariid species (Peichl and Moutairou 1998; Peichl et al. 2001). In these studies, M/L cones reach peak density values of 7,000–11,000 per mm2. These values are no different from cone densities seen in some terrestrial carnivores from which ERG cone signals have been readily recorded, e.g., dog and ferret (Jacobs et al. 1993; Calderone and Jacobs 2003). Thus, it is not obvious that a paucity of cones can account for the recording failure. It is also possible that the physiological integrity of the outer retina in these diving mammals is particularly susceptible to the effects of anesthesia. Isoflurane and halothane are known to have detrimental effects upon the bipolar cells of the retina in some species (e.g., Wasserschaff and Schmidt 1986; Tremblay and Parkinson 2003). If that were the case then, somehow, these effects would have to be more pronounced for cone than for rod-based signals. Finally, although it may seem unlikely, there is the possibility that the failure to record cone-based signals in the ERG accurately reflects events in the retina and thus predicts that these receptors contribute little to the visual lives of pinnipeds. The pupils of some of these species are quite small under even moderately mesopic visual conditions which could dramatically limit retinal illumination (Levenson and Schusterman 1997). Again, good behavioral studies of vision in these amphibious mammals are needed to assess that possibility.
In view of the combined findings from genetics and ERG, it seems that earlier reports of photopic sensitivity and cone-based color discrimination in pinnipeds were likely confounded by the inadvertent inclusion of rod photoreceptor responses, despite testing under apparently photopic conditions. The pinnipeds examined here uniformly lacked S cones, leaving them relatively insensitive to short-wavelength light, and subsequently without the ability to make cone-photoreceptor based color discriminations. The distribution of the S cone loss suggests that the entire pinniped group probably lacks this cone type, a loss that seems to be related to adaptation for aquatic vision. In contrast, the rod and M/L cones of pinnipeds are very much like those of terrestrial carnivores, except for the relatively short-wavelength sensitive rod opsins of the extremely deep-diving elephant seals. This similarity with terrestrial species is most probably related to continued selective pressure to maintain good terrestrial vision, although potential benefits for aquatic vision might also be realized for some species. Overall, the visual pigment complements of pinnipeds are exactly as their amphibious nature would predict: they have rod and M/L cone sensitivities like those of terrestrial carnivores, but lack short-wavelength sensitive cones like the entirely aquatic cetaceans.
References
Adrian ED (1946) Rod and cone components in the electric response of the eye. J Physiol 105:24–37
Asenjo AB, Rim J, Oprian DD (1994) Molecular determinants of human red:green color discrimination. Neuron 12:1131–1138
Bininda-Emonds ORP (2000) Factors influencing phylogenetic inference: a case study using the mammalian carnivores. Mol Phylo Evol 16:113–126
Bowmaker JK (1995) The visual pigments of fish. Prog Retinal Eye Res 15:1–31
Bowmaker JK (1998) Evolution of colour vision in vertebrates. Eye 12:541–547
Calderone JB, Jacobs GH (2003) Spectral properties and retinal distributions of ferret cones. Vis Neurosci 20:11–17
Chiu MI, Zack DJ, Wang Y, Nathans J (1994) Murine and bovine blue cone pigment genes: cloning and characterization of two new members of the S family of visual pigments. Genomics 21:440–443
Collins FD, Morton RA (1950). Studies on rhodopsin. I. Methods of extraction and the absorption spectrum. Biochem J 47:3–10
Cowing JA, Poopalasundaram S, Wilkie SE, Bowmaker JK, Hunt DM (2002) Spectral tuning and evolution of short wave-sensitive cone pigments in cottoid fish from Lake Baikal. Biochem 41:6019–6025
