Abstract
Purpose
In recent pre-clinical studies, biomaterials and bladder tissue engineering have shown promising outcomes when addressing the need for bladder tissue replacement. To date, multiple clinical experiences have been reported. Herein, we aim to review and summarize the reported clinical experience of biomaterial usage and tissue engineering of the urinary bladder.
Methods
A systematic literature search was performed on Feb 2019 to identify clinical reports on biomaterials for urinary bladder replacement or augmentation and clinical experiences with bladder tissue engineering. We identified and reviewed human studies using biomaterials and tissue-engineered bladder as bladder substitutes or augmentation implants. The studies were then summarized for each respective procedure indication, technique, follow-up period, outcome, and important findings of the studies.
Results
An extensive literature search identified 25 studies of case reports and case series with a cumulative clinical experience of 222 patients. Various biomaterials and tissue-engineered bladder were used, including plastic/polyethylene mold, preserved dog bladder, gelatine sponge, Japanese paper with Nobecutane, lypholized human dura, bovine pericardium, amniotic membrane, small intestinal mucosa, and bladder tissue engineering with autologous cell-seeded biodegradable scaffolds. However, overall clinical experiences including the outcomes and safety reports were not satisfactory enough to replace enterocystoplasty.
Conclusion
To date, several clinical experiences of biomaterials and tissue-engineered bladder have been reported; however, various studies have reported non-satisfactory outcomes. Further technological advancements and a better understanding is needed to advance bladder tissue engineering as a future promising management option for patients requiring bladder drainage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The bladder, despite its complex anatomic and physiological pathways, primarily serves as a reservoir to both store and empty the urine excreted from the kidneys. To repeatedly hold and empty urine to its appropriate capacity, it requires a repetitive mechanism of adequate relaxation and contraction with a unique lining to withstand intraluminal pressure and urine solution [1, 2]. The bladder has multiple layers including the inner epithelial lining that protects the underlying stroma from urine and multiple external layers of muscle fibers that coordinate with the external urethral sphincter to function appropriately in storage and voiding [3].
Several congenital and acquired bladder conditions (such as bladder exstrophy, neurogenic bladder, malignancies, and trauma) with anatomical or functional abnormalities require surgery to preserve renal function. Some necessitate either complete removal of the bladder and urinary diversion via conduit or augmentation to increase its storage capacity [4, 5]. To date, these procedures are traditionally performed using a segment of the gastrointestinal (GI) tract. However, the GI tract substitution is associated with local and systematic complications such as urine leak, malignancy, stone formation, and metabolic disturbances due to the different absorptive and secretory properties of the intestinal tract [4,5,6]. Furthermore, the quality of life using the GI tract can be affected significantly due to its related morbidities and sequelae [6,7,8,9,10].
Bioengineering has been exploring an alternative approach to improve outcomes for these patients. Efforts focused on bladder tissue regeneration were initially carried out with experimental studies performed in vitro and in vivo using biomaterials with or without tissue-specific seeded cells. Biomaterials used in bladder tissue engineering are currently either biologic or synthetic scaffolds that serve as the solid support matrix for tissue regeneration [10]. The biologic scaffolds are either naturally derived or acellular tissues; while the synthetic scaffolds are either polymers or silk-based materials [10,11,12]. The approach of using biomaterials seeded with tissue-specific cells or a stem cell source have been studied due to the fact that acellular scaffolding has a higher chance of post-implant contracture and fibrosis [13,14,15]. Biomaterials with stem cells or patient’s autologous tissue cultures have been investigated to avoid tissue fibrosis [13,14,15], and with the created neotissue from autologous stem cells, reduce the immune rejection as previously described with allogeneic cell source [16,17,18].
Research in animal models has shown the feasibility of bladder tissue engineering; however, most of these studies utilized healthy animals in which translational applicability is uncertain [19, 20]. Clinical application of biomaterials and bladder tissue engineering has been attempted and the reports are intriguing. To give an up-to-date overview of the clinical experience, the scope of this review is mainly focused on the clinical experience of biomaterials and bladder tissue engineering. Hence, we aim to present a summary of the feasibility and clinical outcomes of the biomaterial usage and tissue engineering application in bladder regeneration.
