Abstract
Auditory and vestibular functions of otolithic organs vary among vertebrate taxa. The saccule has been considered a major hearing organ in many fishes. However, little is known about the auditory role of the lagena in fishes. In this study we analyzed directional and frequency responses from single lagenar fibers of Dormitator latifrons to linear accelerations that simulate underwater acoustic particle motion. Characteristic frequencies of the lagenar fibers fell into two groups: ≤50 Hz and 80–125 Hz. We observed various temporal response patterns: strong phase-locking, double phase-locking, phase-locked bursting, and non-phase-locked bursting. Some bursting responses have not been previously observed in vertebrate otolithic nerve fibers. Lagenar fibers could respond to accelerations as small as 1.1 mm s−2. Like saccular fibers, lagenar fibers were directionally responsive and decreased directional selectivity with stimulus level. Best response axes of the lagenar fibers clustered around the lagenar longitudinal axis in the horizontal plane, but distributed in a diversity of axes in the mid-sagittal plane, which generally reflect morphological polarizations of hair cells in the lagena. We conclude that the lagena of D. latifrons plays a role in sound localization in elevation, particularly at high stimulus intensities where responses of most saccular fibers are saturated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lagena, one of the otolithic organs, is found in all vertebrate taxa except therian mammals. Mammalian otolithic organs are primarily vestibular sensors though they may also play a minor role in hearing (Fernandez and Goldberg 1976a, 1976b, 1976c; McCue and Guinan 1994). The saccule in nonmammalian vertebrates such as fishes and amphibians is a primary auditory organ (Lewis and Narins 1999; Popper and Fay 1999).
Fishes are divided into two groups based on acoustic inputs and hearing capacities (Fay 1988; Popper and Fay 1999; Lu 2003). Hearing specialists, including otophysans (e.g., the goldfish, Carassius auratus), mormyrids, and clupeids, have specialized auditory accessories (the Weberian ossicles connecting the swim bladder to their inner ears, or gas-filled structures acoustically coupled to the ears) to enhance hearing sensitivity and broaden the frequency response range. Hearing-specialized fishes are sensitive to both acoustic particle motion and pressure components of a sound wave; hearing-generalized fishes are sensitive to acoustic particle motion but much less (or not) sensitive to sound pressure. Hearing generalists (such as the midshipman, Porichthys notatus; sleeper goby, Dormitator latifrons; and toadfish, Opsanus tau) have a relatively long distance between the swim bladder and inner ears, low hearing sensitivity to sound pressure, and a narrow frequency-response range due to the lack of the specialized auditory accessories.
One aspect of fish hearing research is aimed at understanding temporal response patterns and tuning of primary auditory afferents in response to acoustic stimuli produced by an omnidirectional sound source, such as a loudspeaker (Furukawa and Ishii 1967; Fay 1978a, 1978b; Fay and Ream 1986; Lu and Fay 1996; McKibben and Bass 1999, 2001; Suzuki et al. 2002). Another research direction focuses on coding of acoustic directional signals by auditory afferents in teleosts, including the goldfish, midshipman, sleeper goby, and toadfish (Fay 1984; Lu et al. 1996; Fay and Edds-Walton 1997a, 1997b; Lu et al. 1998; Edds-Walton et al. 1999; Lu and Popper 2001; Weeg et al. 2002). These studies on directional hearing in fishes have shown that (1) saccular afferents in both hearing specialized and generalized fishes are very sensitive to linear accelerations as low as 0.039 mm s−2 (RMS), (2) most saccular afferents are directionally sensitive to acoustic particle motion, and (3) the response directionality of these afferents results from the morphological and functional polarity of the hair cells they innervate.
Our understanding of auditory functions of otolithic organs in fishes mainly derives from work on the saccule. We know little about the functions of the lagena and utricle because few studies have been conducted. One reason for the lack of such studies is that the lagenar and utricular nerve bundles are difficult to access in most fish species. We have been studying directional hearing using the sleeper goby as a model system because the three otolithic nerves in this species are completely separated, and each is easily accessible for single-cell recordings (Lu and Popper 1998). The sleeper goby, one of the hearing generalists, shares the fundamental neural mechanisms of sound detection with most teleost fishes. We previously reported the ear structure, response directionality and tuning of saccular nerve fibers, and to what extent the saccule contributes to directional sensitivity in this species (Lu and Popper 1998; Lu et al. 1998; Lu and Popper 2001; Lu and Xu 2002a; Z. Lu et al., unpublished observations).
In the present study we have investigated the response characteristics of single lagenar fibers and compared them with those of saccular fibers in order to reveal if the lagena plays any role in directional hearing in hearing-generalized fishes. Although previous studies have reported some response properties of lagenar fibers in the goldfish (Furukawa and Ishii 1967; Fay and Olsho 1979; Fay 1984), this study is the first systematic investigation to include frequency selectivity, directionality, sensitivity, and temporal response patterns of fish lagenar fibers. Preliminary data from this study were reported in abstract form (Lu and Xu 2002b).
Materials and methods
Animal preparation
Fifty-two sleeper gobies, 100–120 mm in total length, were used in this study. They were imported from Ecuador (South America) and housed in 15-gallon tanks containing fresh water that was aerated, filtered, and maintained at ~20°C. After a fish was anesthetized in a bath of 0.05% MS-222 (Sigma) solution, it was covered with a wet paper towel and placed on a holder in a surgical tray. Air-saturated water irrigated its gills through a mouthpiece at a flow rate of 100 ml min−1 to assist respiration throughout experiments. A 3×3 mm hole was made in the skull to expose the left lagenar nerve bundle. A dose of gallamine triethiodide (3 μg g−1 body weight, Sigma) in 0.7% NaCl solution was intramuscularly injected in the tail to prevent movement during neural recording. The fish remained undisturbed in the holder for at least 1 h before neural recording in order to let it fully recover from anesthesia.
Orientation of the lagena
We perfused nine sleeper gobies through the hearts with 4% paraformaldehyde in 0.02 mol l−1 phosphate-buffered solution and then dissected each head to expose the left lagena. Each lagena was examined from the dorsal view under a Wild dissecting microscope, and its image was taken at a resolution of 1,024×1,024 pixels using a digital camera (Nikon Cool Pix 950) attached to an eyepiece of the microscope. After the image was printed on a sheet or displayed on a computer monitor, the longitudinal axis of the lagena was drawn. The angle between the longitudinal axis of the lagena and longitudinal axis of the fish was calculated, and the mean angle and its standard deviation (SD) were then computed.
