Abstract
The release of HCl is an important parameter for industrial combustion and gasification processes, which must be determined in the ppm range for active process control and optimization. Based on a low power vertical-cavity surface-emitting laser (VCSEL) at 1.74 μm, we developed a new tuneable diode laser absorption spectrometer for calibration-free, absolute in situ HCl detection using the H35Cl (2 ← 0) R(3) absorption line with minimized cross-sensitivity to CO2 and H2O. The spectrometer was applied to in situ measurements in a gasification process (T = 1,130°C, P = 1 atm, L = 28 cm) and yielded an optical resolution of 2.3·10−4, i.e. a HCl sensitivity of 45 ppm (13 ppm·m).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Gasification is a method to convert high amounts of solid or liquid biomass into gaseous products. These products can be used for the synthesis of liquid fuels, H2 and chemicals or simply burned to generate heat and electricity (Balat et al. 2009). At the Karlsruhe Institute of Technology (KIT), a new two-stage process for the production of synthesis gas (syngas) from biomass has been developed, the BIOLIQ® process (Henrich et al. 2004). Straw and other abundant lignocellulosic agricultural by-products are converted to syngas by pyrolysis and the subsequent entrained flow gasification of the pyrolysis product, a highly viscous slurry. From the tar-free syngas, chemical products like synthetic fuels or methanol can be produced. Synthetic fuels can be generated by the Fischer–Tropsch synthesis, e.g. in bubble columns with Fe catalysts. For methanol synthesis, catalysts containing Cu and Zn are used. The catalysts used in these processes are extremely sensitive to contaminants, mainly sulphur, chlorine and nitrogen compounds. The chlorine content of biomass varies significantly. The values of straw are in the range of 0.8–1.8 wt% on a dry basis (Hartmann et al. 2007; Kilgallon et al. 2002). During pyrolysis, the chlorine bound as KCl or NaCl remains in the coke fraction. In the gasifier, the chlorides partly react to HCl and partly remain in the slag. The content of HCl in the syngas must be reduced to below 10 ppb (volume) in order to avoid catalyst poisoning (Leibold et al. 2008). The chlorides in the slag decrease the melting point and, thus, influence the flow behaviour of the slag. Highly corrosive substances such as HCl also influence the choice of the steel quality of the gasifier material and the subsequent gas cleaning equipment increasing the plant lifetime but leading to considerable additional costs (Bjoroey et al. 1996).
Already existing extractive HCl detection methods are difficult to apply due to severe particle loading, and the condensation of the product gases often prevents the extraction itself. Also, the measurement and the gas sample preparation is difficult due to adsorption effects and the high water solubility of HCl. HCl detection in a technical gasification process requires a fast, non-invasive, highly sensitive and selective diagnostic which is—at the same time—economic, robust and easy to use. With respect to these requirements, laser absorption spectroscopy (LAS) is particularly promising owing to the very narrow laser line width and the excellent selectivity and sensitivity (Ebert and Wolfrum 2001; Schulz et al. 2007). One version of laser absorption spectroscopy that combines a minimal setup effort and a sampling-free measurement with high temporal resolution is in situ tuneable diode laser absorption spectroscopy (TDLAS), which was studied intensively in the last few years and successfully used even under complex industrial and technical conditions (Ortwein et al. 2008; Teichert et al. 2003; Wagner et al. 2009). TDLAS-based HCl detection is possible in the rovibrational fundamental band (1 ← 0), as well as in the first (2 ← 0) and the second overtone band (3 ← 0) (see Fig. 1), as was already demonstrated in different publications (Rothman et al. 2009; Corsi et al. 1999; Linnerud et al. 1998; Kim et al. 2003).
Regarding the technical characteristics and the spectrometer components such as laser, detectors, optics and glass fibres, absorption measurements in the region of the HCl 3ν-band are very interesting for industrial applications also for other gas species (Mihalcea et al. 1997; Upschulte et al. 1999). However, the HCl line strengths are nearly 1,000 times smaller than at 3.5 μm, so that the necessary detection limits for gasification and combustions processes require relatively large absorption path lengths or very high optical resolutions, needing considerable setup efforts.
