Abstract
Experimental analyses of thermo-fluid phenomena of micro- and nano-flows with high Knudsen number need the measurement techniques based on interaction of atoms or molecules with photons. The pressure-sensitive paint (PSP) technique has potential as a diagnostic tool for pressure measurement in the high Knudsen number regime because it works as a so-called “molecular sensor”. However, application of PSP to micro devices is very difficult because the conventional PSP is too thick owing to the use of polymer binder and does not have sufficient spatial resolution for pressure measurement of micro-flows. In this study, we have adopted the Langmuir-Blodgett (LB) technique to fabricate pressure sensitive molecular films (PSMFs) using Pd(II) Octaethylporphine (PdOEP) and Pd(II) Mesoporphyrin IX (PdMP) to resolve ordinary PSPs problems, and have tested these PSMFs to evaluate the feasibility of the pressure measurement around micro-devices. It is clarified that the PSMF composed of PdMP has higher sensitivity than that of PdOEP. Since it is also considered that the sensitivity of PSMFs can be increased by introducing arachidic acid (AA) as spacer molecules of LB films to prevent the aggregation of luminescent molecules, we have produced PSMFs with several molar ratio of PdMP to AA. At the most suitable ratio, the PSMF has high sensitivity in the low pressure region with high Knudsen number, even if the amount of the luminescent molecules in the PSMF layer is smaller than that in conventional PSPs. This result indicates that the PSMF is feasible to measure the pressure in high Knudsen number flows such as micro-flows.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A lot of scientists and engineers have focused attention on Micro- and Nano-technologies, and studied those fields for the past few decades. For the development of micro- and nano-technology, it has been desired strongly to understand thermo-fluid phenomena around a device. In the micro-devices, their characteristic lengths are as small as mean free path for gas molecules, and the Knudsen number, the indicator of rarefaction of a gas flow, becomes high. The Knudsen number is defined in the following equation (Bird 1994):
where λ is the mean free path for the gas molecule and L is the characteristic length of the micro-device. The flow must not be treated as a continuum flow but a molecular flow when Knudsen number is greater than 0.01. Experimental analyses of thermo-fluid phenomena with high Knudsen number need molecular sensors based on emission and absorption of photons by atoms or molecules (Mori 2006). However, the experimental techniques are behind in development compared with molecular simulation techniques. In the case of gas flows inside micro-systems, there are no appropriate techniques for measurement of pressure on a solid surface, so the development of measurement techniques for such high Knudsen number regimes has been eagerly anticipated. It is not realistic to apply pressure taps to micro-systems, because dimensions of typical pressure taps are comparable to those of micro-systems.
Pressure sensitive paints (PSPs) have been developed and adopted to measure pressure distribution on solid surfaces (Asai 2001; Liu 1997; Engler 1991; Bell 2001; Niimi 2005). The principle of PSP is based on oxygen quenching of luminescence emitted from the paint irradiated by light with short wavelength (near-ultraviolet, violet, or blue). Because the PSP works as a so-called “molecular sensor”, it seems suitable for analyses of high Knudsen number flows, which require diagnostic tools in the molecular level.
Recently, some groups have been tried to apply PSP technique to micro-device. For example, Huang et al. (2002) applied PSP technique to a micronozzle and compared their experimental result with inviscid analysis. Nagai et al. (2006) also measured the pressure distribution of a micronozzle using PSP technique. It is found that their experimental results agree with the three-dimensional numerical result. However, their studies are limited to submillimeter order. The conventional PSP has serious problems that it is too thick (larger than 5 μm) owing to the use of polymer binder or its spatial resolution is too bad owing to agglutination of luminescent molecules.
In this study, we adopt Langmuir-Blodgett (LB) method (Ulman 1991) to fabricate a pressure sensitive molecular film (PSMF) (Mori 2005) applicable to pfressure measurement around micro-devices. Because the LB method can construct ordered molecular assemblies, a PSMF with nanometer order thickness and high spatial resolution seems suitable for analyses of micro-flows.