Neurophysiological apparatus
The stimulus control system consisted of a Dell Pentium IV computer, a four-channel digital-to-analog and analog-to-digital modular system (Tucker-Davis Technologies, Gainesville, Fla., USA), and a shaker system (Fay 1984; Lu et al. 1996, 1998; Lu and Popper 2001; Lu and Xu 2002a). The shaker apparatus rested on an anti-vibration table (Model 63-540, Technical Manufacture Corporation, Peabody, Mass., USA). A schematic diagram of the stimulus control system and the generation and calibration of directional stimuli were previously published in detail (see Lu et al. 1996). Briefly, the shaker apparatus contains an aluminum experimental dish and five mechanical shakers. Two orthogonal pairs of horizontal mini shakers (Brüel & Kjaer, Type 4810) attached to the sides of the dish, and a vertical large shaker (Type 4809) connected to the center of the bottom of the dish. We positioned the fish in the center of the water-filled experiment dish by securing its head with a holder that was rigidly attached to the experimental dish.
Acoustic signals were pure tones of 500-ms duration and 5-ms cosine rise and fall times at 50, 80, 100, 125, 200, 250, 345, and 400 Hz. They were digitally synthesized by the computer, read out from 16-bit digital-to-analog converters at a rate of 10 kHz, low-pass filtered at 4 kHz, programmably attenuated, and fed to Hafler power amplifiers (P1000). Outputs of the power amplifiers produced the push-pull movement of mechanical shakers. Sinusoids used to drive each pair of horizontal shakers were identical except a 180° shift of the starting phase. Linear motion of the experimental dish and fish was achieved at desired axes in 3-D space by adjusting phases and amplitudes of the signal inputs to three orthogonal shaker channels. Motion produced by the shaker apparatus mimicked underwater acoustic particle motion.
For frequency response experiments, linear accelerations at the eight frequencies were provided along the dorsoventral, longitudinal, and side-to-side axes of the fish. For directional response experiments, 100-Hz stimuli were provided at six axes (−90°, −60°, −30°, 0°, +30°, and +60°) in the horizontal plane. The fish longitudinal axis was defined as 0°, and its side-to-side axis was −90° (or +90°). In the mid-sagittal plane, directional stimuli were provided at five additional axes (30°, 60°, 90°, 120°, and 150°) in reference to the rostral direction of the fish (90° was the dorsoventral axis). Note that 0° in the mid-sagittal plane was the same as 0° in the horizontal plane.
At the beginning of each experiment, linear accelerations of the experimental dish were calibrated and reported in dB re. 1 g using three piezoelectric accelerometers (Model 336C, PCB Piezotronics, Depew, N.Y., USA) mounted on the experimental dish along the three orthogonal axes. Accelerometer sensitivities, which were pre-calibrated by the manufacturer, were 982 mV g−1 (dorsoventral), 1036 mV g−1 (side-to-side), and 1031 mV g−1 (longitudinal). For frequency-response experiments, calibration of the experimental dish movement was performed to achieve constant acceleration rather than constant displacement at the eight frequencies along the longitudinal, side-to-side, and dorsoventral axes of the fish. Search stimuli at 50, 100, and 400 Hz were presented sequentially along the three orthogonal axes once per second at −28 dB re. 1 g (0 dB re. 1 μm at 100 Hz). A custom-written Visual C++ program was used for stimulus calibration and data acquisition.
Neural recording
The lagenar nerve bundle was clearly identified using a Nikon stereomicroscope (see Fig. 1). The lagena is about ten times smaller than the saccule, and the lagenar nerve bundle is half the diameter of the saccular nerve (Lu and Popper 1998). Although the lagenar nerve is small, we were able to access it for single-cell recordings. Action potentials were recorded from single lagenar nerve fibers using a pair of electrodes. The recording microelectrode was made from 1.5-mm glass capillaries (World Precision Instruments, Sarasota, Fla., USA) that were pulled to a tip size of approximately 0.5 μm using a micropipette puller (P-97, Sutter Instrument, Novato, Calif., USA) and then filled with 3 mol l−1 KCl. The recording electrode was mounted on a 3-D hydraulic micromanipulator (WR-6, Narashige, Tokyo, Japan) and lowered down manually, with a step resolution of 0.2 μm, to reach the lagenar nerve bundle. The tip of the reference electrode, a 40-mm long platinum wire (0.5 mm in diameter), was kept in the fluid of the brain cavity. Evoked action potentials recorded by the electrodes were sent to a preamplifier (S-7071A, World Precision Instruments), amplified 100−1,000 times, bandpass filtered between 450 and 4,500 Hz, digitally recorded at 10 kHz, and stored in the computer.
A Photo of the brain, otolithic organs, and otolithic nerves of a sleeper goby from the top view. L lagena; S saccule; U utricle; Cer cerebellum; MB midbrain; VL vagal lobe;Tel telencephalon. B Enlarged photo of the outlined area in A, showing the left lagenar nerve (LN), saccular nerve (SN), utricular nerve (UN), and anterior lateral line nerve (ALLN)
Data acquisition and analysis
Once action potentials from a single lagenar fiber were detected, the average spontaneous rate (SR) and its SD were determined during a 500-ms duration for 50 repetitions. For frequency response experiments, response areas (number of spikes versus stimulus level versus stimulus frequency) of lagenar fibers were constructed at the dorsoventral, longitudinal, and side-to-side axes of the fish. Tuning curves were obtained from their response areas based on criteria of SR plus one or more SDs. Threshold was defined as the lowest stimulus level that evoked a response just exceeding the mean SR by one SD at each stimulus axis. For lagenar fibers without spontaneous activity, threshold was the lowest stimulus level that started to elicit action potentials at a given axis. Characteristic frequency (CF) was the frequency at the lowest threshold among those at the three orthogonal axes.
For directional response experiments, spike rates (number of spikes over the 500-ms stimulus duration) were calculated for lagenar fibers in response to 100-Hz accelerations from −90 to −20 dB re. 1 g with 3- to 5-dB steps at the 11 stimulus axes in the horizontal and mid-sagittal planes to build rate-level functions. A threshold was determined at each stimulus axis, and best sensitivity (BS) was defined as the lowest threshold among those at the six stimulus axes in either the horizontal or mid-sagittal plane.
Rate-level functions were obtained at the 11 stimulus axes in the horizontal and mid-sagittal planes. The slope of a rate-level function was defined as an increase in spike count per decibel, and the slopes at different stimulus axes were averaged. Iso-level spike rate versus stimulus axis functions in both the planes were then constructed from the rate-level functions. For each lagenar nerve fiber, spike rates from different stimulus levels at each stimulus axis were averaged, and a function of average spike rate versus stimulus axis was plotted. Neural responses of a lagenar fiber were defined as directional if there were significant differences among average spike rates at different stimulus axes (one-factor ANOVA, P<0.05). The best response axis (BRA) was defined as the mean angle that was calculated from spike rates at the six axes in either the horizontal or mid-sagittal plane (Eqs. 1.3.7 and 1.3.9 in Batschelet 1981; Lu et al. 1998; Lu and Popper 2001).