Crescitelli F (1958) The natural history of visual pigments. Ann New York Acad Sci 74:230–255
Crognale MA, Levenson D, Ponganis PJ, Deegan JFII, Jacobs GH (1998) Cone spectral sensitivity in the harbour seal (Phoca vitulina) and implications for colour vision. Can J Zool 76:2114–2118
Dehnhardt G, Mauck B, Hanke W, Bleckmann H (2001) Hydrodynamic trail-following in harbor seals (Phoca vitulina). Science 293:102–104
Fasick JI, Applebury ML, Oprian DD (2002) Spectral tuning in the mammalian short-wavelength sensitive cone pigments. Biochem 41:6860–6865
Fasick JI, Lee N, Oprian DD (1999) Spectral tuning in the human blue cone pigment. Biochem 38:11593–11596
Fasick JI, Robinson PR (1998) Mechanism of spectral tuning in the dolphin visual pigments. Biochem 37:433–438
Fasick JI, Robinson PR (2000) Spectral-tuning mechanisms of marine mammal rhodopsins and correlations with foraging depth. Vis Neurosci 17:781–788
Govardovskii VI, Fyhrquist N, Reuter T, Kuzimin DG, Donner K (2001) In search of the visual pigment template. Vis Neurosci 17:509–528
Griebel U, Peichl L (2003) Color vision in aquatic mammals: facts and open questions. Aquat Mammals 29:18–30
Hargrave PA (1982) Rhodopsin chemistry, structure, and topography. Prog Retinal Eye Res 1:1–51
Hillis DM, Moritz C, Mable BK (1996) Molecular systematics, 2nd edn. Sinauer, Sunderland
Hunt DM, Dulai KS, Partridge JC, Cottrill P, Bowmaker JK (2001) The molecular basis for spectral tuning of rod visual pigments in deep-sea fish. J Exp Biol 204:3333–3344
Hunt DM, Fitzgibbon J, Slobodyanyuk SJ, Bowmaker JK (1996) Spectral tuning and molecular evolution of rod visual pigments in the species flock of Cottoid fish in Lake Baikal. Vis Res 36:1217–1224
Jacobs GH (1993) The distribution and nature of colour vision among the mammals. Biol Rev 68:413–471
Jacobs GH, Neitz J, Deegan JFII (1991) Retinal receptors in rodents maximally sensitive to ultraviolet light. Nature 353:655–656
Jacobs GH, Deegan JFII, Crognale MA, Fenwick JA (1993) Photopigments of dogs and foxes and their implications for canid vision. Vis Neurosci 10:173–180
Jacobs GH, Neitz J, Krogh K (1996a) Electroretinogram flicker photometry and its applications. J Opt Soc Am A13:641–648
Jacobs GH, Neitz M, Neitz J (1996b) Mutations in S-cone pigment genes and the absence of colour vision in two species of nocturnal primate. Proc R Soc Lond Biol Sci 263:705–710
Jacobs GH, Fenwick JA, Williams GA (2001) Cone-based vision of rats for ultraviolet and visible lights. J Exp Biol 204:2439–2446
Kirk JTO (1994) Light and photosynthesis in aquatic ecosystems, 2nd edn. Cambridge University Press, Cambridge
Lavigne DM, Bernholz CD, Ronald K (1977) Functional aspects of pinniped vision. In: Harrison RJ (ed) Functional anatomy of marine mammals, vol 3. Academic, London, pp 135–173
Lavigne DM, Ronald K (1972) The harp seal, Pagophilus groenlandicus, part 23—spectral sensitivity. Can J Zool 50:1197–1206
Lavigne DM, Ronald K (1975) Pinniped visual pigments. Comp Biochem Physiol 52B:325–329
Levenson DH, Schusterman RJ (1997) Pupillometry in seals and sea lions: ecological implications. Can J Zool 75:2050–2057
Levenson DH, Dizon A (2003) Genetic evidence for the ancestral loss of short-wavelength-sensitive cone pigments in mysticete and odontocete cetaceans. Proc R Soc Lond Biol Sci B270:673–679
Lythgoe JN, Dartnall HJA (1970) A “deep sea rhodopsin” in a mammal. Nature 227:955–956
Marmor MF, Zrenner E (1999) Standard for clinical electroretinography, 1999 update. Doc Ophth 97:143–156
McFarland WN (1971) Cetacean visual pigments. Vis Res 11:1065–1076
Nathans J (1990) Determinants of visual pigment absorbance: role of charged amino acids in the putative transmembrane segments. Biochem 29:937–942
Oprian DD, Asenjo AA, Lee N, Pelletier S (1991) Design, chemical synthesis, and expression of genes for the three human color vision pigments. Biochem 30:11367–11372