The HCl 2ν-band at 1.7 μm offers 100 times larger line strengths and, thus, considerably better detection sensitivities. Recent improvements in single-mode room-temperature vertical-cavity surface-emitting lasers (VCSELs) in the 1.5–2.3 μm range opened up the practical use of the 2ν-band and, thus, offer a much better compromise between the required absorption path length and the achievable sensitivity (Bachmann et al. 2009; Lackner et al. 2003b). Using long wavelength VCSELs, the detection of different gas species could already be demonstrated (Totschnig et al. 2003; Lackner et al. 2003a). One main advantage of VCSELs is the wide tuning range, so that it is also possible to scan over strongly broadened lines in high-pressure applications, like biomass gasification processes for synthetic fuel production that is done at pressures up to 8 MPa (Balat et al. 2009; Dodge and Biaglow 1986). The high-pressure ability could already be shown during the in situ measurement of potassium (K) atom concentrations in the high temperature (up to 1,650 K) flue gas of a pulverized coal dust combustion system (12 bar) with a 767.5 nm VCSEL (Schlosser et al. 2002).
HCl detection in a gasification process, in which high H2O and CO2 concentrations can occur, suffers from strong spectral interference. Therefore, it is necessary to isolate a spectral region with minimum interference to realize a highly sensitive spectrometer for a quantitative, VCSEL-based HCl absorption measurement in a technical, high temperature gasification process.
2 Experimental details
TDLAS is based on scanning over the absorption line by modulating the laser wavelength and extracting the area of the absorption line to yield the number density of absorbers. To correct the in situ signals for various strong disturbances found under gasification conditions, TDLAS is in our case described by an extended Lambert–Beer`s law (Ebert and Wolfrum 2001; Ebert et al. 2005):
with n being the number density of the molecular absorbers, I 0(ν) the initial laser intensity and I(ν) the intensity detected after the absorbing medium of thickness L. Tr(t) describes the effect of transmission variation due to soot or other particles and E(t) that of additive detection signals, like the thermal and optical background radiation of the gasifier. The absorption line is characterized by the temperature-dependent, spectrally integrated line strength S(T) and the normalized (area = 1) shape function g(ν, ν 0), which is centred at wavelength ν 0. Resolving this equation with respect to the absorber density n while spectrally integrating the absorption line shape g(ν, ν 0) over the wavelength ν leads to:
Thus, the number density can be determined without the need of signal calibration, solely with knowledge of the line strength S(T), the experimental boundary conditions (p, T, Tr(t), E(t)) and the laser tuning coefficient dν/dt.
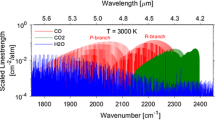
The selection of suitable absorption lines plays an important role in TDLAS and strongly influences the accuracy and quality of the measurements. The large number of possible absorption lines, especially for the interfering species like H2O and CO2, makes a manual selection ineffective. Based on the HITRAN08 database (Rothman et al. 2009), we have developed a line selection software (Wunderle et al. 2006) that calculates a highly resolved absorption spectrum for the expected experimental boundary conditions (pressure, temperature and concentration of the different gas species) in a technical gasification process (see Fig. 2). Based on these data, the H35Cl (2 ← 0) R(3) absorption line at 5,739 cm−1 has proved suitable, and an appropriate 1.74 μm VCSEL (manufactured by VERTILAS GmbH, Germany) was selected.
The 1.74 μm VCSEL offers a maximum optical output power of 0.7 mW. Compared to the commonly used distributed feedback diode lasers (DFB lasers), the output power is rather low. A precise measurement of the HCl concentration requires a comprehensive knowledge of the optical, spectral and electronic characteristics of the VCSEL. The wavelength coverage of the respective laser is limited by the maximum range of operating temperatures (15–35°C) and operating currents (1–6 mA). Within this range, we determined quasi-static, averaged tuning coefficients of dν/dT = 0.45 cm−1/K (I = 5 mA) and dν/dI = 1.75 cm−1/mA (T = 20°C and T = 30°C) (see Figs. 3 and 4).