The BRA of each lagenar fiber in 3-D space was determined from spike rates at the dorsoventral, longitudinal, and side-to-side axes at 10−15 dB above BS. The 3-D BRA was determined by an azimuthal angle in reference to the longitudinal axis of fish and an elevational angle to the horizontal plane. The elevational angle was computed from the equation: atan(N DV/N H) in degrees, where N DV and N H are spike rates at the dorsoventral axis and the horizontal BRA.
The directionality index (DI) of each lagenar fiber was obtained from the spike rate versus stimulus axis curve at a stimulus level between 5 and 30 dB above threshold (see Fig. 2). DI was defined as a ratio of the width over the length of a directional response curve (modified from Lu et al. 1998). The length (“co” in Fig. 2) was the distance between the center of the polar plot and the crossing point of the BRA with the directional response curve; the width (“ab”) of the directional response curve was measured perpendicularly to the BRA at half the “co”. DI is inversely proportional to directional selectivity.
To characterize temporal response patterns of each lagenar fiber, spike times were collected for 50 repetitions to form a raster plot (RP), peri-stimulus-time histogram (PSTH), inter-spike interval histograms (ISIH), and period histogram with a bin width of 1 ms. RPs, PSTHs, ISIHs, and period histograms were obtained at or near horizontal and sagittal BRA at several stimulus levels above threshold. The coefficient of synchronization (R) was calculated from spike time data to represent the strength of phase-locking (Goldberg and Brown 1969; Anderson 1973). The Rayleigh statistic (Z), a combined measurement of the spike count and strength of phase-locking as used by Lu and Fay (1993), Lu et al. (1998), and Lu and Popper (2001), was determined as Z=NR 2, where N is the number of spikes per 50 stimulus presentations. The coefficient of variation (CV), a ratio of SD over average inter-spike intervals, was computed to reveal the regularity of spikes. The adaptation index (S/A), a ratio of the firing rate during the second-half of the stimulus duration over that during the whole stimulus presentation, was also calculated to represent adaptation of neural discharges.
Results
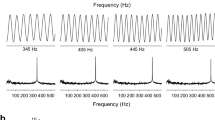
Frequency response data were obtained from 30 single lagenar nerve fibers to reveal CFs. We found that CFs of the lagenar nerve fibers fell into two groups: 80–125 Hz (63%) and ≤50 Hz (37%) (Fig. 3A, panel 1; B, panel 1). For both groups of lagenar nerve fibers, spike rate versus stimulus frequency functions showed a dominant peak in the range 80–125 Hz at supra-threshold (Fig. 3A, panel 2; B, panel 2).
Response areas for two representative lagenar nerve fibers (A, panels 1,2 and B, panels 1,2) with characteristic frequencies (CFs) at 80 Hz and ≤50 Hz. A, panel 1; B, panel 1 Tuning curves plots based on spontaneous rate (SR) plus 1 standard deviation (SD) (solid), 2 SDs (dashed), and 3 SDs (dotted); the size of circles indicates the spike rate. A, panel 2; B, panel 2 Spike rate versus stimulus frequency curves at different stimulus levels (dB re. 1 g), which were converted from (A, panel 1) and (B, panel 1)
For directional response experiments, complete data including SRs, BS, BRA, slopes of rate-level functions, spike rates, R, Z, CV, S/A, PSTHs, ISIHs, and period histograms were obtained from 128 single lagenar nerve fibers in response to 100-Hz accelerations in various axes in both the horizontal and mid-sagittal planes (see Table 1). These lagenar fibers were classified into strong phase-lockers (Z≥6.91 and R≥0.5; 63%), weak phase-lockers (Z≥6.91 and R<0.5; 29%), and non-phase-lockers (Z<6.91 and R<0.5; 8%) based on their responses to 100-Hz stimuli at 10–15 dB above threshold at or near BRA (Fig. 4).
Rayleigh statistic (Z) versus coefficient of synchronization (R) plots for 128 lagenar nerve fibers. Z and R were computed based on neural responses of each lagenar nerve fiber to 50, 100-Hz stimulus presentations at 10 or 15 dB above threshold along or near its best response axis (BRA). The horizontal line, Z 0.001, 500=6.91, divides phase-lockers (circles, squares) and non-phase-lockers (triangles). The vertical line, R=0.5, separates strong phase-lockers (squares) from weak phase-lockers (circles)
Figure 5 shows response properties of a strong phase-locker. It was directionally sensitive to acoustic stimuli in both the horizontal and mid-sagittal planes with its horizontal and sagittal BRA at −13° and 164°(Fig. 5A). It had both the horizontal and sagittal BS at −58 dB re. 1 g along the longitudinal axis and became saturated at 20 dB above threshold (Fig. 5B). This strong phase-locker had a sustained temporal response pattern, i.e., it discharged one spike at almost every stimulus cycle (Fig. 5C). The ISIH of the lagenar fiber had preferred peaks at the time intervals that corresponded to the stimulus period (10 ms) and its multiples; the period histogram had a single peak. The phase of the peak in each period histogram tended to decrease with stimulus level while R increased with stimulus level.
Response properties of a strong phase-locker to 100-Hz stimuli. A Spike rate versus stimulus axis functions at several stimulus levels in the horizontal and mid-sagittal planes. The lines indicated by arrowheads are the horizontal BRA (−13°) and sagittal BRA (164°). B Rate-level functions at different stimulus axes in both the planes. Horizontal and sagittal best sensitivity (BS): −58 dB re. 1 g. C RPs, PSTHs, ISIHs, and period histograms at three stimulus levels along the longitudinal axis of the fish. The vertical line on the ISIHs indicates the stimulus period of 10 ms. φ: the phase at which the lagenar nerve fiber was phase-locked. Two sweeps of 100-Hz stimulus waveforms are shown below the peri-stimulus time histogram (PSTH) and period histogram. Histogram bin: 0.1 ms
The weak phase-locker shown in Fig. 6A was most sensitive to the horizontal axis at −14° and the mid-sagittal axis at 136°. The rate-level functions of the lagenar fiber were monotonic with its BS at −48 dB re. 1 g along the longitudinal axis (Fig. 6B). This weak phase-locker showed sustained temporal responses with little or no adaptation (Fig. 6C). The ISIH of the lagenar fiber had a major peak at the time interval that was unrelated to the stimulus period. Since inter-peak intervals were much less than the stimulus period, they appeared to fire more than one spike per stimulus cycle. The period histogram had more than one preferred peak, and both the peak phases and the inter-peak intervals decreased with stimulus level.
