Abstract
The purpose of this work is to get fundamental knowledge and to understand experimentally the fluorescence phenomenon usually used in laser-based imaging diagnostics. A complete review of thermodynamic effects (pressure, temperature, oxygen and tracer concentration) on biacetyl fluorescence is presented for a wide range of temperature and pressure. Biacetyl fluorescence increases with pressure and then levels off at high pressure when the relaxation is complete. The influence of the temperature is explained by a competition between the intersystem crossing and fluorescence. The effect of oxygen fluorescence quenching is important at high pressure and is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Laser induced fluorescence (LIF) technique allows quantitative determination of scalar fields (concentration, temperature), however, the aerothermodynamic surrounding conditions involve complex fluorescence phenomena. Before setting quantitative fuel concentration measurement by LIF on engines, the influence of preponderant parameters (concentration of oxygen, tracer, temperature, pressure, laser power, combination of tracer and excitation wavelength...) on the fluorescence signal must be characterized. The objective of this study is thus to investigate the LIF experiments in a constant volume vessel at high temperature and high pressure to underline the influencing parameters over a wide range of pressure, temperature, tracer concentration, in conditions close to those encountered in internal combustion (IC) engines.
Laser induced fluorescence presents many applications in spectroscopy. This technique serves as a sensitive monitor for the absorption of laser photons in fluorescence excitation spectroscopy; it is suited to gain information on molecular states and allows the spectroscopic study of collision processes (Demtröder 1982). Moreover, planar laser induced fluorescence (PLIF) has been widely developed for imaging fuel distributions in IC engines (Zhao and Ladommatos 1998), for concentration (Lozano et al. 1992; Thurber and Hanson 2001) and temperature measurements (Thurber et al. 1997; Koch and Hanson 2003). For fuel concentration measurement by LIF, the technique usually adopted is fluorescence from a tracer molecule, which presents satisfactory spectroscopic and thermodynamic properties similar to the fuel. For instance, a tracer must present a limited absorption of the available laser wavelength, satisfactory fluorescence yield, low quenching rate by oxygen, low toxicity, good stability and solubility in fuel, with a boiling point and a latent heat of vaporisation similar to the fuel. A number of studies have been conducted to find suitable tracers for IC engines (Zhao and Ladommatos 1998). The required absorption wavelength in the visible and UV region restricts the tracers to two main categories: aromatic and carbonyl compounds. The fluorescence intensity of the aromatic tracer is much stronger than that of the carbonyl compounds (Krämer et al. 1998). The lowest-lying transitions of aromatic hydrocarbons are of the π–π* type and are characterized by high molar absorption coefficients and relatively high fluorescence quantum yields (Valeur 2002). A π orbital is formed from two p atomic orbitals overlapping laterally, that involves a π bond. The transition π–π* corresponds to an absorption of a photon with appropriate energy, which promotes one of the π electrons to an antibonding orbital π*. Aromatic compounds fluoresce in the broad range of 270–640 nm, with an intense fluorescence for unsubstituted aromatics in the ultraviolet (benzene, toluene, naphthalene) or visible region (anthracene). As the degree of conjugation increases (an increase in the extent of the π-electron system), the intensity of fluorescence often increases and a shift to longer wavelengths is observed (Valeur 2002). Among the carbonyl compounds, aldehydes, ketones and diones are used. These compounds fluoresce in the spectral region of 300–550 nm as a result of the n–π* transition in the C=O group (Grossmann et al. 1996; Ossler and Alden 1997; Yuen et al. 1997), which corresponds to a promotion of a non-bonding electron to an antibonding orbital. The transition can be realised with sufficient coupling between the magnetic moments of orbital and spin. Transitions of the n–π* type are characterized by molar absorption coefficients which are at least a factor of 102 smaller than those of π–π* transitions (Valeur 2002). For example, ketones are excited from a singlet ground electric state (S0, n) to a first excited singlet state (S1, π*), and then transit to an excited triplet state (T1) whose energy level is lower than S1 by intersystem crossing without radiation. The fluorescence results from the relaxation from S1 to S0. For carbonyl compounds, the transition from S1 to T1 by intersystem crossing has a high probability, whereas the probability of S1 to S0 is very low. The intersystem crossing to the triplet state is the most important process for the excited singlet ketone (Shortridge et al. 1971). On the contrary, for aromatic compounds the intersystem crossing probability is low and hence they emit strong fluorescence. Quenching by molecular oxygen makes the fluorescence intensities inversely proportional to oxygen concentration (Koban et al. 2002). Since the lifetime of excited states is controlled by a rapid intersystem crossing, the influence of collisional quenching of the S1 state by O2 is higher for aromatic compounds than for carbonyl compounds (Einecke et al. 2000). Thus, aromatics such as toluene or xylene are strongly quenched by oxygen (Reboux et al. 1994; Fujikawa et al. 1997) and the oxygen quenching rate is particularly elevated at high pressure.