2 Photophysical property of PSP
The pressure measurement technique using PSP is based on oxygen quenching of luminescent molecules. When the PSP layer applied to the surface is illuminated with light of a proper wavelength, the luminescent molecules are excited to a higher energy state. The excited electrons undergo transition back to the ground state through radiative or radiationless processes. The radiative emission is called luminescence, which can be affected by concentration quenching, energy transfer quenching, and so on. In particular, some materials used as luminescent molecule of PSP can return to ground state by interacting with an oxygen molecule (oxygen quenching). The luminescent intensity decreases as an increase in partial pressure of oxygen. Pressure on the solid surface can be derived from the relationship between the pressure and the luminescence intensity (Stern-Volmer plot)(Liu 1997; Engler 1991; Bell 2001).
where I is the luminescence intensity, P is the oxygen pressure and T is the temperature of the sample. I ref is the luminescence intensity at the known reference pressure P ref. A n are the constants called as Stern-Volmer coefficients determined by calibration tests. The Stern-Volmer coefficients A n have temperature dependence, because the radiationless deactivation and the oxygen diffusion in a polymer, which is limited to a conventional polymer PSP, are temperature-dependent. Hence, the calibration test has to take into account of the temperature dependence. The luminescence intensity I of the ideal PSP depends inversely on P following to Eq. 2, but the actual PSPs have nonlinear dependence of 1/I on P. Therefore, the following equation considering the nonlinearity should be employed:
In practice, a second-order polynomial (N = 2) is commonly used.
3 Experimental method
3.1 Formation of PSMF
The PSP technique seems suitable for analyses of high Knudsen number flows. However, it is difficult to apply conventional PSP to pressure measurement inside micro-devices, because thickness of a PSP paint layer is of the order of microns (Bell 2001). Moreover, the spatial resolution is too bad to measure pressure distribution in micro-devices, because of the nonuniformity of the thickness of the layer and the aggregation of the luminescent molecules in the layer.
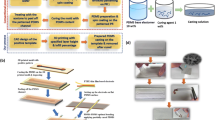
In this study, we have adopted LB method (Ulman 1991) to fabricate a PSMF applicable to pressure measurement around micro-devices. The LB method, systematized by Langmuir (1917) and Blodgett (1935), provides us with capability to construct ordered molecular assemblies. Molecular films are fabricated according to the following procedure. At first, a drop of a dilute solution of amphiphilic molecules in a volatile solvent is spread on the interface between air and subphase (deionized water is used in this study). After the solvent is evaporated, a monolayer of the molecules remains on the interface and the ordered molecular film is constructed by compressing the monolayer using a barrier. The monolayer is transferred to a substrate with keeping the surface pressure constant so as to control the order of the molecules (see Fig. 1).
Palladium(II) Octaethylporphine (PdOEP) and Palladium(II) Mesoporphyrin IX (PdMP) have been adopted as luminescent molecule of PSMF. A conventional PSP composed of PdOEP has high pressure sensitivity in low pressure region (Mori 2005). It is difficult to form stable LB film of PdOEP, because PdOEP is hydrophobic. PdMP, on the other hand, is amphiphilic, it is expected that stable LB film of PdMP can be obtained.
All solutions of PdOEP and arachidic acid (AA) or PdMP and AA in an organic solvent (chloroform:methanol = 9:1 by volume) were prepared as spreading solutions, and the concentration of them were adjusted at 0.01 mM. We have adopted AA to control the intermolecular spacing of luminescent molecule and to form high quality and stable LB film, because the LB film of AA is stable and high quality. PdOEP and PdMP were purchased from Frontier Scientific, Inc. An LB trough (Filgen, LB200-MWC) was used for the formation of PSMFs. The resistivity of the subphase pure water was 18.2 MΩ cm and the temperature was kept at 293 K. The monolayer was deposited onto a silanized hydrophobic glass slide by vertical dipping method at a constant surface pressure of 30 mN/m. Three types of samples with 2-layer, 6-layer and 20-layer PSMFs of PdOEP/AA and PdMP/AA were prepared, and tested their pressure sensitivity and influence of containing AA for PSMF.