Furthermore, we measured the modulation-frequency-dependent, so-called dynamic tuning characteristics of the laser for modulation frequencies from 0.5–6,000 Hz. Ideally, under linear current modulation, a constant laser tuning coefficient dν/dt would be desirable (Lytkine et al. 2006). However, most lasers deviate strongly from linearity—particularly for higher modulation frequencies—making an efficient tuning linearization algorithm indispensable. In this work, the laser was characterized using a planar, air-spaced (L = 10 cm) precision etalon. The tuning coefficients were determined using a semi-automated fringe fitting software.
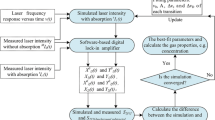
The spectrometer for the HCl concentration measurements (see Fig. 5) consisted of a function generator (Agilent 33220A) supplying the laser driver with a periodic, triangular signal. For the in situ measurements, the laser was scanned across the absorption line with a modulation frequency of 1,040 Hz to optimize suppression of transmission interferences due to soot or other particles. The laser is temperature stabilized at 305 K using a built-in Peltier element that is mounted in the laser scan. The laser is collimated using an AR-coated aspheric lens (f = 4.5 mm, diameter 6.35 mm) and directed through the gasifier. The transmitted laser beam is detected by an extended InGaAs photodiode (diameter 1 mm, peak wavelength 1.75 μm), and the photo current is amplified by a low-noise transimpedance amplifier (Femto DLPCA 200, 103 V/A, 500 kHz bandwidth). The resulting signal is digitized with a 12 bit A/D-converter (60 MS/s) and evaluated using our LABVIEW-based line fitting code as described in detail in Ebert et al. 2005.
The application of the spectrometer in a gasification process was accomplished at the atmospheric, pilot scale entrained flow gasifier REGA (Research Entrained Flow Gasifier) (Santo et al. 2007). The process flow sheet is shown in Fig. 6.
The reactor consists of a ceramic tube with an inner diameter of 0.28 m and a length of 3 m. The heat loss of the system is minimized by electric heating of the reactor walls in three zones up to 1,200°C allowing nearly adiabatic operating conditions. The reactor has a thermal load of 60 kW. A maximum of 100 m³/h syngas is produced. In order to achieve well-defined experimental conditions, the tests are carried out with ethylene glycol as a non-toxic reference fuel. Model slurries are mixed from ethylene glycol and finely ground charcoal. Oxygen enriched air is used as a gasification agent. The burner is equipped with an external mixing nozzle for the atomization of the fuel. Along the reactor axis, flanges are mounted for sampling probes as well as optical access to the reacting gas phase. With the vertically moveable burner construction, concentration and temperature profiles can be measured at variable distances from the nozzle. The syngas leaving the reactor is cooled by a tube-in-tube heat exchanger and a scrubber. Subsequently, the produced gas is burned in a flare. Research is focused on the characterization of the thermal conversion of biomass-based slurries under technically relevant gasification conditions. Hereby, the influence of fuel specification and atomization of fuel on the syngas quality, characterized by the conversion of hydrocarbons and the cold gas efficiency, are investigated (Kolb and Zarzalis 2008). During the gasification of biomass-based slurries, HCl is generated, which leads to corrosion damage at the reactor walls. HCl sources are mainly salts, originating from the ubiquitous NaCl and all chloric hydrocarbon compounds. Hence, HCl generation is a very important parameter especially due to the reducing conditions during the gasification process. For this application, TDLAS is a well-suited analysis method due to its capability of in situ HCl detection without influencing the gasification process.
In the air flow gasifier REGA, optical access is given directly downstream the burner, which can be mounted in four different vertical positions. The horizontal distance between the access windows is 140 cm, and the length of the in situ absorption path is 28 cm determined by the reactor tube diameter (see Fig. 7).
To meet the criteria needed for a laser-based absorption measurement, we developed a specialized optical access unit for both reactor sides, equipped with 1° wedged 2′′ sapphire windows. To avoid intrusion of process gases into the access pipes, which would modify the effective absorption length and contaminate the sapphire windows, the whole access unit is purged with nitrogen. Hence, the nitrogen also intrudes into the reactor itself but due to the low purging volume (0.5 m3/h) and the high gas velocity (0.1 m/s) inside the reactor it was verified that this has no significant effect on the absorption path length. The gas temperature was determined with a type S thermoelement in the centre of the absorption path.
