Abstract
The honeybee queen pheromones promote both worker sterility and worker-like pheromone composition; in their absence workers become fertile and express the queen pheromones. Which of the queen pheromones regulate worker pheromone expression and how, is still elusive. Here we investigated how two queen pheromones, the mandibular and Dufour’s, singly or combined, affect worker ovarian activation and occurrence of queen-like Dufour’s esters. Although queen mandibular pheromone (QMP) alone, or combined with Dufour’s secretion, inhibited to some extent worker reproduction, neither was as effective as the queen. The effect of the queen pheromones on worker pheromone expression was limited to workers with developed ovaries. Here too, QMP and Dufour’s combined had the greatest inhibitory effect. In contrast, treatment with Dufour’s alone resulted in augmentation of esters in the workers. This is another demonstration that a pheromone emitted by one individual affects the rates of its production in another individual. Ester production was tightly coupled to ovarian development. However fertile workers from queenright or QMP-treated colonies had significantly higher amounts of esters in their Dufour’s gland than untreated queenless colonies. The fact that the queen or QMP exert greater suppression on signal production than on ovary activation, suggests disparate regulatory pathways, and presents a challenging ultimate as well as proximate questions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The honeybee queen pheromones have a profound effect on worker physiology and behavior, as evident from the dramatic changes in these upon removal or death of the queen, and their partial restoration by applying queen extracts (Winston 1987). Accordingly, there are a multitude of queen-specific pheromones including that of the mandibular gland (QMP), tergal glands (Wossler and Crewe 1999a), Dufour’s gland (Katzav-Gozansky et al. 1997) and feces (Page et al. 1988). The complexity of the queen signals presents a challenging ultimate and proximate questions regarding the regulation of queen–worker interactions in general and queen-reproductive monopoly in particular. These pheromone-effects are especially intriguing in light of the multiple evidence that both the mandibular and Dufour’s gland queen pheromones may also occur in QL workers, in particular in egg-laying workers (Crewe and Velthuis 1980; Plettner et al. 1996; Katzav-Gozansky et al. 2000a; Simon et al. 2001). Biosynthetic studies with Dufour’s gland demonstrated that queens but not QR workers synthesize esters, while QL egg-laying workers become queen-like in that they biosynthesize fair amounts of these esters. Studies in vitro shed further light on the regulatory system, revealing that Dufour’s gland of all sources biosynthesized the queen-like esters, irrespective of whether they belonged to queens, QR, or QL workers (Katzav-Gozansky et al. 2000b). This implies that ester biosynthesis in the gland is the default situation, but is inhibited in the presence of the queen, similar to workers’ reproduction.
Reproductive monopoly of the queen honeybee is generally attributed to a tight regulation of worker sterility by queen pheromones [but see Keller and Nonacs (1993) for a different interpretation of the queen pheromone role in this process]. However, the mechanisms underlying this process are only partially understood, and several lines of evidence suggest that more than one pheromone may be involved. For example, the large variability among colonies in the response to QMP (Hoover et al. 2003), suggests that the QMP alone cannot fully explain worker ovary inhibition. Additional studies showing that the tergal glands as well as brood pheromones have such an inhibitory effect (Wossler and Crewe 1999b; Mohammedi et al. 1998) corroborate the multiple pheromone hypotheses. These multiple studies also suggest that some of the queen pheromones that may not have a direct effect on worker ovary inhibition may exert an indirect effect on worker reproductive strategy, thereby leading to sterility.
The fact that QL workers concomitantly develop ovaries and produce the queen pheromones may provide a tool for understanding the effects of particular pheromones when presented singly or as multiple pheromone complexes. For example, evidence suggests that the tight coupling between the occurrence of queen characteristic Dufour’s esters and ovary activation indicates that the former acts as a fertility signal (Katzav-Gozansky et al. 2004; Dor et al. 2005). Likewise, the mandibular glands were suggested to indicate reproductive dominance among competing QL workers (Plettner et al. 1993; Moritz et al. 2000, 2004).