Response properties of a weak phase-locker to 100-Hz stimuli. A Spike rate versus stimulus axis functions at several stimulus levels in the horizontal and mid-sagittal planes. The lines indicated by arrowheads are the horizontal BRA (−14°) and sagittal BRA (136°). B Rate-level functions at different stimulus axes in both the planes. Horizontal and sagittal BS: −48 dB re. 1 g. C Raster plots (RPs), PSTHs, inter-spike interval histograms (ISIHs) and period histograms at three stimulus levels along the longitudinal axis of the fish. The ISIHs show dominant peaks at 2.2, 1.6, and 1.6 ms at −43, −38, and −33 dB re. 1 g. The phase of each peak shown in the period histograms was calculated based on the maximum number of spikes in a bin width of 0.1 ms. Two sweeps of 100-Hz stimulus waveforms are shown below the PSTH and period histogram. Histogram bin: 0.1 ms
Although non-phase-lockers were also directionally sensitive to linear accelerations, they had irregular functions of spike rate versus stimulus axis, unlike those of weak phase-lockers and strong phase-lockers (Fig. 7A). Most non-phase-lockers had monotonic rate-level functions; a few (n =3) showed non-monotonic rate-level functions (Fig. 7B). Non-phase-lockers often had phasic responses with strong adaptation and did not synchronize to stimulus waveforms (Fig. 7C).
Response properties of a non-phase-locker to 100-Hz stimuli. A Spike rate versus stimulus axis functions at several stimulus levels in the horizontal and mid-sagittal planes. B Rate-level functions at different stimulus axes in both the planes. Horizontal BS: −38 dB re. 1 g, and sagittal BS: −48 dB re. 1 g. C RPs, PSTHs, ISIHs and period histograms at three stimulus levels along 120° in the mid-sagittal plane. Two sweeps of 100-Hz stimulus waveforms are shown below the PSTH and period histogram. Histogram bin: 0.1 ms
Based on spike waveforms, RPs, PSTHs, ISIHs, and period histograms, we categorized the temporal responses of 125 lagenar nerve fibers into entrained phase-lockers (n=25), double phase-lockers (n=21), phase-locked bursters (n=68), and non phase-locked bursters (n=11) (Fig. 8 and Table 2). Note that the CVs shown in Fig. 8 gradually increase from the entrained phase-locking to non-phase-locked bursting, indicating that the regularity of responses decreases with the changes in temporal response. All entrained phase-lockers had a single peak in the period histogram and a single dominant peak at the time interval that is equal to the stimulus period in the ISIH (Fig. 8A, also Fig. 5C). Strong phase-lockers included entrained phase-lockers, double phase-lockers, and phase-locked bursters. Fifty-nine strong phase-lockers had sustained responses during the 500-ms stimulus presentation with little adaptation (S/A≥0.3), while 22 showed phasic responses with adaptation over the stimulus duration and repetitions (S/A<0.3).
Four temporal response patterns of lagenar nerve fibers, each showing a sweep of spikes, RP, PSTH, ISIH and period histogram. Stimuli: 100 Hz at 10–15 dB above threshold and 50 presentations. A Strong phase-locker. Its period histogram shows a single peak, and its ISIH has a major peak at the time interval that is equal to the stimulus period. B Double phase-locker. The period histogram shows two preferred peaks that are 180° apart. The ISIH has a major peak at the time interval that is half the stimulus period, indicating that the lagenar fiber fired two spikes within a stimulus cycle. C Phase-locked burster. The period histogram has multiple peaks with regular inter-peak intervals, and the ISIH has a major peak at the time that corresponds to the bursting rate rather than the stimulus period. Note that the first spike of each burst is phase-locked. D Non-phase-locked burster. The period histogram has no preferred peak; the ISIH has a dominant peak at the time that is associated with the bursting rate. Two sweeps of stimulus waveforms are shown below the PSTH and period histogram of the non-phase-locked burster. Histogram bin: 0.1 ms
Double phase-lockers had the period histogram with two preferred peaks that were located 180° apart (Fig. 8B). They were able to fire two spikes that were ~180° out of phase within a stimulus cycle. The majority of double phase-lockers (n=12) fell into the category of strong phase-lockers, each having a minor peak at the time interval that was equal to half the stimulus period in the ISIH and two peaks with different heights in the period histogram (not shown in Fig. 8). Other double phase-lockers (n=9) were weak phase-lockers, each having a major peak at half the stimulus period and several minor peaks at the stimulus period and its multiples in the ISIH (Fig. 8B). The period histogram of these double phase-lockers had two peaks with similar heights. The double phase-locking tended to adapt into single phase-locking over the stimulus duration.
The phase-locked bursting is a combination of phase-locking and bursting (Fig. 8C, also Fig. 6C). Phase-locked bursters fired bursts, each of which contained two to many spikes. Of each burst, the amplitude of the first spike was often significantly greater than that of the succeeding spikes which were more uniform in amplitude. The first spike of each burst fired approximately at the same phase in a stimulus period, and the inter-spike intervals remained about constant within each burst at a given stimulus level. These led to two to several preferred peaks in period histograms. Like phase-locked bursters, non-phase-locked bursters fired bursts in response to a sweep of sinusoidal waveforms (Fig. 8D, also Fig. 7C). However, non-phase-locking fibers fired the first spikes of individual bursts at random phases in the stimulus cycle.
Of 79 lagenar nerve fibers with bursting responses, sixty-one fired bursts with small inter-spike intervals (i.e., 0.9 ms) at the beginning of each burst and then reached a constant inter-spike interval of 1.4 ms for the rest of the burst (Fig. 9A). The initial inter-spike interval of 0.9 ms was independent of stimulus level and varied little among these lagenar fibers. Sixteen lagenar fibers had bursting responses with an increase in inter-spike interval over time within bursts. Among them, some fibers (n=9) showed a steady decrement in discharge rate over the bursting duration except the very end of the burst (Fig. 9B), while others (n=7) had bursting responses at the beginning of the stimulus but gradually transformed into phase-locking responses (Fig. 9C). Two other lagenar fibers did not fall into these classifications.