3 Results and discussion
During the experiments, the average gas temperature was 1,130°C. Figure 8 shows a typical radial gas temperature profile at different distances from the burner at gasification conditions comparable to those during the HCl measurements. The temperature profiles show rotational symmetry and with increasing distance from the burner, the profiles become more uniform along the reactor cross-section due to mixing of the reactants and smoothening of the flow field. The HCl measurements were taken at a distance of 682 mm from the burner to minimize the effect of the radial temperature behaviour on the path integrated TDLAS measurement. The average temperature of the radial temperature profile for this burner position is 1,193°C with a standard deviation of 18 K (1σ). The standard deviation is important to minimize effects by the temperature-dependent line strength on the concentration determination.
Due to the use of glycol, the scattering loss during our measurements was low (20%). But also a measurement in a highly particle loaded process is possible as could already be shown during measurements in a full-sized coal-fired power plant (Teichert et al. 2003).
Figure 9 shows a measured and transmission, offset and amplitude corrected TDLAS line profile of the H35Cl R(3) absorption line during the gasification of glycol (without any additives) at a gas temperature of 1,130°C and a pressure of 1 atm. The measured absorption profiles were averaged over 25 scans and fitted with the Voigt line shape model using a non-linear Levenberg–Marquart algorithm. The Gaussian width was calculated using the measured temperature inside the reaction chamber. This procedure led to a time resolution of 1 s to measure, evaluate and save the absorption profile.
Measured and fitted line profile of the H35Cl R(3) absorption line in the gasifier REGA at 1,130°C gas temperature. The scan is transmission and emission corrected and normalized with the initial laser intensity. A minimum detectivity of 2.3·10−4, i.e. a signal to noise ratio of 40 can be derived from the residual
The measured absorption profile agrees very nicely with the Voigt model yielding an optical resolution of 2.3·10−4 and a signal to noise ratio (=peak height/residual) of 40. Hence, the resulting measured HCl concentration of 1,885 ppm corresponds to a very good concentration resolution of 45 ppm, i.e. 13 ppm·m with a time resolution of only 1 s.
The gas temperature for the fitting procedure was measured at a single point at the centre of the reactor. According to Fig. 8, this could result in a 10 K higher maximum temperature than the average temperature. This leads to an overestimation of the concentration due to the temperature dependence of the line strength (see Fig. 11 and Eq. 2). In the range of 1,400 ± 50 K, the temperature dependence of the line strength can be assumed as a linear function, i.e. a change in the line strength of 0.15%/K. An assumed standard deviation for the radial temperature behaviour of 20 K and an overestimation of the temperature of 10 K, therefore, lead to a systematic uncertainty of 28 ppm for the measured HCl concentration, which lies within the determined concentration resolution.
Figure 10 depicts the temporal evolution of the measured HCl concentration beginning with the start-up process, the switch into the gasification mode and finally the reactor shut down. Most of the time glycol was used as the fuel, at t = 45 min glycol with 0.8% NaCl was added to increase the HCl generation. Even prior to the start-up process (first 3 min in Fig. 10), we determined a HCl concentration of 200 ppm. This is due to electrical heating of the reactor and the fact that the reactor has already been operated before our experiments. In the following 12 min, the reactor was operated in the combustion mode, which resulted in a fast increase in the HCl concentration. Although pure glycol was used as a fuel, we measured significant HCl concentrations in a range of about 1,000 ppm. Then, the reactor was switched to the gasification mode by continuously decreasing the air number below 1 (λ = 0.43) and increasing the amount of fuel (9.5 kg/h) and the oxygen fraction in the supplied air up to 50%. These variations also led to an oscillation in the HCl concentration. After 40 min, a stationary operation point was reached with a constant HCl concentration of 1,750 ppm. Due to the reducing atmosphere during the gasification, this value is significantly higher than in the combustion mode. For this stationary phase (see also Fig. 10), we determined a concentration noise of Δc = 40 ppm, i.e. 11 ppm·m, which is a conservative sensitivity estimate since this value still includes real HCl concentration fluctuations.