In spite of the accumulating knowledge regarding worker pheromonal plasticity, the cues and mechanisms regulating this plasticity are still unknown. In this respect, the honeybee system provides a rare case in which it appears that the production of sets of pheromones is dependent on the perception of these very same pheromones. Furthermore, the phenomenon seems to be unidirectional: i.e., the occurrence of these pheromones in workers is modulated by their emission by the queen, but not vice versa.
In the present study we investigated the effects of two queen pheromones, the mandibular and Dufour’s, singly or combined, on worker ovarian activation and the production by workers of the queen-like Dufour’s esters. We further tested which of the two pheromone systems, if any, modulates the occurrence of the queen-esters in workers.
Material and methods
Bees
All the experiments were conducted with colonies of Apis mellifera ligustica, which were either obtained from Nir-Galim Apiary, or reared at the I. Meier Segals Garden for Zoological Research at Tel Aviv University, between March and September of 2001. Thirty-eight QL and QR colonies of approximately 3,000 workers each were set in four-comb mini-hives (22×27×24 cm3) each separated in the middle by a queen excluder (QR as well as QL). Half of each hive contained one comb with empty cells and the other half a comb with honey and pollen. All combs were broodless to eliminate possible effect of brood pheromone on worker ovarian development. The queen was placed in the inner compartment of the hive (not the free-foraging side). The experiment was repeated twice, comprising 15 and 23 colonies, respectively.
Experimental setup
To test the effect of the queen pheromones on worker reproduction and Dufour’s gland secretion, QL colonies were treated with: (1) queen Dufour’s gland secretion, (2) synthetic queen mandibular pheromone (QMP), or (3) a combination of both. Two control groups were composed: untreated QL colonies and untreated QR colonies (with a queen excluder as previously described).
Synthetic QMP was applied at the beginning of the experiment using bee boost (Phero Tech, Inc. Canada; slow release lure containing 10 queen equivalents (Qeq) QMP with a release rate of about 1/3 Qeq per day; Ledoux et al. 2001). Dufour’s glands were removed from mated queens and extracted in dichloromethane. One queen equivalent (20 μg/gland; Katzav-Gozansky et al. 1997) mixed with candy (mixture of sugar and honey) was provided daily after solvent evaporation. The pheromones were placed in the inner compartment (the one containing the queen in the QR control hives). The combs in the queen-excluded parts were inspected every other day for the appearance of eggs. Onset of worker egg-laying was defined as such when more than five eggs appeared in a comb. We observed that at the onset of oviposition, when only a few eggs were deposited, they were usually destroyed, rendering it difficult to determine precisely when egg-laying had started. The occurrence of five eggs per comb, on the other hand, represents a more reliable, although conservative, parameter for initiation of worker reproduction (Katzav-Gozansky et al. 2004). Sampling of bees started when worker egg-laying started in the QL-control colonies (about 8 days after colony establishment). Fifteen workers were collected from each colony every 2 days for 10 additional days (total of five collections). The bees were dissected for determination of ovary development and Dufour’s gland extraction. Ovarian development was classified as: stage 1 = undeveloped, stage 2 = early stage of development, stage 3 = ovaries with full-size egg.
Chemical analyses
Workers’ Dufour’s glands were extracted in 50 μl of dichloromethane containing 100 ng of eicosane as internal standard. Glands from bees with ovaries at stage 3 were extracted individually, whereas glands from bees with ovaries at stage 1 or 2 were extracted in pairs to obtain enough material for proper quantification. The amount was calculated as ng/gland. Samples were stored at −20°C until chemical analysis. Glandular components were quantified by gas chromatography (Varian CP 3800) equipped with DB-1 fused silica column temperature programmed from 150°C to 300°C at 5°C/min. Esters were identified according to Katzav-Gozansky et al. (1997, Table 2; note that tetradecyl dodecanoate and tetradecyl-(Z)-9-tetradecenoate were added).
Statistical analyses
Statistical analyses were performed using Statistica for Windows; version 7.0, Statsoft, Inc. χ2-test was used to compare the onset of egg-laying between QR and QL colonies. Kruskal–Wallis test was used to compare onset of egg-laying among the QL colonies. One-way ANOVA followed by Scheffé post hoc test was used to compare treatment effect on the percentage of egg-laying workers. To convert percentages to continuous values, they were transformed using Arcsin sqrt x (Sokal and Rohlf 1995).