Ketones commonly used are mainly acetone and 3-pentanone (Yuen et al. 1997; Thurber et al. 1998; Thurber and Hanson 1999). As shown by Fujikawa et al. (1997), both tracers have higher fluorescence yields than aldehydes (e.g. propionaldehyde, n-valeraldehyde). Moreover, ketones are preferred over aldehydes as tracers due to their low reactivity under high pressure and high temperature environment such as in an IC engine (Zhao and Ladommatos 1998). Acetone and 3-pentanone are relatively chemically stable and dissociate only at high temperatures in the flame front (Grossmann et al. 1996). These compounds are commonly studied for pressures up to 1.5 MPa and in the temperature range of 293–723 K (Fujikawa et al. 1997; Thurber et al. 1998). In a pressure range of 0.1–1.5 MPa, the fluorescence intensity per ketone molecule increases with increasing pressure and excitation wavelength. The increase in the fluorescence quantum yield with pressure may be the result of a combination of incomplete vibrational relaxation in the excited singlet level and the dependence of the singlet–triplet intersystem crossing rate on the vibrational level in the singlet manifold (Reboux et al. 1994; Yuen et al. 1997). High-excitation wavelengths involve weaker pressure and composition dependences of the fluorescence yield. The observed decrease in the fluorescence quantum yield with increasing temperature (Yuen et al. 1997; Thurber and Hanson 1999; Koch and Hanson 2003) is the result of the increase in the intersystem crossing rate for vibrational levels with higher energy in the excited singlet manifold.
Diones, such as biacetyl (2,3-butanedione), present longer excitation and fluorescence wavelengths (Horowitz et al. 2001). The fluorescence emission is broadband in the blue with a lifetime of 15 ns, the phosphorescence is long-lived (lifetime of 1.5 ms). When excited by a laser beam at 355 nm, biacetyl is generally selected as the tracer of iso-octane (2,2,4-trimethylpentane), a reference compound of gasoline (Baritaud and Heinze 1992; Deschamps et al. 1994; Itoh et al. 1995). This tracer decomposes slowly and polymerizes in presence of impurities, that involves a decrease of the fluorescence efficiency with time. Biacetyl has been widely used as a PLIF tracer in low-pressure (0.1–0.5 MPa) and low-temperature (293–373 K) environments (Heicklen 1959; Van Cruyningen et al. 1990; Lawrenz et al. 1992; Deschamps et al. 1994). The fluorescence efficiency is very low when the excitation wavelength is shorter than 320 nm.
For applications in IC engines (for fuel distribution imaging and study of the mixture homogeneity), tracers usually used are: ketones, aldehydes, aromatics and diones. Acetone and 3-pentanone are generally selected because of their property of showing the weakest temperature and pressure dependence of LIF intensity compared to all other tracers. However, their pumping requires the use of lasers at low wavelength (KrF laser at 248 nm, Nd:YAG laser at 266 nm, XeCl laser at 308 nm), in comparison with biacetyl excited at 355 nm by third harmonic Nd:YAG laser and ionized argon Ar++ laser. This continuous laser source was used for concentration measurements in a pressurized and heated gas mixture flow (Guibert et al. 2002) and for LIF application in engine (Rochelle and Perrard 1999). Biacetyl presents longer excitation and fluorescence band than ketones and has thermodynamic properties similar to iso-octane: at atmospheric pressure, the boiling point is 361 K for biacetyl, 329 K for acetone and 373 K for iso-octane.
For low laser intensity, the fluorescence signal, in number of photons collected, is given by:
where E is the laser fluence, (hc/λ) is the energy of a photon at the excitation wavelength λ, ηopt is the overall efficiency of the collection optics, dV C is the collection volume, σ is the molecular absorption cross-section of the tracer, Φ is the fluorescence quantum yield equal to the number of fluorescence photons per absorbed photon. The bracketed term is the biacetyl number density, given as the product of mole fraction X biacetyl and the total pressure divided by the product of the Boltzmann constant k and the temperature. From Eq. 1, biacetyl fluorescence can be modeled. It is then necessary to characterize the dependences of fluorescence quantum yield and the molecular absorption cross-section on temperature, pressure, composition and excitation wavelength. For isothermal and isobaric conditions, and a given excitation wavelength, the fluorescence signal is proportional to mole fraction.