Peichl L, Behrmann G, Kroger RHH (2001) For whales and seals the ocean is not blue: a visual pigment loss in marine mammals. Eur J Neurosci 13:1520–1528
Peichl L, Moutairou K (1998). Absence of short-wavelength sensitive cones in the retinae of seals (Carnivora) and African giant rats (Rodentia). Eur J Neurosci 10:2586–2594
Rice DW (1998) Marine mammals of the world: systematics and distribution. Society for Marine Mammalogy, Lawrence, KS
Robinson PR, Griffith K, Gross JM, O’Neil MC (1999) A back-propagation neural network predicts absorption maxima of chimeric human red:green visual pigments. Vis Res 39:1707–1712
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Nat Acad Sci USA 74:5463–5467
Schusterman RJ, Kastak D, Levenson DH, Reichmuth CJ, Southall BL (2000) Why pinnipeds don’t echolocate. J Acoust Soc Am 107:2256–2264
Shi Y, Yokoyama S (2003) Molecular analysis of the evolutionary significance of ultraviolet vision in vertebrates. Proc Nat Acad Sci USA 100:8308–8313
Southall KD, Oliver GW, Lewis JW, Le Boeuf BJ, Levenson DH, Southall BL (2002) Visual pigment sensitivity in three deep diving marine mammals. Mar Mam Sci 18:275–281
Swofford DL (2001) Phylogenetic analysis using parsimony, version 4. Sinauer, Sunderland
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucl Acid Res 22:4673–4680
Tremblay F, Parkinson JE (2003) Alteration of electroretinographic recordings when performed under sedation or halogenate anesthesia in a pediatric population. Doc Opthalm 107:271–279
Walls G (1942) The vertebrate eye and its adaptive radiation. The Cranbrook Institute of Science, Bloomfield Hills
Wasserschaff M, Schmidt JGH (1986) Electroretinographic responses to the additional of nitrous oxide to halothane in rats. Doc Ophthalm 64:347–354
Yokoyama S, Radlwimmer FB (1998) The “five sites” rule and the evolution of red and green color vision in mammals. Mol Biol Evol 15:5660–5667
Yokoyama S, Radlwimmer FB (1999) The molecular genetics of red and green color vision in mammals. Genetics 153:919–932
Yokoyama S, Radlwimmer FB (2001) The molecular genetics and evolution of red and green color vision in vertebrates. Genetics 158:1697–1710
Yokoyama S, Yokoyama R (1996) Adaptive evolution of photoreceptors and visual pigments in vertebrates. Ann Rev Ecol Sys 27:543–567
Acknowledgments
Our principal thanks go to the marine mammal stranding program of SeaWorld, San Diego, CA for providing rehabilitated animals for electroretinographic (ERG) examination, as well as tissue samples from several species for genetic study. Representatives of the U.S. Fish and Wildlife Service (Fairbanks, AK), Alaska Department of Fish and Game (Anchorage, AK), California Department of Fish and Game (Santa Cruz, CA), and Dr. Siniff’s Antarctic research group (McMurdo station, Antarctica) also provided valuable samples for genetic examination. We appreciate the valuable input of the dissertation committee members of DHL who reviewed earlier versions of this manuscript. We thank the graduate student support for DHL provided by the NSF Office of Polar Programs (NSF 98-14794). Financial support for this project provided by the San Diego Achievement Rewards for College Scientists (ARCS) program to DHL, NSF (IBN 00-78540) and the UCSD Chancellors Fund grants to PJP, and an NIH grant (EY002052) to GHJ. All animal husbandry and experimental procedures were conducted following protocols approved by the National Marine Fisheries Service (NMFS) Office of Protected Resources (permit 732–1487), the University of California, San Diego Institutional Animal Care and Use Committee (IACUC; permit S00092), and the NIH Principles of animal care, publication No. 86-23 (1985).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Levenson, D.H., Ponganis, P.J., Crognale, M.A. et al. Visual pigments of marine carnivores: pinnipeds, polar bear, and sea otter. J Comp Physiol A 192, 833–843 (2006). https://doi.org/10.1007/s00359-006-0121-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-006-0121-x