For analysis of the chemical data the bees with ovarian levels 2 or 3 were pooled together and termed as bees with developed ovaries then analyzed, using t test for differences in total amount and ester proportion (all esters included). Mann–Whitney U test was used to compare between the amounts of esters found in QR versus QL bees. Global effects of the various treatments on chemical composition were tested for significance using Kruskal–Wallis followed by multiple comparisons post hoc tests. Statistical significance was accepted at P=0.05. Data are presented as means ± SE.
Results
Worker reproduction
Worker egg-laying in the QL hives was observed in 80–100% of the colonies, depending on the treatment, between seven to 12 days after onset of the experiment. In two out of 10 QR colonies (20%) egg-laying occurred also in the queen-excluded compartment, and worker dissections revealed that 12±5.8% of the bees (n=37) in these colonies had developed ovaries, compared to 8±2.3% in all QR colonies (range 0–18.2%). Since egg transfer between cells is very rare in honeybees we consider these eggs as worker-laid. Among QL colonies the percentage of colonies with worker oviposition was the lowest (80%), however, not statistically significant. Global comparison of all hives revealed that none of the queen pheromones were as effective as the queen in inhibiting worker egg-laying; two out of 10 colonies in the QR colonies versus 26 out of 28 colonies in the QL colonies (all treatments combined) started egg laying (χ2=20.17, df=1, P<0.001). Furthermore, there was no treatment effect on the onset of oviposition among the QL colonies (pheromone treatment and control; Kruskal–Wallis test H 3,26=4.02, P=0.26).
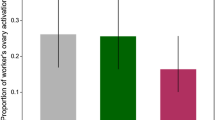
By day 16 the number of colonies in which workers started to lay eggs had stabilized (reached a plateau in no. of colonies with laying workers) and therefore we monitored ovarian development of the sampled workers in the queen-excluded compartment on this day. Figure 1 presents the average percentage of bees with developed ovaries in each of the hives. The queen’s presence was the most effective of all treatments in inhibiting worker ovarian development (ANOVA F 4,17=11.8, P<0.0001; Scheffé post hoc test P<0.002, 0.002, and 0.0001 for QR–QL, QR–QMP, and QR–Dufour’s gland, respectively). QMP but not queen Dufour’s secretion had some inhibitory effect on workers’ ovarian development; however, this difference was not statistically significant. Treatment with the two pheromones combined was even more effective; however, none were significantly different from the QL control (Fig. 1).
The effect of queen glandular source on ovary development in the queen-excluded compartment in colonies with queen, or QL treated with QMP, Dufour’s gland extract or both at day 16 (n=38 colonies in total). Numbers represent the number of colonies. Statistical analysis of the percent of developed ovaries was performed by ANOVA F 4,17=11.8, P<0.0001 followed by Scheffé post hoc test. Different letters denote statistical differences at P=0.05
The effect of the queen glandular secretion on Dufour’s gland composition
As expected, the presence of the queen affected the amount of queen-like esters found in Dufour’s gland. Only 16% (n=204) of the QR workers, [12 (63%) and 20 (37%) bees with developed and undeveloped ovaries, respectively] had detectable amounts (>1 ng) of esters in their gland, compared to 69% (n=214) among QL workers (of which 119 (81%) and 28 (19%) had developed and undeveloped ovaries, respectively). If we consider only bees with fully developed ovaries (stage 3), QR workers had only 12±5.2 ng esters per gland compared to 95±34.2 in QL bees (Mann–Whitney U test Z=−3.67, P<0.0001).
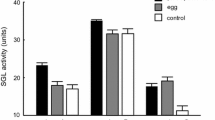
Comparing all the QL bees irrespective of treatment revealed that the total amount of secretion found in Dufour’s gland was ovary-dependent; namely, significantly higher in bees with developed ovaries as compared to bees with undeveloped ovaries (Fig. 2 inserted table). The total amount found in bees with undeveloped ovaries was 236±67.7 ng (n=337) compared to 488±25 ng (n=340), among bees with developed ovaries (t=7.16, df=1352, P<0.001). This difference was even more profound when comparing ester proportion (4±1.5% vs. 13±1.6%, in bees with undeveloped and developed ovaries, respectively; t=−84.1, df=1352, P<0.001).