Pioneering studies of biacetyl photophysics (Okabe and Noyes 1957; Heicklen 1959) have detected little dependence of biacetyl fluorescence on pressure and incident wavelength, over a weak domain. Heicklen (1959) reported the increase of the fluorescence intensity with increasing pressure when the excitation wavelength was 365.5 nm. On the other hand, at 435.8 and 404.7 nm, fluorescence efficiencies were independent of pressure. Amongst the numerous experimental and theoretical studies on the biacetyl emission characteristics (Brand and Mau 1974; Kamei et al. 1986; Senent et al. 1994), Ten et al. (2002) have compared the experimental spectra of the biacetyl absorption and fluorescence with theoretical spectra calculated from the method of hybrid atomic orbitals and semi-empirical correlations. With a satisfactory agreement between experimental and theoretical spectra, the model shows the structural and dynamical changes occurring in molecules under electronic excitation. Recent results (Guibert et al. 2002) have shown the effects of the temperature on the fluorescence intensity. These effects are similar to those observed with ketones and the LIF signal decreases with increasing temperature. Concerning the phosphorescence characteristics, biacetyl triplets phosphoresce with high efficiency, except in an oxygenated environment where the oxygen molecule quenches the biacetyl phosphorescence rapidly and strongly (Jiang-Bang et al. 1988).
In the following sections, the experimental setup (a high temperature and high pressure facility) developed to follow instantaneously the fluorescence signal is described with the optical system. The fluorescence signal is studied for biacetyl. Absorption and emission spectra are characterized. Moreover, a parametric study with the influence of different parameters (laser power, pressure, temperature, mole fraction of tracer, percentage of oxygen) on the fluorescence signal is presented, in order to underline the significant parameters for an optimal application of biacetyl LIF diagnostics. The influence of each parameter is discussed separately by underlining the important effects of the biacetyl photophysics.
2 Experimental setup
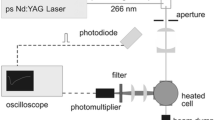
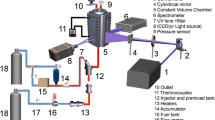
To evaluate the influence of preponderant parameters on the fluorescence signal, experiments are conducted in a high temperature and high pressure constant volume vessel, equipped with different sensors and an optical system (cf. Fig. 1).
2.1 High temperature and high pressure facility
The maximum operating pressure of the experimental facility is 5 MPa and the maximum temperature is 773 K. The vaporization of the liquid tracer is achieved by reaching the tracer vapor pressure through a void pump. The liquid tracer is injected by means of different types of syringes at airtight piston. An electrical heating system regulates the temperature of the chamber. Temperature is controlled inside the chamber by three thermocouples that ensure uniformity of the temperature field. During the LIF experiments, the following uncertainties are encountered on measuring the tracer volume, the ambient temperature and the ambient pressure: ΔV/V=1%, 0.05%<ΔT/T<0.08%, 0.5%<ΔP/P<20%.
2.2 Optical system
The chamber is equipped with three optical quartz windows for absorption and fluorescence signal measurements. Two types of excitation sources are used. The first one used for fluorescence measurements is a continuous argon laser in an UV domain (ionized argon Ar++, discrete wavelengths of 352.9 and 365.2 nm, maximum power output of 100 mW) coupled to an optical fiber of 200 μm diameter. The second one used for the absorption measurements is a high-pressure xenon lamp with a wide wavelength range (from 250 to 1,100 nm) coupled to a liquid optical fiber (Series 380, Lumatec) with a core diameter of 2 mm, a light conducting liquid core, transparent from 270 to 720 nm and more efficient than quartz fiber for transmission in UV range. A focusing lens system allows obtaining an incident coherent light beam of 1 mm diameter approximately in the chamber center. A second optical fiber of 400 μm diameter collects the emitted fluorescent signal along the axis perpendicular to the incident light beam and is coupled to a high sensitivity miniature spectrometer with a permanently installed diffraction grating (2,048 pixels, a spectral range of 200–850 nm), UV detector upgrade, longpass sorting filter and entrance slit of 10 μm. The spectrometer is connected to a PC, that allows to analyze the absorption and fluorescence spectra accurately.
2.3 Experimental conditions
In the current work, biacetyl (CH3–(CO)2–CH3) is tested as a fluorescence tracer for its satisfactory spectroscopic qualities and thermodynamic properties (boiling point at atmospheric pressure of 361 K, density of 980 kg m−3, molecular weight of 86×10−3 kg mol−1, saturated vapour pressure at 293 K of 6,960 Pa). The experiments are conducted in nitrogen or reconstituted air (80% vol. N2 + 20% vol. O2) for a constant biacetyl volume and for different biacetyl mole fractions. The constant biacetyl volume is fixed at 400 μl, that corresponds to 3.57×1018 molecules cm−3, a value close to those used in the literature (for example: fluorescence of various tracers with a concentration between 1015 and 1018 molecules cm−3 (Grossmann et al. 1996; Bryant et al. 1997), fluorescence of acetone and 3-pentanone with a concentration of 3.1×1017 molecules cm−3 (Krämer et al. 1998).