3.2 Experimental apparatus
Figure 2 shows the experimental apparatus composed for this study. The PSMF samples are set inside a vacuum chamber evacuated by a scroll pump (Ulvac, DVS-631) and a turbo molecular pump (Ulvac, UTM-300). Oxygen gas (99.9%) is supplied into the chamber to control the oxygen pressure in the chamber, and the pressure is monitored by a capacitance manometer (Ulvac, CCMT-10A) and an ionization vacuum gauge (Ulvac, GI-M2). A xenon-arc lamp (Ushio, UXL-500SX) with a band-pass filter (400 ± 20 nm) is used as an excitation light source irradiating the sample via an optical fiber. The luminescence is filtered by a long-pass filter (540 nm) to eliminate the light from the xenon lamp, and is detected by an Electron Multiplying CCD (EMCCD) camera (Andor Technology, DV887AC-UV). The temperature of CCD is cooled to 208 K and the exposure time is 5 s. The temperature of PSMF sample is kept at 293 K. The image of the luminescence is processed by a personal computer to obtain the pressure map on the sample. In this study, 1 mm on the sample corresponds to 11.7 pixels, and the intensity data of 100 × 150 pixels were averaged for calculating the Stern-Volmer plot. The surface roughness of PSMF is measured by an atomic force microscope (AFM; Seiko Instruments, Nanopics2100).
4 Results and discussions
4.1 Property of PSMF composed of PdOEP
In this study, we have fabricated PSMF composed of PdOEP. As mentioned in Sect. 3.1, PdOEP is hydrophobic, and it is difficult to construct a stable LB film. We have introduced AA to form high quality and stable LB film. Four PSMF samples, composed of PdOEP and AA, were prepared. The molar ratios of PdOEP:AA were 1:2, 1:6, 1:20 and 1:60. The LB film of AA was also prepared.
The limiting molecular area of AA film, that is, the average area occupied by a single AA molecule in the monolayer, was 2.3 × 10−1 nm2 which agrees with previous measurements by Li et al. (2000) and Sostarecz et al. (2004). The limiting molecular areas were 1.8 × 10−1, 2.0 × 10−1, 2.2 × 10−1 and 2.1 × 10−1 nm2 for LB films composed of the mixture of PdOEP and AA whose molar ratios are 1:2, 1:6, 1:20 and 1:60, respectively. However, an area per molecule of 6.0 × 10−1 nm2 for porphyrins is reported (Luk 1988; Jones and Tredgold 1983) when the porphyrin rings are packed perpendicular to the surface, and the area per molecule for PdOEP is larger than that for AA. Because of this reason, the limiting molecular area of compounds should become larger, as the molar fraction of PdOEP is increased. As it was, the limiting molecular area of compounds hardly changed, even if the molar fraction of PdOEP was increased. These results suggest that the aggregation of PdOEP happens in the mixed LB films and the formation of stable LB film composed of PdOEP is very difficult.
We have tested the pressure sensitivity of these PSMFs in the pressure condition below 1.3 × 102 Pa, and it was found that the pressure sensitivity of these PSMFs were not high enough to measure in low pressure flows with high Knudsen number. Figure 3 shows the Stern-Volmer plots and Fig. 4 shows the luminescence intensity for PSMF of 2-layer, 6-layer and 20-layer, when the molar ratio (PdOEP:AA) is 1:20, whose pressure sensitivity was the highest in this study. The horizontal axis of Stern-Volmer plots is the pressure P, the vertical axis is the inverse luminescent intensity ratio I ref/I, where the I ref is the luminescence intensity at the reference pressure P ref = 1.0 × 10−2 Pa. As is shown, the pressure sensitivity of PSMF composed of PdOEP was worse than conventional PSPs (Mori 2005). It is because the LB film of PdOEP was unstable and it would cause concentration quenching (Vanderkooi 1987; Sapunov 1994). This result indicates that it is difficult to apply PSMF of PdOEP/AA to measure pressure distribution around micro-devices because the number of collision between gas molecules and solid surfaces become small in high Knudsen number flows such as miro-flows as well as flows under low pressure conditions.