Total amount of queen-like esters (ng/gland) in Dufour’s gland of workers receiving a different glandular source treatment in bees with developed and undeveloped ovaries (n=340 and 337 bees; respectively). The table refers to the total amount of secretion in the gland. Numbers represent the number of bees. Kruskal–Wallis performed statistical analysis followed by multiple comparisons P values. For statistical detail see Results. Different letters denote statistical differences at P=0.05
There was no treatment effect on both the total amounts and ester amounts among bees with undeveloped ovaries [Fig. 2, white bars and inserted table; Kruskal–Wallis H(3,N=337)=2.7, and 4.24; respectively, P>0.5]. Considering only bees with developed ovaries, both the total secretion and ester amounts found in Dufour’s gland were affected by the treatment with queen pheromone [Fig. 2, black bars and inserted table; Kruskal–Wallis H(3,N=340)=9.26, P=0.03 and H(3,N=340)=8.6, P=0.035, respectively]. However, due to large within-treatment variation among bees, between-treatment differences were not highly significant. Although bees treated with a combination of QMP + Dufour’s had the lowest amount of esters, it was not significantly different from that of bees treated with QMP alone or untreated QL workers. Treatment with Dufour’s gland secretion alone resulted in bees possessing the highest amount of esters, but this was not significantly different either from treatment with QMP or from untreated QL bees. The only significant difference was between bees treated with Dufour’s gland secretion and those treated with QMP + Dufour’s (P<0.05).
Discussion
The fact that queen-like pheromone components are expressed in workers once they start to develop ovaries indicates a tight evolutionary association between ovarian development and the expression of royal pheromones. Two of the glands producing such pheromones have been extensively studied, and it appears that the mandibular glands pheromones are associated with reproductive dominance whereas that of Dufour’s gland constitutes a reliable fertility signal (Plettner et al. 1993; Simon et al., 2005; Dor et al. 2005). It also implies that under hopeless queenless conditions these royal pheromones may be used in worker–worker competition just as they are used in queen–worker competition under normal conditions. Although the effect of QMP on worker reproduction has been studied throughout the years (Butler 1959; Hoover et al. 2003), data on the pheromonal regulation of worker exocrine expression are limited if present at all. This is of particular interest not only because of the evolutionary association of these pheromones with reproduction, but because it constitutes a unique case in which we can examine the role of pheromones as modulators of the expression of the same pheromones in other individuals.
We addressed this issue by exposing hopeless QL bees to either QMP or queen Dufour’s secretion, or their combination, and analyzed both worker ovarian development and Dufour’s gland composition. Although QMP alone, or combined with Dufour’s secretion, inhibited to some extent worker reproduction, neither was as effective as the queen. QMP partially suppressed ovarian development in QL workers, but this was not significant, unlike previous reports (Hoover et al. 2003). This may stem from differences in the experimental setup; the previous study used small groups of 30 QL bees from which a sample of five bees was analyzed. Here we used small hives containing 1,000–2,000 bees of which we sampled 15 bees found in the queen-excluded compartment. Accordingly, we present here the percentage of bees with developed ovaries and not ovary score as a measure of worker reproduction. This also enabled us to compare the data with reports in fully-fledged hives (Visscher 1996). In two of the QR colonies we observed egg-laying, and the proportion of bees with activated ovaries was 8.3% (in all the QR colonies), in agreement with what was found in fully-fledged hives (Visscher 1996). It is important to mention that these eggs were observed in the queen-excluded compartment, indicating that under QR conditions workers may manage to escape the control of the queen and sneak eggs away from her direct control. These eggs seem also to evade policing by nest mates.