Table 1 summarizes pressure, temperature and mole fraction ranges explored. For given experimental conditions of pressure, temperature and tracer mole fractions, the fluorescence signals are averaged from five spectra registered with back noise and reference spectrum. The integration time is of 2,500 ms, every experiment is reproduced three times and the signal stability in time is checked. A fresh sample of biacetyl is used regularly to avoid the decomposition and polymerization of biacetyl in the presence of impurities. Test experiments were investigated for different heights of the incident laser beam in the chamber, which ensures a good homogeneity of the measurement volume.
2.4 Spectra of absorption and emission
To underline satisfactory spectroscopic qualities of biacetyl excited with Ar++ laser (limited absorption of the laser beam, sufficient spectral shift of the fluorescence signal relative to the laser excitation wavelength), the absorption spectrum of biacetyl is determined, by using the high-pressure xenon lamp with a large wavelength range. Horowitz et al. (2001) decomposed the absorption spectrum of biacetyl with two bands, the first one between 210 and 325 nm and the second one between 350 and 480 nm. The absorption cross-section noted σ at a given wavelength λ is derived from the Beer–Lambert’s law:
with l the optical path length, C the tracer concentration, I 0(λ) and I(λ) respectively, the light intensities of the beams entering and leaving the absorbing medium.
As presented in Fig. 2, only the second absorption band is observed experimentally, due to the insufficient power of the high-pressure xenon lamp for wavelengths lower than 350 nm. Deduced absorption cross-sections of vapor biacetyl are in a satisfactory agreement with values obtained in previous studies. For a temperature of 373 K, the largest absorption cross-section σ is equal to 6.44×10−20 cm² molecule−1, in comparison with values of the literature at 298 K: σ = 6.92×10−20 cm² molecule−1 (Plum et al. 1983) and σ = 7.62×10−20 cm² molecule−1 (Horowitz et al. 2001). For the studied temperature range between 373 and 573 K, the absorption spectrum increases very slightly in magnitude and width with increasing temperature. This evolution is due to the following effects: the increasing of the ground state vibrational energy with temperature, which involves the absorption spectrum to grow and the decreasing of the energy required to the electronic transition with added energy in the ground state, which involves the absorption spectrum to broaden. The temperature influence on biacetyl absorption cross-section is very low, when compared to ketonic tracers. For example, the peak absorption of 3-pentanone increases by a factor of two between 300 and 1,000 K and by 25% between 300 and 540 K (Koch 2005), compared with the largest cross-section of biacetyl increasing by 7% between 373 and 573 K. As shown in Fig. 3, biacetyl excited with Ar++ laser radiation presents a fluorescence emission band between 440 and 510 nm with a peak at 485 nm (emission from the first excited singlet state) and a phosphorescence band between 510 and 600 nm with a peak at 517 nm (emission from an excited triplet state by intersystem crossing). From the emission characteristics of biacetyl, the signal is integrated between 440 and 510 nm to discuss the influence of physical parameters on the fluorescence only. For every experiment reproduced three times, the fluorescence signal is averaged from five spectra. The normalized standard deviations in percent at a given pressure and temperature obtained from 15 integrated fluorescence signals in nitrogen for a constant biacetyl volume of 400 μl (3.57×1018 molecules cm−3) are summarized in Table 2. With different integration limits of 440–500 nm or 440–510 nm, the uncertainty of the integrated fluorescence signals varies between 6 and 15% for the explored experimental pressure and temperature range.
3 Influence of different parameters on the biacetyl fluorescence signal
3.1 Linear fluorescence regime
As expressed in Eq.1, the fluorescence signal depends on excitation wavelength. To explain the observation of decreasing fluorescence quantum yield with decreasing excitation wavelength, a multi-step model was developed by Thurber and Hanson (1999) and discussed by Schulz and Sick (2005). The fluorescence quantum yield is decreased with increasing vibrational excitation in the excited state, which gets preferentially populated with short excitation wavelengths. The relaxation of the laser-coupled upper state is treated as a multi-step process, which depends on fluorescence, vibrational relaxation, intersystem crossing and collisional quenching. The wavelength dependence of biacetyl LIF is not studied in this work where the excitation wavelength is fixed by the ionized argon laser.
According to the intensity of the ionized argon laser used as the excitation source, the fluorescence regime has been characterized. As shown in Fig. 4, the fluorescence signal is linearly proportional to the incident laser power; the studied regime is a linear fluorescence regime with a spectral intensity of the incident laser light strongly lower than the saturation intensity. For the results presented in this work, the power of the continuous argon laser is fixed to the maximum value of 100 mW. When the incident laser intensity is strongly higher than the saturation intensity, the fluorescence regime called the “saturation fluorescence regime” is ideal, the excited population does not depend on the quenching rate nor on the laser intensity. However, a high-incident laser power is necessary to investigate such a regime. The spatial and temporal repartition is not uniform, which induces a partially saturation fluorescence regime then an important limitation to its use. In this work, with a linear regime in energy, the quenching rate could be evaluated to achieve quantitative fluorescence measurements. The maximum ionized argon laser power varies between 90 and 110 mW for a series of experiments, hence the drift in laser power is negligible. The percentage of laser absorption is bounded by 7–11 % depending on the ambient gas (nitrogen, pure oxygen or reconstituted air). For the above reasons, no correction on the incident laser energy is introduced in the results presented in the following sections.