4.2 Property of PSMF composed of PdMP
In the previous section, it is shown that the formation of PSMF composed of PdOEP is difficult and the concentration quenching occurs leading to low pressure sensitivity. In this section, we apply Pd(II) Mesoporphyrin IX (PdMP), which is one of the amphiphilic palladium porphyrins, as a luminophore of the PSMF, because amphiphiles can form stable LB films. We have prepared five samples of PSMF deposited on glass slides. The molar ratios of PdMP:AA were set for 1:0, 1:3, 1:9, 1:15, 1:21 and 1:45.
The limiting molecular areas of mixed LB films containing AA as spacer molecules are shown in Table 1. The limiting molecular area of PdMP film was 6.1 × 10−1 nm2 which agrees with the expected molecular area for porphyrins (Luk 1988; Jones and Tredgold 1983). Here, the average limiting molecular area of the compounds is caluculated by the following additive relation
where N PdMP and N AA are the molar fractions of PdMP and AA while A L,PdMP and A L,AA are the corresponding limiting molecular area. As is shown in Table 1, the limiting area of mixed LB films agree well with theoretical values of Eq. 4, indicating there is no aggregation of molecules in the layers and the formation of stable LB films were successful. Moreover, these results suggest that the intermolecular spacing of luminescent molecule can be controlled by introducing AA into the film.
Figure 5a–f shows the Stern-Volmer plots for PSMFs of 2-layer, 6-layer and 20-layer in the pressure condition below 1.3 × 102 Pa, when the molar ratio is (PdMP:AA) 1:0, 1:3, 1:9, 1:15, 1:21 and 1:45, respectively, and Fig. 6 shows the luminescence intensity for PSMF(PdMP : AA = 1:9) of 2-layer, 6-layer and 20-layer. The horizontal axis of each Stern-Volmer plot is the pressure P, the vertical axis is the inverse luminescent intensity ratio I ref/I, where the I ref is the luminescence intensity at the reference pressure P ref = 1.0 × 10−2 Pa. It is shown that the PSMF has high pressure sensitivity in the low pressure region with high Knudsen number, even if the amount of the luminescent molecules in PSMF layer whose thickness is of the order of nanometer is smaller than that in conventional PSPs whose thickness is of the order of micron. When the molar ratio (PdMP : AA) is 1:0, it is found the pressure sensitivity of PSMF is much worse compared with the others. The presssure sensitivity of PSMFs composed of PdMP is higher than that of PSMFs composed of PdOEP, except for the molar ratio (PdMP: AA) of 1:0 and 1:45.
Stern-Volmer plots for PSMF of PdMP below 1.3 × 102 Pa at 293 K, where I ref is the luminescent intensity at P ref (= 1.0 × 10−2 Pa). a Stern-Volmer plot for PSMF of PdMP : AA = 1:0. b Stern-Volmer plot for PSMF of PdMP : AA = 1:3. c Stern-Volmer plot for PSMF of PdMP : AA = 1:9. d Stern-Volmer plot for PSMF of PdMP : AA = 1:15. e Stern-Volmer plot for PSMF of PdMP : AA = 1:21. f Stern-Volmer plot for PSMF of PdMP : AA = 1:45
Figures 5a–d and 3a show that the pressure sensitivity of the 2-layer PSMF is higher than that of the 6-layer and the 20-layer PSMFs, because the decrease of luminescent intensity caused by oxygen quenching is almost equal regardless of the number of layers as shown in Figs. 4 and 6. The fact indicates that the oxygen molecules interact only with the outermost layer of each PSMF, that is, oxygen quenching occurs at the outermost layer only. For the further improvement of pressure sensitivity, it is important to improve the oxygen gas permeability of the PSMFs.
The pressure sensitivity of PSMF increases as the quantity of AA increases as shown in Fig. 5a–d. This result shows the effectiveness of introducing AA to control the intermolecular spacing of luminescent molecule. When the molar ratio is 1:0, it is considered that the concentration quenching occurs because the luminescent molecules lie close to one another, leading to the extremely low pressure sensitivity. On the other hand, when the molar ratio is 1:9 and 1:15, the pressure sensitivity is relatively high. In these cases, the intermolecular spacing of PdMP is enlarged by introducing AA into the film, and as a result, the concentration quenching is prevented.