The tight coupling between ovarian development and the occurrence of the queen-specific esters (Katzav-Gozansky et al. 1997; Dor et al. 2005) in QL workers is also apparent from the present results. However, the results also point to the fact that the queen and/or queen pheromones may modulate the amounts of queen components in the Dufour’s gland independently of ovarian development. QR workers that did develop ovaries (stage 3, representing eggs in the ovaries ready for laying) still had significantly lower amounts of esters than those of QL bees exhibiting the same degree of ovarian development. This is also apparent from the experiments in which we tested the ability of the various queen pheromones (QMP and Dufour’s gland pheromones) to replace a queen. Although in all of the QL bees, treated and untreated, the occurrence of the queen-specific esters in Dufour’s gland was linked to ovarian development treatment with the queen pheromones, which had an effect on their quantity, reminiscent of the queen effect. Treatment with QMP did not reduce the amounts of esters compared to untreated QL workers, but treating QL bees with a combination of both pheromone bouquets resulted in lower ester amounts in these bees, albeit not significant. This result and that of QR bees with developed ovaries, further emphasizes that in order to successfully replace a queen more than one queen pheromone is needed. Unexpectedly, treatment with Dufour’s gland alone had a stimulatory effect on Dufour’s gland secretion of QL bees. Moreover, bees treated with Dufour’s gland alone revealed a significant increase in the amount of esters compared to those treated with the combined pheromones. This suggests that the reaction to the queen pheromone may be context dependent. Exposure to a complex pheromone system (QMP and Dufour’s in the present case), better mimicked the queen’s presence resulting in worker suppression, both with respect to ovarian development and royal pheromone expression. Exposure to a single queen pheromone on the other hand had a differential effect. Exposure to QMP alone may have caused partial suppression of the worker ovaries, but did not affect ester production. On the other hand, Dufour’s pheromone alone did not affect ovarian development, but did enhance ester production in the Dufour’s gland. We can explain this enhancement in terms of worker–worker competition, and via the role of Dufour’s gland pheromone as a fertility signal. Under a “queenless hopeless situation” there is a short window for worker reproduction before the colony breaks down. Workers that advertise their fertility status may thus gain advantage in the race for reproduction, and sensing high levels of Dufour’s gland pheromone in the hive may catalyze higher rates of production, especially in workers at a particular physiological state, that is, at advanced ovarian development. We can exclude the possibility that the higher amounts of esters in these bees is due to their sequestration from an external source, because bees that were exposed to both QMP and Dufour’s gland had an external source of esters, but nevertheless showed diminished amounts in their Dufour’s gland. Another conclusion from this finding is that the workers’ perception of Dufour’s gland esters affected their own expression of these esters. This, to our knowledge, is the first demonstration that a pheromone emitted by one individual affects the rates of its production in another individual.
The findings presented above demonstrate that queen regulation of worker physiology is multi-signal dependent. While a single pheromone blend may have a particular effect on worker physiology and behavior, the reaction to multiple blends seems to be more profound and mimic better the presence of the queen. The results also suggest that although ovary activation and the occurrence of queen pheromones appear coupled in workers, their regulation may be uncoupled. The fact that the queen or QMP exert greater suppression on signal production than on ovary activation (QR or QMP treated QL workers with developed ovaries had a weaker Dufour’s signal than that of untreated QL workers with the same degree of ovarian development), presents a challenging ultimate as well as proximate questions. Finally, it seems that the perception of Dufour’s gland pheromone affects the regulation of its production in workers. Again, both the evolutionary significance and the mechanisms involved remain elusive and reiterate the complexity of the role of caste-specific pheromones in social insect biology.