Three types of bursting responses of lagenar fibers, each showing a sweep of evoked spikes and a plot of inter-spike interval versus time. Stimuli: 100 Hz at 10–15 dB above threshold. A Burster with regular inter-spike intervals. B Burster with a gradual increase of inter-spike intervals. C Onset burster. The dotted line indicates the end of bursting. A sweep of stimulus waveforms is shown below the onset burster
All lagenar nerve fibers recorded were directionally sensitive to linear accelerations. Figure 10 shows that DI distributions of 109 lagenar fibers ranged from 0.18 to 2.32 (median=0.93) in the horizontal plane and from 0.20 to 2.46 (median=1.06) in the mid-sagittal plane. Directional selectivity of lagenar fibers decreased as stimulus level increased (Fig. 10A, panel 2 and B, panel 2). At the stimulus level about 10–15 dB above threshold, many lagenar fibers had DI of about 1.00, indicating that they had approximately circular spike rate versus stimulus axis functions. Other lagenar fibers (n=19) showed bimodal or irregular spike rate versus stimulus axis functions (Fig. 7A), and shapes of spike rate versus stimulus axis curves were independent of stimulus level.
Directionality index (DI) distributions of 109 lagenar fibers in the horizontal plane (A, panel 1) and mid-sagittal plane (B, panel 1). DI versus stimulus level plots in the horizontal plane (A, panel 2) and the mid-sagittal plane (B, panel 2) for 20 lagenar fibers (dotted curves) that were randomly selected. The solid curves in (A, panel 2) and (B, panel 2) are average curves for the 109 lagenar fibers, showing increases of DI with stimulus level. Linear equations (Y=0.02X+0.59 and Y=0.03X+0.65) fit the average curves in (A, panel 2) and (B, panel 2)
The angles between the longitudinal axis of the left lagena and the longitudinal axis of the fish varied from −26° to −3° (median=−16°, n=9). Horizontal BRA of left lagenar fibers clustered around −13° (median), which is close to the longitudinal axis of the left lagena (Fig. 11A). Sagittal BRA distributed in a variety of axes from 0° to 150°, which is generally consistent with morphological polarizations of hair cells in the lagena (the insert in Fig. 11B). Fig. 11C shows the “northern hemisphere” of a sphere with crossing points of lagenar 3-D BRA penetrating the spherical surface upward. The stimulus axes on the “equator” are shown as −90°, −60°, −30°, 0°, 30°, and 60°. Concentric circles indicate the horizontal plane altitudes such as 90° (the north pole), 75°, 60°, 45°, 30°, 15°, and 0°(the equator).
Directional threshold versus BRA in the horizontal plane (A) and the mid-sagittal plane (B) for 128 lagenar nerve fibers. The length of each black line represents threshold (dB re. 1 g) for a lagenar fiber. The line indicated by the arrowhead in A is the average longitudinal axis of nine left lagenas. The insert in B shows the lagenar macula with morphological polarizations of hair cells (modified from Lu and Popper 1998). Response directionality in three dimensional space in C illustrates the north pole of a sphere with a sleeper goby in the center of the sphere. Concentric circles represent elevations from 0° (the equator) to 90° (the north pole) with 15° steps. Crosses indicate the locations on the northern hemisphere where the 3-D BRA of lagenar fibers penetrate the spherical surface upward
Many lagenar fibers (39%) did not have any spontaneous activity while others had SRs up to 268 spikes s−1 (median=0 spike s−1, n=128). SRs of saccular fibers ranged from 0 to 162 spikes s−1 (median=1 spike s−1, n=293, Lu et al. 1998; Lu and Popper 2001) (Fig. 12A). Lagenar fibers had slightly lower SRs than saccular fibers (Mann-Whitney U-test, P<0.05).
Comparisons of SR, BS, and BRA between saccular and lagenar fibers. Stimuli: 100 Hz. A SR distributions. B, D BS distributions in the sagittal and horizontal planes. C, E BRA distributions in the mid-sagittal and horizontal planes. Saccular fiber data were adapted from Lu et al. (1998) and Lu and Popper (2001)
Horizontal BS of the lagenar fibers ranged from −79 to −31 dB re. 1 g (mean±SD=–50±10 dB), and sensitivity at the dorsoventral axis varied from −78 to −33 dB re. 1 g (mean±SD=−52±10 dB). There was no significant difference between them (paired two-sample t test, P>0.05). Horizontal BS of the lagenar fibers was significantly less than that of the saccular fibers (Lu et al. 1998; −83±14 dB, one-tailed t-test, P<0.001) (Fig. 12B). Sagittal BS of the lagenar fibers was also significantly less than that of the saccular fibers (Lu and Popper 2001; −87±11 dB, one-tailed t-test, P<0.001) (Fig. 12D).
Like those of saccular fibers, sagittal BRA of lagenar fibers distributed in a 180° range (Fig. 12C). However, horizontal BRA of lagenar fibers had a non-random distribution clustering at −16° off the longitudinal axis of the fish (Poisson goodness-of-fit test, P<0.001). Horizontal BRA of saccular fibers also had a non-random distribution but clustering at −36° (Lu and Popper 1998; Poisson goodness-of-fit test, P<0.001) (Fig. 12E). Lagenar fibers had significantly smaller horizontal BRA than saccular fibers (one-tailed t-test, P<0.05).
Discussion
Sensitivity
It is thought that acoustic particle motion directly reaches otolithic organs in fishes including hearing specialists and generalists (Popper and Fay 1999). Although the three otolithic organs in the goldfish vary greatly in size and shape, the three otolithic nerve fibers have similar best displacement thresholds (i.e., about 0.1 nm at 140 Hz) (Fay 1984). Similar thresholds have also been observed for saccular fibers in several hearing generalized fishes (the toadfish, Fay and Edds-Walton 1997a; the sleeper goby, Lu and Popper 1998; the midshipman, Weeg et al. 2002). It was hypothesized that all otolithic organs in fishes have best displacement thresholds of about 0.1 nm (Popper and Fay 1999). However, results of the present study do not support this hypothesis. Clearly, our results show that lagenar fibers in the sleeper goby are significantly less sensitive than saccular fibers. In many hearing generalized fishes, including the sleeper goby, the saccule is remarkably larger than the other two otolithic organs (Popper 1977; Lu and Popper 1998; Popper and Fay 1999 for a review). It appears that there is a correlation between the size of otolithic organs and their sensitivity for the sleeper goby. Nerve fibers innervating smaller otolithic organs (i.e., the lagena and utricle; Z. Lu, unpublished data for utricular fibers) are less sensitive than the larger otolithic organ (the saccule). We predict that this correlation exists in other hearing generalized fishes, such as the midshipman and toadfish.