3.2 Influence of the tracer mole fraction on fluorescence
As presented in Fig. 5, the fluorescence signal increases with increasing tracer mole fraction and reaches a saturation level from a biacetyl mole fraction of 3.13×10−1 at 423 K and 3.87×10−1 at 523 K. The effect of the tracer mole fraction on the fluorescence emission is due to the reabsorption process. The fluorescence of the excited biacetyl can be reabsorbed by another molecule of biacetyl at fundamental state, all the more probable phenomenon when the tracer quantity is at a high concentration. Moreover, this reabsorption changes the fluorescence spectrum, the highest frequencies are only reabsorbed. Another effect of the tracer mole fraction on the fluorescence emission is due to the self-quenching, more important at high mole fractions. To characterize biacetyl–biacetyl collisions, the reference collision frequency can be calculated from the following equation (Thurber and Hanson 1999):
where Z coll is the Lennard–Jones collision frequency (Reid et al. 1987).
For a temperature of 423 K and a pressure of 0.1 MPa, the frequency of biacetyl–biacetyl collisions is 1.65×109 s−1 for a biacetyl mole fraction of 0.2 against 6.35×109 s−1 for a biacetyl mole fraction of 0.8.
For the following studies, in order to avoid the saturation phenomenon, the fluorescence signal will be studied for different concentrations of biacetyl with a maximum value of 3.57×1018 molecules cm−3.
3.3 Influence of the pressure on fluorescence
The pressure dependence of biacetyl fluorescence has been studied in nitrogen, for the pressure range of 0.1–4 MPa, the temperature range of 373–573 K and for a constant biacetyl concentration of 3.57×1018 molecules cm−3. As shown in Fig. 6, the fluorescence signal increases between 0.1 and 2.5 MPa for every studied temperature, and then levels off when relaxation is complete. Between 0.1 and 2.5 MPa, the increase of the fluorescence with pressure is more important at a high temperature. With nitrogen as a buffer gas, Yuen et al. (1997) have observed that the acetone fluorescence quantum efficiency increases with pressure from 0.6 to 5 Pa and remains constant between 5 and 8 Pa. Thurber and Hanson (1999) have shown a similar effect of pressure on acetone fluorescence in nitrogen with excitation at 248, 266 and 308 nm: an initial increase of the fluorescence at low pressure then a stagnation to asymptote towards a high pressure limit.
With increasing pressure, the collisional vibrational relaxation will allow to return more rapidly from molecules with high-lying, initially-excited singlet states to molecules with less vibrational states. Since the intersystem crossing is slower from these lower-lying levels, the fluorescence yield and the fluorescence signal will be higher.
3.4 Influence of the temperature on fluorescence
As observed experimentally in Fig. 7, for the studied pressure range, the fluorescence signal increases with temperature between 373 and 423 K at low pressure and between 373 and 473 K at high pressure, and then decreases with increasing temperature.
For the biacetyl molecule which presents a transition of type n–π* (molecular orbital n and orbital π*) between the singlet ground electric state S0 and the first excited singlet state S1, the intersystem crossing is efficient. For an excited triplet state T1 whose energy level is lower than S1, the intersystem crossing from the state S1 to the state T1 can be followed by a thermal excitation from the triplet state T1 to the state S1. This transfer from T1 to S1 occurs when the energy difference between both levels is low and the lifetime of T1 is long. In this case, the fluorescence presents two parts: the first one with a classic deexcitation time, the second one with a slightly lower lifetime than the state T1. The fluorescence intensity then increases with temperature.
On the other hand, at a high temperature, the biacetyl molecule at the singlet ground state will acquire more vibrational energy, to reach a state S1 than the triplet state T2, higher than the state T1. In this case, the intersystem crossing between S1 and T2 is activated thermally and is in competition with the fluorescence when the temperature increases, which will be translated into a decrease in fluorescence signal.
3.5 Influence of the oxygen concentration on fluorescence
To characterize the influence of the oxygen concentration on the fluorescence signal, two ambient gases are used: pure nitrogen and a mixture of 80% nitrogen and 20% oxygen. Figure 8 compares the results obtained in nitrogen and reconstituted air, for a temperature of 473 K and a pressure range of 0.1–4 MPa, for biacetyl mole fraction of 2.9×10−3, 3.8×10−3, 4.8×10−3, that corresponds to equivalence ratios of 0.6, 0.8, 1. The studied conditions are close to those in IC engines, with high pressures and temperatures for lean and stoichiometric mixtures.