Abbreviations
- QMP:
-
Queen mandibular pheromone
- QR:
-
Queenright
- QL:
-
Queenless
References
Butler CG (1959) The source of the substance produced by a queen honeybee (Apis mellifera) which inhibits development of the ovaries of the workers of her colony. Proc R Soc Lond B 34:137–138
Crewe M, Velthuis HHW (1980) False queens: a consequence of mandibular gland signals in worker honeybee. Naturwissenschaften 67:467–469
Dor R, Katzav-Gozansky T, Hefetz A (2005) Dufour’s gland pheromone as a reliable fertility signal among honeybee (Apis Mellifera) workers. Behav Ecol Sociobiol 58:270–276
Hoover SER, Keeling CI, Winston ML, Slessor KN (2003) The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften 90:477–480
Katzav-Gozansky T, Boulay R, Soroker V, Hefetz A (2004) Queen-signal modulation of worker pheromonal composition in honeybees. Proc R Soc Lond B 271:2065–2069
Katzav-Gozansky T, Soroker V, Hefetz A (2000a) Plasticity in caste-related exocrine secretion biosynthesis in the honey bee (Apis Mellifera). J Insect Physiol 46:993–998
Katzav-Gozansky T, Soroker V, Hefetz A (2000b) Plasticity in caste-related exocrine secretion biosynthesis in the honey bee (Apis mellifera). J Insect Physiol 46:993–998
Katzav-Gozansky T, Soroker V, Hefetz A Cojocaru M, Erdmann DH, Francke W (1997) Plasticity of caste-specific Dufour’s gland secretion in the honey bee (Apis mellifera L.). Naturwissenschaften 84:238–241
Keller L, Nonacs P (1993) The role of queen pheromones in social insects: queen control or queen signal? Animal Behav 45:787–794
Ledoux MN, Winston ML, Higo H, Keeling CI, Slessor KN, Leconte Y (2001) Queen pheromonal factors influencing comb construction by simulated honey bee (Apis Mellifera L.) Swarms. Ins Soc 48:14–20
Mohammedi A, Paris A, Crauser D, LeConte Y (1998) Effect of aliphatic esters on ovary development of queenless bees (Apis mellifera L). Naturwissenschaften 85:455–458
Moritz RFA, Lattorff HMG, Crewe RM (2004) Honeybee workers (Apis Mellifera Capensis) compete to produce queen-like pheromone signals. Proc R Soc Lond B (Suppl) 271:98–100
Moritz RFA, Simon UE, Crewe RM (2000) Pheromonal contest between honeybee workers (Apis Mellifera Capensis). Naturwissenschaften 87:395–397
Page RE, Blum MS, Fales HM (1988) O-aminoacetophenone, a pheromone that repels honeybees (Apis mellifera L.). Experienta 44:270–271
Plettner E, Slessor KN, Winston ML, Oliver JE (1996) Caste-selective pheromone biosynthesis in honeybees. Science 271:1851–1853
Plettner E, Slessor KN, Winston ML, Robinson GE, Page RE (1993) Mandibular gland components and ovarian development as measures of caste differentiation in the honey bee (Apis mellifera L.). J Insect Physiol 39:235–240
Simon UE, Moritz RFA, Crewe RM (2005) Reproductive dominance among honeybee workers in experimental groups of Apis mellifera capensis. Apidologie 36:413–419
Simon UE, Moritz RFA, Crewe RM (2001) The ontogenetic pattern of mandibular gland components in queenless worker bees (Apis Mellifera capensis Esch.). J Insect Physiol 47:735–738
Sokal RR, Rohlf FJ (1995) Biometry. Freeman and Company, New York
Visscher PK (1996) Reproductive conflict in honey bees: a stalemate of worker egg-laying and policing. Behav Ecol Sociobiol 39:237–244
Winston ML (1987) The biology of the honey bee. Harvard University Press, Cambridge
Wossler TC, Crewe RM (1999a) The releaser effects of tergal gland secretion of queen honeybees (Apis Mellifera). J Insect Behav 12:343–351
Wossler TC, Crewe RM (1999b) Honeybee queen tergal gland secretion affects ovarian development in caged workers. Apidologie 30:311–320
Acknowledgments
The Israel Science Foundation founded by the Israel Academy of Sciences and Humanities and a George S. Wise postdoctoral fellowship (to RB) supported this research. We thank Armin Ionescu for his statistical help, Tovit Simon and Lea Falco for their technical help, Josef Kamer and Haim Efrat from Tzrifin Apiary and Shaike Wise from Nir-Galim Apiary for assistance in establishing of experimental hives and Naomi Paz for editorial assistance. This manuscript is contribution no (507/05) from the Volcani Center, Israel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamar, KG., Raphaël, B., Victoria, S. et al. Queen pheromones affecting the production of queen-like secretion in workers. J Comp Physiol A 192, 737–742 (2006). https://doi.org/10.1007/s00359-006-0110-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-006-0110-0