As shown in Fig. 5e, f, when the molar ratios of PdMP : AA are 1:21 and 1:45, the pressure sensitivity shows the different tendency, that is, the 2-layer PSMF has the lowest pressure sensitivity unlike the above results for the low molar fraction. It is also clarified that the pressure sensitivity becomes lower for all cases (2-, 6- and 20-layer PSMFs) as an increase in the molar fraction of AA. These results are probably attributed to the relatively small amount of luminescent molecules in the PSMFs compared with the PSMFs of the other molar ratios. In other words, most of the excited molecules in the 2-layer PSMFs are quenched even at low pressure conditions, that is, the quenching process is saturated, resulting in very low pressure sensitivity because the absolute number of the luminescent molecules is small. On the other hand, in the cases of 6-layer and 20-layer PSMFs, the luminescent molecules are quenched partially in the inner layers and the slope of Stern-Volmer plots become smaller with increasing pressure because of the slower permeation of oxygen molecules. Hence, the 6-layer and 20-layer PSMFs show higher oxygen pressure sensitivity than the 2-layer PSMF with the same molar fraction. As shown in Fig. 5c–e, the pressure sensitivity of the 6-layer and the 20-layer PSMFs of PdMP : AA = 1:21 looks comparative to that of the 2-layer PSMF of PdMP : AA = 1:9 or 1:15, but it should be mentioned that the lumminescent intensity of the PSMFs of PdMP : AA = 1:21 is very low, leading to low SN ratio because the amount of luminescent molecules is small.
As a result, it is clarified that PSMF composed of PdMP is stable and the pressure sensitivity of PSMF composed of PdMP is higher than that of PSMF composed of PdOEP in low pressure region with high Knudsen number. Moreover, it is shown that controlling the intermolecular spacing of luminescent molecules to prevent concentration quenching is an effective technique to improve the pressure sensitivity of PSMF. In this study, it is found that the suitable molar ratio of luminescent molecules and spacer molecules (PdMP : AA) is between 1:9 and 1:15.
4.3 Surface roughness of PSMF
In this study, the surface roughness of 2-layer PSMF (PdMP : AA = 1:9), which has high pressure sensitivity, was measured by AFM. The area of 10 μm × 10 μm was scanned for each sample. The surface roughness of substrate and the conventional PSP (PdOEP/poly(TMSP)) were also measured to compare with that of PSMF. The AFM images of the substrate, the PSMF and the PdOEP/poly(TMSP) are shown in Fig. 7a–c, respectively. The image size of Fig. 7a, b are 10 μm × 10 μm × 8 nm, while the image size of Fig. 7c is 10 μm × 10 μm × 150 nm, so the surface of PdOEP/poly(TMSP) looks smoother at first glance. Table 2 shows arithmetical mean roughness (R a ) and maximum height (R y ) of each samples. As is shown in Table 2, it is found that R a and R y of PSMF are same order of those of the substrate, while R a and R y of the conventional PSP are much larger compared with those of the substrate. Since the R a of PSMF is smaller than that of substrate, it is suggested the LB film is deposited bridging jogs of the substrate. These results show the effect of the PSMF on the micro-flow field is vanishingly small.
5 Conclusions
We have examined the fundamental properties of PSMFs using palladium porphyrins as luminophores in low pressure conditions, to clarify the feasibility of PSMF for measurement of surface pressure in high Knudsen number flows such as micro-flows. The following concluding remarks are obtained:
-
1.
It is shown that the PSMF fabricated in this study has high sensitivity in the low pressure region with high Knudsen number, even if the amount of the luminescent molecules in PSMF is smaller than that in conventional PSP.
-
2.
It is clarified that the PSMF composed of Pd(II) Mesoporphyrin IX (PdMP) has higher sensitivity than that of Pd(II) Octaethylporphine (PdOEP). The pressure sensitivity of the 2-layer PSMF is higher than that of the 6-layer and the 20-layer PSMFs, because oxygen quenching occurs at the outermost layer only.