The following sharp concentration increase at t = 46 min results from the change from pure glycol to a 0.8% NaCl in glycol composition and nicely demonstrates the high time resolution and sensitivity of the spectrometer. Directly after the change, the reactor was shut off, and the HCl concentration decreased rapidly to the level before the start-up process.
In a technical gasification in larger reactors, much higher temperatures up to 2,000 K can occur. To apply the VCSEL-based spectrometer also in high temperature processes, the temperature sensitivity of the used absorption line is of significant interest. Using the HITRAN08 data for the temperature-dependent line strength of the H35Cl R(3) absorption line, it is possible to conclude on the detection limits of the presented spectrometer also for technical gasifications with higher temperatures (see Fig. 11).
The calculations are based on a conservative assumption for an optical resolution of 10−3. For a temperature of 1,800 K and a pressure of 1,000 hPa, this results in a detection limit of 58 ppm·m. In the air flow gasifier REGA, we typically reached more than 4 times better detection limits. Assuming an optical resolution of 2.3 10−4 based on our measurements, it is possible to reach a HCl detection limit of even 13 ppm m.
As already mentioned, technical gasifications are planned at pressures up to 8 MPa. A sensitive HCl measurement in pressurized high temperature gasification processes, thus, requires a widely tuneable laser to cover also strongly pressure broadened absorption lines. While distributed feedback diode lasers (DFB) have also been used for measurements in high-pressure measurements, it is no longer possible to scan over a complete absorption line for high pressures and one therefore requires additional evaluation effort or the use of different direct absorption techniques like wavelength modulation spectroscopy (Rieker et al. 2007; 2009).
Recently, we demonstrated the application of the 1.74 μm VCSEL in the first laser-based high-pressure study on the room temperature pressure broadening of HCl by He, N2 and O2 covering pressures of up to 1 MPa (T = 293 K) (Ortwein et al. 2009). With rising process temperature the line width decreases for constant pressure (~ (1/T)s with s = 0.5 as derived from the kinetic gas theory). Thus, the measured line width at 1 MPa and 293 K corresponds at a temperature of 1,800 K to a pressure of 2.5 MPa.
Figure 12 shows a simulated absorption spectrum for the same boundary conditions as in Fig. 2 except for the pressure and the temperature being 2.5 MPa and 1,800 K, respectively. As can be seen, the H35Cl (2 ← 0) R(3) absorption line suffers from spectral interference by H2O for high pressures but is still separated and can therefore be scanned by the 1.74 μm VCSEL. Due to the significant line widths, it will be necessary to use a multi-line Voigt fit to account for spectral overlap with the neighbouring H2O and HCl lines. The 10-cm−1 tuning range of the VCSEL allows the inclusion of the neighbouring lines. As some lines that are also contributing to the measured signal are located outside the absorption scan they have to be calculated using HITRAN 2008. The effectiveness of this procedure was already demonstrated in (Ortwein et al. 2009). Additionally, it is also possible to use a second VCSEL to simultaneously measure more neighbouring lines. Therefore, our VCSEL-based spectrometer is well suited to cover also strongly broadened lines.
4 Conclusion
In this paper, we demonstrated the first laser-based, calibration-free and absolute in situ detection of HCl in a high temperature gasification process. The measurements showed that the new spectrometer based on a low power 1.74 μm VCSEL is well applicable for highly sensitive HCl detection. We demonstrated a HCl detection limit of 45 ppm (13 ppm m) with a time resolution of only 1 s. This enables the spectrometer for HCl monitoring also in technical gasification and combustion processes. The wide tuning range of the VCSEL also establishes the possibility for already planned measurements in pressurized high temperature gasification processes.