In the sleeper goby, the sensitivity difference between saccular and lagenar fibers might derive from the following aspects. First, the mass and size of the saccular otolith are significantly greater than those of the lagena. Second, mechanical properties of otolithic organs and mechanical coupling between the otolithic membrane and hair cell ciliary bundles may vary among different otolithic organs (Blair and Lewis 1986). In the sleeper goby, the saccular otolith has a deep groove in the medial side that connects hair cell ciliary bundles via the otolithic membrane, while the lagenar otolith has a relatively flat and smooth medial surface. Third, hair cells in various otolithic organs could have different sensitivities in response to acoustic particle motion. However, the innervation ratio of otolithic fibers and hair cells in a given otolithic organ may not contribute to the sensitivity difference between lagenar and saccular afferents because previous studies did not show any correlation between sensitivity of saccular fibers and number of hair cells innervated by the saccular fibers for both the toadfish and sleeper goby (Edds-Walton et al. 1999; Lu and Popper 2001).
At 100 Hz, sagittal BS of lagenar fibers in the sleeper goby ranged from 3.1 to 555.7 nm, corresponding to 1.2–219.4 mm s−2. Lagenar fibers of the goldfish responded to 100-Hz vertical accelerations with BS ranging from 0.3 to 100.0 nm, corresponding to 0.1–3.9 mm s−2 (estimated from Fay and Olsho 1979). Fay (1984) also showed that the goldfish lagenar fibers responded to140-Hz displacements as small as 0.1 nm. Thus, the sleeper goby’s lagenar fibers are less sensitive to acoustic particle motion than goldfish’s lagenar fibers. We do not know what aspects contribute to this sensitivity difference between these two species. It is clear that the Weberian ossicles are unlikely to be a contributing factor because these ossicles are involved in pressure detection but not particle motion detection.
Temporal response patterns
We found remarkable differences in temporal response patterns between lagenar and saccular fibers. Almost all saccular fibers were strongly phase-locked and had sustained responses with little or no adaptation (Lu et al. 1998; Lu and Popper 2001). Although the majority of the lagenar fibers (91%) were phase-locked to stimuli throughout the stimulus duration, 9% of the lagenar fibers showed little or no phase-locking. In addition, 61% of the lagenar fibers had bursting patterns, which were rarely observed for saccular fibers in the sleeper goby and other hearing generalized fishes, such as the toadfish and midshipman (Fay and Edd-Walton 1997a, 1997b; Lu et al. 1998; Weeg et al. 2002). However, the bursting response that resembles the phase-locked bursting observed in this study was reported on saccular afferents of hearing specialized fishes, the goldfish and mormyrids (Furukawa and Ishii 1967; Suzuki et al. 2002).
In this study we also discovered two types of bursters that have not been reported for otolithic fibers in any vertebrates. Non-phase-locking bursters had irregular directional response profiles and non-monotonic rate-level functions (Figs. 7, 8D). We believe that those fibers are likely efferents, originating from the medulla to innervate the lagena, rather than lagenar afferents. Onset bursters shown in Fig. 9C transformed the bursting response into the entrained response. We do not think that this onset bursting response resulted from abnormal discharging due to fiber injury by the microelectrode because we were able to repeat the recording many times and retain the response up to 1 h.
Another type of temporal response we encountered is the double phase-locking with the period histogram having two peaks that are separated by 180°. This suggests that double phase-lockers may innervate two groups of hair cells, in the striolar region of the lagena, with opposing morphological polarizations (Fig. 11B, insert). The ISIH of many double phase-lockers had a major peak in the time interval that is equal to half the stimulus period, indicating that they fired two spikes per stimulus cycle. The double phase-locking has been observed for saccular fibers in the goldfish and all three otolithic nerves in the sleeper goby (Furukawa and Ishii 1967; Lu et al. 1998; Z. Lu, unpublished data for utricular fibers). This response feature is preserved at the level of the fish midbrain (Fig. 11 in Lu and Fay 1993).
Auditory role of the lagena
The lagena has been found in fishes, amphibians, reptiles, birds, and some mammals (Retzius 1881, 1884; Harada et al. 2001a). The function of lagena varies among different vertebrate groups. Nerve fibers innervating the striolar and extrastriolar regions in the lagenar macula of the frog respond to acoustic and vestibular signals, respectively (Narins 1975; Caston et al. 1977; Lewis et al. 1982; Blair and Lewis 1986; Cortopassi and Lewis 1998). However, the lagenar functions have not been well studied in other vertebrate groups. Some neurophysiological studies showed that the lagena in birds plays no auditory role (Oeckinghaus 1985; Manley et al. 1991), indicating that the bird lagena may be an equilibrium sensor. Recent studies have suggested the possibility that lagenas in some birds and fishes are involved in the geomagnetic sense that could help their migration (Harada et al. 2001b; Harada 2002).
Our previous study on the sleeper goby showed that bilateral removal of saccular otoliths reduced hearing sensitivity up to 35 dB at different stimulus axes at 100 Hz, but did not completely wipe out hearing in this species, indicating that other otolithic organs may play partial roles in hearing (Lu and Xu 2002a). Interestingly, this amount of hearing loss correlates with the sensitivity difference (about 35 dB) between saccular and lagenar fibers, suggesting that the lagena, possibly as well as the utricle, of the sleeper goby also contributes to hearing.
Physiological studies on the lagena have only been conducted on a hearing specialized fish, the goldfish (Furukawa and Ishii 1967; Fay and Olsho 1979; Fay 1984). Our study is the first to provide neurophysiological evidence regarding the auditory role of the lagena in hearing generalized fishes that are primarily sensitive to acoustic particle motion. We found that lagenar fibers in the sleeper goby are most responsive to acceleration frequencies between 80 and 125 Hz. Frequency range criteria that distinguish audition from equilibrium have not been well defined. Traditionally, auditory sensors are considered to be sensitive to high frequencies (>10 Hz); vestibular sensors are sensitive to low frequencies of <10 Hz (Cortopassi and Lewis 1998). The frequency response range of the lagenar fibers presented in this study falls into the auditory scope.
Cortopassi and Lewis (1996, 1998) reported that auditory fibers in the frog lagena were bandpass with dynamic orders >3.0 and vestibular fibers were lowpass with dynamic orders <3.0. We have shown that lagenar afferents in the sleeper goby act like narrow bandpass filters with steep slopes at both low- and high-frequency edges in the frequency range we tested (see Fig. 3). We also calculated dynamic orders of 24 lagenar fibers, and they ranged from 1.0 to 18.0 (mean 7.8±3.4). All these lagenar fibers except two had dynamic orders higher than 3.0. Therefore, based on Cortopassi and Lewis’ findings we believe that the lagena of fishes, at least the sleeper goby, is primarily an acoustic particle motion detector.