The fluorescence signal is normalized by the atmospheric pressure value for every biacetyl concentration. As plotted in Fig. 8, it increases linearly with increasing pressure when the ambient gas is nitrogen. On the other hand, with air bath gas, from a pressure of 1 MPa, the fluorescence signal does not increase linearly with pressure, and it is strongly quenched by oxygen molecules, with fluorescence data for air rising very slightly above 1 MPa. For a pressure of 4 MPa, a temperature of 473 K and a biacetyl mole fraction of 2.9×10−3 (equivalence ratio of 0.6), the maximum LIF intensity is twice higher in nitrogen than in air. Experimental results for the three equivalence ratios differ significantly in nitrogen, which would characterize a self-quenching and define the influence of biacetyl–biacetyl collisions.
The increase in the fluorescence signal with increasing pressure could be attributed to the vibrational relaxation toward higher fluorescence quantum yield levels. However, the quenching of the excited singlet state by oxygen could compete effectively with this vibrational relaxation. The excited singlet state of biacetyl is quenched by collisions with the oxygen molecule. A similar fluorescence quenching effect of oxygen at high-partial pressures is also observed with a constant biacetyl concentration of 3.57×1018 molecules cm−3, which corresponds to a mole fraction between 4.6×10−3 (at 4 MPa and 373 K) and 25.76×10−2 (at 0.1 MPa and 523 K) (cf. Fig. 9).
Oxygen often presents a quenching behavior different from other molecules: oxygen occurs naturally as a ground-state triplet, with two low-lying singlet states. Nau and Scaiano (1996) have studied oxygen quenching of excited aliphatic ketones (acetone, 2-pentanone) and diketones (biacetyl). They propose an oxygen-assisted intersystem crossing, which proceeds through an intermediate encounter complex, as the mechanism for oxygen quenching of emission from the excited singlet state of ketones. The formation of a charge-transfer complex with the ketone involves the electronegative oxygen atom of the excited carbonyl bond and electrons of the double bond.
The experimental results obtained with biacetyl in air are compared to that obtained with tracers, such as acetone and 3-pentanone excited at different wavelengths (248 and 266 nm). Figure 10 shows the influence of pressure and Fig. 11, the influence of temperature. As shown in Fig. 10, these results are in agreement with those reported in the literature for acetone and 3-pentanone: a rise in fluorescence at low pressures and a decrease or stagnation in fluorescence signal at high pressures (Fujikawa et al. 1997). For a constant concentration of 2.6×1017 molecules cm−3 of 3-pentanone, Grossmann et al. (1996) have observed an initial increase of the fluorescence signal up to 0.5 MPa, in an air bath gas for a temperature of 473 K and an excitation wavelength of 248 nm. From a pressure of 0.5 MPa, the fluorescence signal keeps a constant value up to the studied maximum pressure of 2.5 MPa. With a constant number of 1.7×1017 molecules cm−3 of acetone excited at 266 nm in air, Yuen et al. (1997) report similar results: a rise in fluorescence with pressure between 0.06 and 0.5 MPa, then a slight decrease with increasing pressure.
Comparison of pressure dependence of the normalized fluorescence signal in air for different tracers: biacetyl (this work), acetone and 3-pentanone (Fujikawa et al.1997) and for a temperature of 473 K
Comparison of temperature dependence of the normalized fluorescence signal in air for different tracers: biacetyl (this work), acetone and 3-pentanone (Fujikawa et al. 1997) and for a pressure of 1 MPa
According to the excitation wavelength, the temperature dependence of the fluorescence signal differs strongly (cf. Fig. 11). For example, Thurber et al. (1998) have shown a continuous transition from a steady increase with temperature at 320 nm to a steady decrease at 248 nm for the acetone fluorescence signal at atmospheric pressure in nitrogen. Grossmann et al. (1996) have studied the temperature dependence of 3-pentanone in synthetic air using 277 and 312 nm excitation wavelength at atmospheric pressure: in the temperature range of 383–573 K, the fluorescence intensity decreased by 15% per 100 K for 277 nm excitation wavelength, but increased by 80% for 312 nm excitation. On the other hand, for each excitation wavelength, Fujikawa et al. (1997) have underlined a decrease of the acetone and 3-pentanone fluorescence signal with increasing temperature in air at atmospheric pressure.
4 Conclusions
The experimental study on the LIF allowed to characterize the influence of parameters such as pressure, temperature, concentration of tracer and oxygen, on the fluorescence signal intensity of biacetyl. This step is necessary before applying the LIF technique on IC engines in order to obtain quantitative measurements of fuel concentration over the chamber. The fluorescence of biacetyl, usually selected as the dopant of iso-octane and excited with Ar++ laser, is studied for a pressure range of 0.1–4 MPa, a temperature range of 373–573 K, and for different biacetyl mole fractions in air and nitrogen in a high pressure and high temperature facility. The studied regime is a linear fluorescence regime with a spectral intensity of the incident laser light strongly lower than the saturation intensity. Due to the reabsorption process, the fluorescence signal reaches a saturation level from a biacetyl mole fraction of 3.13×10−1 at 423 K and 3.87×10−1 at 523 K. The fluorescence is then studied for different concentrations of biacetyl with a maximum value of 3.57×1018 molecules cm−3.