References
Bachmann A, Arafin S, Kashani-Shirazi K (2009) Single-mode electrically pumped GaSb-based VCSELs emitting continuous-wave at 2.4 and 2.6 μm. New J Phys 11(12):1367–2630
Balat M, Balat M, Kirtay E, Balat H (2009) Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 2: Gasification systems. Energy Convers and Manage 50(12):3158–3168. doi:10.1016/j.enconman.2009.08.013
Bjoroey O, Haugholt KH, Jäger T (1996) Diode laser spectroscopy of gaseous HCl. Quantum Electron 26(12):1090–1092. doi:10.1070/QE1996v026n12ABEH000880
Corsi C, Inguscio M, Chudzynski S, Ernst K, D’Amato F, De Rosa M (1999) Detection of HCl on the first and second overtone using semiconductor diode lasers at 1.7 μm and 1.2 μm. Appl Phys B 68(2):267–269. doi:10.1007/s003400050616
Dodge LG, and Biaglow JA (1986) Effect of elevated temperature and pressure on sprays form simplex swirl atomizer. Journal of Engineering for Gas Turbines and Power 108(1):209–215
Ebert V, Wolfrum J (2001) Absorption spectroscopy. In: Mayinger F, Feldmann O (eds) Optical measurements—techniques and applications. Springer, Heidelberg, pp 227–265
Ebert V, Teichert H, Strauch P, Kolb T, Seifert H, Wolfrum J (2005) Sensitive in situ detection of CO and O2 in a rotary kiln-based hazardous waste incinerator using 760 nm and new 2.3 μm diode lasers. Proc Combust Inst 30(1):1611–1618. doi:10.1016/j.proci.2004.08.224
Hartmann H, Böhm T, Maier L (2007) Naturbelassene biogene Festbrennstoffe-umweltrelevante Eigenschaften und Einflussmöglichkeiten. Schriftenreihe “Materialien” des Bayerischen Staatsministeriums für Umwelt, Gesundheit und Verbraucherschutz 154
Henrich E, Dinjus E, Kögel A, Raffelt K, Stahl R, Weirich F (2004) A two stage process for synfuel from biomass. ScientificCommons. http://opac.fzk.de:81/de/oai_frm.html?titlenr=57073&server=//127.0.0.1&port=81
Kilgallon P, Simms NJ, Oakey JE (2002). Fate of trace contaminants from biomass fuels in gasification systems. Proceedings of the 7th Liège Conference, Part I, Materials for Advanced Power Engineering 2002, Schriften des Forschungszentrum Jülich, Reihe Energietechnik/Energy Technology Volume 21, Part I, ISBN 3-89336-312-2: 903ff
Kim S, Klimecky P, Jeffries JB, Terry FL Jr, Hanson RK (2003) In situ measurements of HCl during plasma etching of poly-silicon using a diode laser absorption sensor. Meas Sci Technol 14(9):1662–1670
Kolb T, and Zarzalis N (2008) Entrained-Flow Gasification of Biomass-Based Slurry-Investigations on Atomization and Fuel Conversion. 7th High Temperature Air Combustion and Gasification International Symposium
Lackner M, Totschnig G, Winter F, Ortsiefer M, Amann MC, Shau R, Rosskopf J (2003a) Demonstration of methane spectroscopy using a vertical-cavity surface-emitting laser at 1.68 μm with up to 5 MHz repetition rate. Meas Sci Technol 14(1):101–106
Lackner M, Winter F, Totschnig G, Ortsiefer M, Rosskopf J, Amann MC, Shau R (2003b) Spektroskopischer einsatz neuer langwelliger (bis 2 μm) diodenlaser (VCSEL) für schwierige bedingungen. Technisches Messen 70(6):294–305
Leibold H, Hornung A, Seifert H (2008) HTHP syngas cleaning concept of two stage biomass gasification for FT synthesis. Powder Tech 180(1–2):265–270. doi:10.1016/j.powtec.2007.05.012
Linnerud I, Kaspersen P, Jaeger T (1998) Gas monitoring in the process industry using diode laser spectroscopy. Appl Phys B 67(3):297–305. doi:10.1007/s003400050509
Lytkine A, Jäger W, Tulip J (2006) Frequency tuning of long-wavelength VCSELs. Spectrochim Acta A Mol Biomol Spectrosc 63(5):940–946. doi:10.1016/j.saa.2005.11.004