Directional hearing
Most lagenar fibers we recorded were directionally sensitive to acoustic particle motion. Directional selectivity of lagenar fibers decreases as stimulus level increases (Fig. 10A, panel 2 and B, panel 2). At stimulus levels just above threshold, lagenar fibers are more directionally selective with oval-shaped directional response profiles. At stimulus levels 10─15 dB above threshold, spike rate versus stimulus axis functions are close to circles, indicating that most lagenar fibers innervate lagenar hair cells with the same or similar morphological polarizations. As previously reported for saccular afferents in the toadfish and sleeper goby (Edds-Walton et al. 1999; Lu and Popper 2001), each lagenar fiber likely innervates hair cells in a small region of the lagenar macula. At stimulus levels 20 dB above threshold or higher, the directional response profile became close to a half circle due to response saturation, resulting in loss of directional selectivity. Dependence of the response directionality on stimulus level has also been observed for saccular fibers in the sleeper goby and midshipman (Lu et al. 1998; Lu and Popper 2001; Weeg et al. 2002).
We realize that the results from this study only allow us to estimate 3-D best response axes rather than best response directions because the fish was accelerated along axes. Each 3-D BRA has two directions that penetrate the northern and southern hemispheres, respectively. Figure 11C only shows upward directions of lagenar BRA penetrating the surface of the northern hemisphere and does not illustrate downward directions of the BRA penetrating the southern hemisphere.
The lagenar epithelium of the sleeper goby is positioned approximately perpendicular to the horizontal plane, and the lagenar longitudinal axis deviates about 16° off the fish longitudinal axis. Our neurophysiological data show BRA of left lagenar fibers clustering at an azimuth of −13°(Fig. 11A), which is close to the longitudinal axis of the left lagena. The wide distribution of sagittal BRA of lagenar fibers shown in Fig. 11B is consistent with our previous findings of morphological polarizations of hair cells in the lagena (Lu and Popper 1998). The correlation of the neural response directionality and the morphological polarity of hair cells has been reported for saccular fibers of the toadfish and sleeper goby (Fay and Edds-Walton 1997a; Lu et al. 1998; Edds-Walton et al. 1999; Lu and Popper 2001). In the sleeper goby, both the lagenar and saccular epithelia are oriented vertically. However, the longitudinal axis of the saccule deviates from the fish longitudinal axis at a larger angle (about 40°) than that of the lagena. Both saccular and lagenar epithelia have hair cells with a wide range of morphological polarizations. Therefore, these anatomical and physiological data suggest that both the saccule and lagena in the sleeper goby play a role in sound localization in elevation.
Although the orientation of the goldfish lagenar macula in 3-D space is not available, the diversity of BRA of the goldfish lagenar fibers is consistent with morphological polarizations of hair cells in its lagena (Platt 1977; Fay 1984). Since BRA of the goldfish saccular fibers cluster in a small area (Fay 1984), the goldfish saccule alone is not sufficient to encode acoustic particle motion in elevation. In the goldfish, the lagena but not the saccule appears to play a dominant role in sound localization in elevation.
Conclusions
Results from this work show that lagenar fibers of the sleeper goby are most responsive to acceleration frequencies between 80 and 125 Hz with the lowest threshold of about 2.8 nm, corresponding to 1.1 mm s−2. Lagenar fibers have a diversity of temporal response patterns and are directionally selective to acoustic particle motion. They had preferred response axes different from saccular fibers and are less sensitive than saccular fibers. At ~−45 dB re. 1 g or higher stimulus levels, most saccular fibers in the sleeper goby are no longer able to encode the directional information due to response saturation while lagenar fibers are still directionally selective. We conclude that the lagena in the sleeper goby is an acoustic directional detector that apparently extends the dynamic range of saccular directional coding.
Based on this and other studies, we suggest that all three otolithic organs in fish contribute to directional hearing to some extent. Although directional sensitivity may vary among different otolithic organs, we can predict directional selectivity based on orientations of otolithic epithelia and morphological polarizations of hair cells in the otolithic organs. One otolithic organ in fish may not be sufficient to encode acoustic particle motion in 3-D space. All three pairs of otolithic organs with different spatial orientations together enhance the capability of directional detection. In most fishes, the saccule and/or lagena contributes significantly to directional hearing in elevation while the utricle may play a major role in directional hearing in azimuth.
Abbreviations
- BRA:
-
best response axis/axes
- BS:
-
best sensitivity
- CF:
-
characteristic frequency
- CV:
-
coefficient of variation
- DI:
-
directionality index
- ISIH:
-
inter-spike interval histogram
- PSTH:
-
peri-stimulus time histogram
- SR:
-
spontaneous rate
References
Anderson DJ (1973) Quantitative model for the effects of stimulus frequency upon synchronization of auditory nerve discharge. J Acoust Soc Am 54:361–364
Batschelet E (1981) The Rayleigh test. In: Batschelet E (ed) Circular statistics in biology. Academic Press, New York, pp 54–58
Blair RA, Lewis ER (1986) Correspondences between afferent innervation patterns and response dynamics in the bullfrog utricle and lagena. Brain Res 369:48–64
Caston J, Precht W, Blanks RHI (1977) Response characteristics of frog’s lagena afferents to natural stimulation. J Comp Physiol A 118:273–289
Cortopassi KA, Lewis ER (1996) High-frequency tuning properties of bullfrog lagenar vestibular afferent fibers. J Vestib Res 6:105–119
Cortopassi KA, Lewis ER (1998) A comparison of the linear tuning properties of two classes of axons in bullfrog lagena. Brain Behav Evol 51:331–348
Edds-Walton PL, Fay RR, Highstein SM (1999) Dendritic arbors and central projections of physiologically characterized auditory fibers from the saccule of the toadfish, Opsanus tau. J Comp Neurol 411:212–38
Fay RR (1978a) Coding of information in single auditory-nerve fibers of the goldfish. J Acoust Soc Am 63:136–46
Fay RR (1978b) Phase-locking in goldfish saccular nerve fibers accounts for frequency discrimination capacities. Nature 275:320–322