The results obtained with biacetyl are in agreement with previous studies in the literature about the pressure dependence of acetone: the fluorescence signal increases at low pressure and decreases slightly or stagnates at high pressure. The increase of biacetyl fluorescence with pressure is attributed to the vibrational relaxation of levels with higher fluorescence quantum yields. At high pressure, the vibrational relaxation is faster than the intersystem crossing. As far as the temperature effect is concerned, the fluorescence signal increases and then decreases with increasing temperature resulting from the competition between the intersystem crossing and the fluorescence. From a pressure of 1 MPa, the excited singlet state of biacetyl is strongly quenched by collisions with the oxygen molecule, which involves a decrease of the fluorescence signal. In this case, the vibrational relaxation, which explains the increase of fluorescence with pressure is not more significant, compared to the fluorescence quenching effect of oxygen.
Concerning the application of PLIF diagnostics, the results observed in this work for the variation of fluorescence with pressure, temperature, concentration of tracer and oxygen should allow to optimize experimental measurements. The next step of this study consists of the proposal of an analytic model in order to process direct fluorescence images obtained in specific thermodynamic conditions in order to extract quantitative information.
Abbreviations
- c:
-
light velocity (m s−1)
- C:
-
tracer concentration (molecule cm−3)
- E:
-
laser fluence (J cm−2)
- h:
-
Planck constant (J s)
- I:
-
light intensity (J)
- k:
-
Boltzmann constant (J K−1)
- l:
-
optical path length (cm)
- P:
-
pressure (MPa)
- T:
-
temperature (K)
- V:
-
tracer volume (μl)
- Vc :
-
collection volume (cm3)
- X:
-
mole fraction
- Zcoll :
-
Lennard–Jones collision frequency (cm3 s−1)
- Φ:
-
fluorescence quantum yield
- λ:
-
excitation wavelength (nm)
- ηopt :
-
overall efficiency of the collection optics
- σ:
-
absorption cross section (cm² molecule−1)
References
Baritaud T, Heinze T (1992) Gasoline distributions measurements with PLIF in a SI engine. SAE Paper 922355
Brand JCD, Mau AWH (1974) Low-lying electronic states of biacetyl. J Am Chem Soc 96:4380–4385
Bryant RA, Donbar JM, Driscoll JF (1997) Acetone LIF for flow visualization at temperatures below 300 K. AIAA Paper 97–0156
Demtröder W (1982) Laser spectroscopy. Springer, Berlin Heidelberg New York
Deschamps B, Snyder R, Baritaud T (1994) Effect of flow and gasoline stratification on combustion in a 4-valve SI engine. SAE Paper 941993
Einecke S, Schulz C, Sick V (2000) Measurement of temperature, fuel concentration and equivalence ratio fields using tracer LIF in IC engine combustion. Appl Phys B 71:717–723
Fujikawa T, Hattori Y, Akihama K (1997) Quantitative 2-D fuel distribution measurements in a SI engine using laser-induced fluorescence with suitable combination of fluorescence tracer and excitation wavelength. SAE Paper 972944
Grossmann F, Monkhouse PB, Ridder M, Sick V, Wolfrum J (1996) Temperature and pressure dependences of the laser-induced fluorescence of gas-phase acetone and 3-pentanone. Appl Phys B 62:249–253
Guibert P, Perrard W, Morin C (2002) Concentration measurements in a pressurized and heated gas mixture flow using laser induced fluorescence. J Fluids Eng 124:512–522
Heicklen J (1959) The fluorescence and phosphorescence of biacetyl vapor and acetone vapour. J Am Chem Soc 81:3863–3866
Horowitz A, Meller R, Moortgat GK (2001) The UV-VIS absorption cross sections of the α-dicarbonyl compounds: pyruvic acid, biacetyl and glyoxal. J Photochem Photobiol A Chem 146:19–27
Itoh T, Kakuho A, Hishinuma H, Urushiahara T, Takagi Y (1995) Development of a new compound fuel and fluorescent tracer combination for use with laser-induced fluorescence. SAE Paper 952465
Jiang-Bang L, Qi P, Chang-Sheng L, Jie-Rong S (1988) Principles of flow field diagnostics by laser induced biacetyl phosphorescence. Experiments Fluids 6:505–513
Kamei S, Okuyama K, Abe H, Mikami N, Ito M (1986) Mode selectivity in intersystem crossing: glyoxal, methylglyoxal, and biacetyl. J Phys Chem 90:93–100
Koban W, Schorr J, Schulz C (2002) Oxygen-distribution imaging with a novel two-tracer laser-induced fluorescence technique. Appl Phys B 74:111–114