-
3.
The effectiveness of introducing AA to control the intermolecular spacing of luminescent molecule is shown. It is shown that controlling the intermolecular spacing of luminescent molecules to prevent concentration quenching is an effective technique to improve the pressure sensitivity of PSMF. In this study, it is found that the suitable molar ratio of luminescent molecules and spacer molecules (PdMP : AA) is from 1:9 to 1:15.
-
4.
It is found that arithmetical mean roughness and maximum height of PSMF are same order of those of the substrate, and PSMF does not affect the micro-flow structure.
As a result, the PSMF composed of PdMP is feasible to measure the pressure in high Knudsen number flows such as micro-flows.
References
Bird GA (1994) Molecular gas dynamics and the direct simulation of gas flows. Oxford university press, New York
Mori H et al (2006) Pressure sensitive paint suitable to high Knudsen number regime. Meas Sci Technol 17(1):1242–1246
Asai K (2001) Status and prospect of pressure-sensitive paint technology. Kashika Joho Gakkaishi (J Vis Soc Jpn) 21(83):203–208
Liu T et al (1997) Temperature- and pressure-sensitive luminescent paints in aerodynamics. Appl Mech Rev 50(4):227–246
Engler RH et al (1991) Description and assessment of a new optical pressure measurement system (OPMS) demonstrated in the high speed wind tunnel of DLR in Göttingen, DLR-FB 92-24
Bell JH et al (2001) Surface pressure measurements using luminescent coatings. Annu Rev Fluid Mech 33:155–206
Niimi T et al (2005) Application of pressure-sensitive paints to low-pressure range. J Thermophys Heat Transf 19(1):9–16
Huang CY et al (2002) Molecular sensors for MEMS, AIAA2002-0256. In: 40th aerospace sciences meeting & exhibit, Reno, NV
Nagai H et al (2006) Flow diagnostic using PSP and TSP technique ina supersonic micronozzle. In: 12th international symposium on flow visualization, Göttinge, Germany
Ulman A (1991) An introduction to ultrathin organic films from Langmuir-Blodgett to Self-Assembly. Academic, New York
Mori H et al (2005) Oxygen sensitive films and its production method. Patent Pending 2005-278731 (Japan)
Langmuir I (1917) The constitution and fundamental properties of solids and liquids. ii. Liquids. J Am Chem Soc 39(9):1848–1906
Blodgett KB (1935) Films built by depositing successive monomolecular layers on a solid surface. J Am Chem Soc 57(6):1007–1022
Mori H et al (2005) Molecular number flux detection using oxygen sensitive luminophore. Phys Fluids 17(10):100610
Li H et al (2000) AFM study of templated growth of cadmium sulfide nanoparticles using pure and mixed arachidate films. Thin Solid Films 358:62–72
Sostarecz AG et al (2004) Depth profiling of Langmuir-Blodgett films with a buckminsterfullerene probe. Anal Chem 76(22):6651–6654
Luk SY et al (1988) Preparation and characterization of Langmuir-Blodgett films of mesoporphyrin ix dimethylester indium chloride. Thin Solid Films 157:69–79
Jones R, Tredgold RH (1983) Langmuir-Blodgett films of simple esterified porphyrins. Thin Solid Films 99:25–32
Vanderkooi JM et al (1987) An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J Biol Chem 262(12):5476–5482
Sapunov VV (1994) Concentration quenching of the triplet state of Pd-octaethylporphin. Opt Spectrosc 77(5):696–701
Acknowledgments
The present work was supported by a grant-in-aid for Scientific Reseach of Ministry of Education, Culture, Sports, Science and Technology. The authors would like to express our gratitude to Prof. Tokuji Miyashita and Prof. Masaya Mitsuishi in Tohoku University and Prof. Takahiro Seki in Nagoya University for their helpful advice about LB film technique.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuda, Y., Mori, H., Niimi, T. et al. Development of pressure sensitive molecular film applicable to pressure measurement for high Knudsen number flows. Exp Fluids 42, 543–550 (2007). https://doi.org/10.1007/s00348-007-0259-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00348-007-0259-5