Mihalcea RM, Baer DS, Hanson RK (1997) Diode laser sensor for measurements of CO, CO2, and CH4 in combustion flows. Appl Opt 36(33):8745–8752. doi:10.1364/AO.36.008745
Ortwein P, Gisi M, Eberhard M, Kolb T, Wagner S, Ebert V (2008) 2.3 μm diode laser absorption spectrometer for absolute CO measurements in gasification processes. VDI Ber 2047:195–200
Ortwein P, Woiwode W, Wagner S, Gisi M, and Ebert V (2009) Laser-based measurements of line strength, self- and pressure-broadening coefficients of the H35Cl R(3) absorption line in the first overtone region for pressures up to 1 MPa. Applied Physics B: Lasers and Optics. doi:10.1007/s00340-009-3862-8
Rieker G, Li H, Liu X, Jeffries JB, Hanson RK, Allen MG, Wehe SD, Mulhall PA, Kindle HS (2007) A diode laser sensor for rapid, sensitive measurements of gas temperature and water vapour concentration at high temperatures and pressures. Meas Sci Technol 18(5):1195–1204. doi:10.1088/0957-0233/18/5/005
Rieker G, Jeffries J, Hanson R (2009) Measurements of high-pressure CO2 absorption near 2.0 μm and implications on tunable diode laser sensor design. Appl Phys B 94(1):51–63. doi:10.1007/s00340-008-3280-3
Rothman LS, Gordon IE, Barbe A, Chris Benner D, Bernath PF, Birk M, Boudon V et al (2009) The HITRAN 2008 molecular spectroscopic database. J Quant Spectrosc Radiat Transf 110(9–10):533–572. doi:10.1016/j.jqsrt.2009.02.013
Santo U, Kuhn D, Wiemer H-J, Pantouflas E, Zarzalis N, Seifert H, Kolb T (2007) Erzeugung von Synthesegas aus biomassestämmigen Slurries im Flugstromvergaser. Chemie Ingenieur Technik 79(5):651–656. doi:10.1002/cite.200700037
Schlosser E, Fernholz T, Teichert H, Ebert V (2002) In situ detection of potassium atoms in high-temperature coal-combustion systems using near-infrared-diode lasers. Spectrochim Acta A Mol Biomol Spectrosc 58(11):2347–2359. doi:10.1016/S1386-1425(02)00049-5
Schulz C, Dreizler A, Ebert V, and Wolfrum J (2007) Combustion diagnostics. In: Tropea C, Foss JF, Yarin A (eds) Springer handbook of experimental fluid mechanics, Springer, Hidleberg, pp 1241–1316
Teichert H, Fernholz T, Ebert V (2003) Simultaneous In situ measurement of CO, H2O, and gas temperatures in a full-sized coal-fired power plant by near-infrared diode lasers. Appl Opt 42(12):2043–2051. doi:10.1364/AO.42.002043
Totschnig G, Lackner M, Shau R, Ortsiefer M, Rosskopf J, Amann MC, Winter F (2003) 1.8 μm vertical-cavity surface-emitting laser absorption measurements of HCl, H2O and CH4. Meas Sci Technol 14(4):472–478
Upschulte BL, Sonnenfroh DM, Allen MG (1999) Measurements of CO, CO2, OH, and H2O in room-temperature and combustion gases by use of a broadly current-tuned multisection ingaasp diode laser. Appl Opt 38(9):1506–1512. doi:10.1364/AO.38.001506
Wagner S, Fisher BT, Fleming JW, Ebert V (2009) TDLAS-based in situ measurement of absolute acetylene concentrations in laminar 2D diffusion flames. Proce Combust Inst 32(1):839–846. doi:10.1016/j.proci.2008.05.087
Wunderle K, Fernholz T, Ebert V (2006) Selektion optimaler absorptionslinien für abstimmbare laserabsorptionsspektrometer. VDI Ber 1959:137–148
Acknowledgments
This paper is dedicated to Prof. Dr. J. Wolfrum on the occasion of his 70th birthday. This work was funded by the German Ministry of Education and Research (BMBF) under the project number 03SF0320D.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ortwein, P., Woiwode, W., Fleck, S. et al. Absolute diode laser-based in situ detection of HCl in gasification processes. Exp Fluids 49, 961–968 (2010). https://doi.org/10.1007/s00348-010-0904-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00348-010-0904-2