Fay RR (1984) The goldfish ear codes the axis of acoustic particle motion in three dimensions. Science 225:951–954
Fay RR (1988) Hearing in vertebrates: a psychophysics databook. Hill-Fay, Winnetka, IL
Fay RR, Edds-Walton PL (1997a) Directional response properties of saccular afferents of the toadfish, Opsanus tau. Hear Res 111:1–21
Fay RR, Edds-Walton PL (1997b) Diversity in frequency response properties of saccular afferents of the toadfish, Opsanus tau. Hear Res 113:235–46
Fay RR, Olsho LW (1979) Discharge patterns of lagenar and saccular neurons of the goldfish eighth nerve: displacement sensitivity and directional characteristics. Comp Biochem Physiol 62:377–386
Fay RR, Ream TJ (1986) Acoustic response and tuning in saccular nerve fibers of the goldfish (Carassius auratus). J Acoust Soc Am 79:1883–95
Fernandez C, Goldberg JM (1976a) Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol 39:970–984
Fernandez C, Goldberg JM (1976b) Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. II. Directional selectivity and force-response relations. J Neurophysiol 39:985–995
Fernandez C, Goldberg JM (1976c) Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. III. Response dynamics. J Neurophysiol 39:990–1008
Furukawa T, Ishii Y (1967) Neurophysiological studies on hearing in goldfish. J Neurophysiol 30:1377–1403
Goldberg JM, Brown PB (1969) Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol 32:613–636
Harada Y (2002) Experimental analysis of behavior of homing pigeons as a result of functional disorders of their lagena. Acta Otolaryngol 122:132–137
Harada Y, Kasuga S, Tamura S (2001a) Comparison and evolution of the lagena in various animal species. Acta Otolaryngol 121:355–363
Harada Y, Taniguchi M, Namatame H, Iida A (2001b) Magnetic materials in otoliths of birds and fish lagena and their function. Acta Otolaryngol 121:590–595
Lewis ER, Baird RA, Leverenz EL, Koyama H (1982) Inner ear: dye injection reveals peripheral origins of specific sensitivities. Science 215:1641–1643
Lewis ER, Narins PM (1999) The acoustic periphery on amphibians: anatomy and physiology. In: Fay RR, Popper AN (eds) Comparative hearing: fish and amphibians. Springer, Berlin Heidelberg New York, pp 101–154
Lu Z (2003) Neural mechanisms of hearing in fishes. In: Emde G von der, Mogdans J, Kapoor BG (eds) The senses of fishes: adaptations for the reception of natural stimuli. Narosa Publishing House, New Delhi, India, pp 147–172
Lu Z, Fay RR (1993) Acoustic response properties of single units in the torus semicircularis of the goldfish, Carassius auratus. J Comp Physiol A 173:33–48
Lu Z, Fay RR (1996) Two-tone rate interaction of primary afferents and midbrain neurons of the goldfish, Carassius auratus. Aud Neurosci 2:257–273
Lu Z, Popper AN (1998) Morphological polarizations of sensory hair cells in the three otolithic organs of a teleost fish: fluorescent imaging of ciliary bundles. Hear Res 126:47–57
Lu Z, Popper AN (2001) Neural response directionality correlates of hair cell orientation in a teleost fish. J Comp Physiol A 187:453–465
Lu Z, Xu Z (2002a) Effects of saccular otolith removal on hearing sensitivity in the sleeper goby (Dormitator latifrons). J Comp Physiol A 188:595–602
Lu Z, Xu Z (2002b) Encoding of acoustic particle motion by lagenar afferents of the sleeper goby, Dormitator latifrons. Soc Neurosci Abstr 162.5
Lu Z, Popper AN, Fay RR (1996) Behavioral detection of acoustic particle motion by a teleost fish (Astronotus ocellatus): sensitivity and directionality. J Comp Physiol A 179:227–233
Lu Z, Song J, Popper AN (1998) Encoding of acoustic directional information by saccular afferents of the sleeper goby, Dormitator latifrons. J Comp Physiol A 182:805–815
Manley GA, Haeseler C, Brix J, Gleich O (1991) Innervation patterns and spontaneous activity of afferent fibers to the lagenar macula and apical basilar papilla of the chick’s cochlea. Hear Res 56:211–226
McCue MP, Guinan JJ Jr (1994) Acoustically responsive fibers in the vestibular nerve of the cat. J Neurosci 14:6058–6070
McKibben JR, Bass AH (1999) Peripheral encoding of behaviorally relevant acoustic signals in a vocal fish: single tones. J Comp Physiol A 184:563–76
McKibben JR, Bass AH (2001) Peripheral encoding of behaviorally relevant acoustic signals in a vocal fish: harmonic and beat stimuli. J Comp Physiol A 187:271–85
Narins PM (1975) Electrophysiological determination of the function of the lagena in terrestrial amphibians. Biol Bull 149:438
Oeckinghaus H (1985) Modulation of activity in starling cochlear ganglion units by middle-ear muscle contractions, perilymph movements and lagenar stimuli. J Comp Physiol A 157:643–655
Platt C (1977) Hair cell distribution and orientation in goldfish otolith organs. J Comp Neurol 172:283–298
Popper AN (1977) A scanning electron microscopic study of the sacculus and lagena in the ears of fifteen species of teleost fishes. J Morphol 153:397–418
Popper AN, Fay RR (1999) The auditory periphery in fishes. In: Fay RR, Popper AN (eds) Comparative hearing: fish and amphibians. Springer, Berlin Heidelberg New York, pp 43–100
Retzius G (1881) Das Gehörorgan der Wirbelthiere. I. Gehörorgan der Fische und Amphibien. Samson and Wallin, Stockholm
Retzius G (1884) Das Gehörorgan der Wirbelthiere. II. Das Gehörorgan der Reptilien, der Vögel und der Säugethiere. Samson and Wallin, Stockholm
Suzuki A, Kozloski J, Crawford JD (2002) Temporal encoding for auditory computation: physiology of primary afferent neurons in sound-producing fish. J Neurosci 22:6290–6301
Weeg MS, Fay RR, Bass AH (2002) Tuning and directional responses of saccular afferents of the midshipman. J Comp Physiol A 188:631–641
Acknowledgements
This work was supported by University of Miami start-up funds and grants R29 DC03275 and R01 DC03275 to Z.L. from the National Institute of Deafness and Other Communication Disorders of the National Institutes of Health (NIH). William Evoy and Seth Tomchik provided helpful comments on the manuscript. Seth Tomchik assisted in the measurement of lagenar orientation. Two anonymous reviewers provided specific comments to improve the clarity of the manuscript. The animal care and use protocol used in this study complies with the Principles of Animal Care, publication No. 86-23, revised 1985 of the NIH, and was approved by the University of Miami Animal Care and Use Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Z., Xu, Z. & Buchser, W.J. Acoustic response properties of lagenar nerve fibers in the sleeper goby, Dormitator latifrons . J Comp Physiol A 189, 889–905 (2003). https://doi.org/10.1007/s00359-003-0462-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-003-0462-7