Koch JD, Hanson RK (2003) Temperature and excitation wavelength dependencies of 3-pentanone absorption and fluorescence for PLIF applications. Appl Phys B 76:319–324
Koch JD (2005) Fuel tracer photophysics for quantitative planar laser induced fluorescence. Thesis, Stanford University
Krämer H, Einecke S, Schulz C, Sick V, Nattrass SR, Kitching JS (1998) Simultaneous mapping of the distribution of different fuel volatility classes using tracer-LIF and NIR-tomography in an IC engine. SAE Paper 982467
Lawrenz W, Köhler J, Meier F, Stolz W, Wirth R, Bloss WH, Maly R, Wagner E, Zahn M (1992) Quantitative 2-D LIF measurements of air/fuel ratios during the intake stroke in a transparent SI engine. SAE Paper 922320
Lozano A, Yip B, Hanson RK (1992) Acetone: a tracer for concentration measurements in gaseous flows by planar laser-induced fluorescence. Experiments Fluids 13:369–376
Nau WM, Scaiano JC (1996) Oxygen quenching of excited aliphatic ketones and diketones. J Phys Chem 100:11360–11367
Okabe H, Noyes WA (1957) The relative intensities of fluorescence and phosphorescence in biacetyl vapor. J Am Chem Soc 79:801–806
Ossler F, Alden M (1997) Measurements of picosecond laser induced fluorescence from gas phase 3-pentanone and acetone: implications to combustion diagnostics. Appl Phys B 64:493–502
Plum CN, Sanhueza E, Atkinson R, Carter WPL, Pitts JN (1983) OH radical rate constants and photolysis rates of α-dicarbonyls. Environ Sci Technol 17:479–484
Reboux J, Puechberty D, Dionnet F (1994) A new approach of planar laser induced fluorescence applied to fuel/air ratio measurement in the compression stroke of an optical SI engine. SAE Paper 941988
Reid RC, Prausnitz JM, Poling BE (1987) The properties of gases and liquids, 4th edn. McGraw-Hill Book Company, New York
Rochelle P, Perrard W (1999) Fuel consumption and emission reduction of a small two-stroke engine through air-assisted fuel injection and delayed-charging SAE Paper 99P−141
Schulz C, Sick V (2005) Tracer-LIF diagnostics: quantitative measurement of fuel concentration, temperature and fuel/air ratio in practical combustion systems. Prog Energy Combustion Sci 31:75–121
Senent ML, Moule DC, Smeyers YG, Toro-Labbé A, Peqalver FJ (1994) A theoretical spectroscopic study of the A~1Au(S1)←X~1Ag(S0), n→π* transition in biacetyl, (CH3CO)2. J Mol Spectrosc 164:66–78
Shortridge RG, Rusbult CF, Lee EKC (1971) Fluorescence excitation study of cyclobutanone, cyclopentanone, and cyclohexanone in the gas phase. J Am Chem Soc 93:1863–1867
Ten GN, Nechaev VV, Baranov VI (2002) Structure of excited biacetyl molecule and analysis of its absorption and fluorescence spectra. Opt Spectrosc 92:375–382
Thurber MC, Grisch F, Hanson RK (1997) Temperature imaging with single- and dual-wavelength acetone planar laser-induced fluorescence. Opt Lett 22:251–253
Thurber MC, Grisch F, Kirby BJ, Votsmeier M, Hanson RK (1998) Measurements and modeling of acetone laser-induced fluorescence with applications for temperature-imaging diagnostics. Appl Opt 37:4963–4978
Thurber MC, Hanson RK (1999) Pressure and composition dependences of acetone laser-induced fluorescence with excitation at 248, 266, and 308 nm. Appl Phys B 69:229–240
Thurber MC, Hanson RK (2001) Simultaneous imaging of temperature and mole fraction using acetone planar laser-induced fluorescence. Experiments Fluids 30:93–101
Valeur B (2002) Molecular fluorescence, principles and applications. Wiley-VCH Verlag, Weinheim
Van Cruyningen I, Lozano A, Hanson RK (1990) Quantitative imaging of concentration by planar laser induced fluorescence. Experiments Fluids 10:41–49
Yuen LS, Peters JE, Lucht RP (1997) Pressure dependence of laser induced-fluorescence from acetone. Appl Opt 36:3271–3277
Zhao H, Ladommatos N (1998) Optical diagnostics for in-cylinder mixture formation measurements in IC engines. Prog Energy Combustion Sci 24:297–323
Acknowledgments
Vincent Modica is supported by a joint PhD grant from the French Ministry of National Education, Research and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guibert, P., Modica, V. & Morin, C. Influence of pressure, temperature and gas phase composition on biacetyl laser-induced fluorescence. Exp Fluids 40, 245–256 (2006). https://doi.org/10.1007/s00348-005-0064-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00348-005-0064-y