Methodology
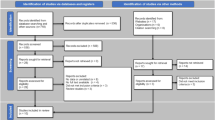
A literature search was performed in PubMed on February 1, 2019, with the search strategy used: (“urinary bladder”[MeSH Terms] OR (“urinary”[All Fields] AND “bladder”[All Fields]) OR “urinary bladder”[All Fields] OR “bladder”[All Fields]) AND (“tissue engineering”[MeSH Terms] OR (“tissue”[All Fields] AND “engineering”[All Fields]) OR “tissue engineering”[All Fields]). We did not restrict identification of relevant studies to the English language; however, foreign language studies were only summarized and assessed according to the available English reports. Assessment of relevance was accomplished by reviewing abstracts and identifying clinical studies. Screening on the identified records was categorized into clinical studies, animal experiments, and review articles. Records of review papers were gathered for cross-referencing and identification of relevant clinical studies. Full-text articles were ordered and included for the summary of literature specifically focused on reports on bladder tissue engineering clinical experience. The studies with reported clinical experience were then summarized for the biomaterial used, number of patients involved, outcomes, and follow-ups. When multiple publications of clinical experience were identified, only the latest and or most complete data reported were included in the summary of the clinical experience.
Results
Out of 1113 retrieved records, after screening, there were 190 animal studies available and 25 clinical studies with 222 patients. Studies are mainly case reports and case series with the application of various biomaterials. Table 1 summarizes the details of clinical experiences on various biomaterials usages and tissue engineering in bladder regeneration.
Clinical experience on biomaterials (synthetics and natural) alone for bladder regeneration
In 1957, Bohne and Urwiller reported seven patients’ clinical experience with a plastic mold used as a bladder matrix, which was implanted after cystectomy and then explanted after a few weeks. However, the result was disappointing with serious morbidities and all pseudo-bladders formed over the plastic mold eventually contracted over time [21]. Subsequent clinical reports using a plastic mold also described similar outcomes [22, 23]. Although these studies did not show a promising outcome, they found that, although urothelial tissue regenerates, the muscle fibers do not, which lead to eventual contraction and fibrosis of the formed tissues.
Preserved dog bladders anastomosed orthotopically to patient’s after cystectomy was being reported by Tsuji et al. in 1963 and 1967. He described major morbidities in most patients [24, 25]. The same author group also reported using Gelatin sponge sutured to the bladder edge or urethra of patients’ post-cystectomies. Similarly, the results were disappointing with all patient’s developing bladder contractures needing a urinary diversion or tumor reoccurrence [25]. Orisaka et al. in 1970 then further modified the biomaterial using Gelatine sponge with Nobecutane, which is ethyl acetate and acrylic resin compound with tetra-methylthiuram disulfide acting as a sealant and bactericide [26]. They reported four out of five patients with satisfactory outcomes, although a later study reported that bladder calculi seem to be a common complication with this biomaterial [26]. Further modification of the biomaterial was reported by Taguchi et al. [27] and Fujita [28] using Japanese rice paper (Tetrapanax papyrifer) with Nobecutane. Taguchi et al. had up to 5.5-year follow-up with favorable outcomes, most patients with a post-op bladder capacity of > 200 cc [27]. However, no subsequent follow-up studies were carried out with this biomaterial.
From 1974 to 1995, several various studies from different institutions described clinical experiences with the use of lypholized human dura patch as a biomaterial for bladder substitution among patients with bladder tumors and/or contracted bladders [29,30,31,32,33,34,35]. The majority of the studies reported post-operative reasonable bladder enlargement and no serious complications; however, urine leakage and tumor recurrence were common with the overall outcome determined to be not fully satisfactory.
Other natural biomaterials include Bovine pericardium and Amniotic membrane, both used as a bladder patch for repair of enterovesical or vesicovaginal fistula [36, 37]. Although the studies described that the bladder wall remained intact with no recurrence of fistula on the last follow-up, both showed contracture of the graft. Mansson et al. also described using a synthetic biocarbon (Carbon Polymer) as a stoma prosthesis implant for 13 patients with urinary conduit or cutaneous ureterostomy. However, four out of five patients reported to having good outcomes had died when the study was being reported. The remaining patients had the implant removed due to various complications [38].
In 2012–2014, three studies reported using porcine small intestinal submucosa, commercially available as (SIS [Oasis®], Cook Biotech). It is an acellular, native collagen-based extracellular matrix (ECM) of submucosal layer being used among patients with neurogenic or congenital myogenic bladders [43,44,45]. All studies have reported increased bladder volumes; however, functional outcomes were described to be only partially satisfactory, in which some authors have concluded that SIS cannot substitute the enterocystoplasty [43, 44].
Clinical experience on bladder tissue engineering (biomaterials seeded with autologous cells)
In 2006, Atala et al. reported their clinical experience of seven patients with myelomeningocele using collagen only or composite collagen, polyglycolic acid bladder acellular matrix seeded with autologous urothelial and muscle cells [39]. The study highlights the importance of using an autologous cell tissue source to avoid an adverse immune-response while including muscle tissue in the seeded cell to enhance the presence of all bladder tissue components, as seen in their full-thickness bladder biopsies. Furthermore, the importance of an omental wrap for vascular supply was described with better outcomes. However, another three studies sponsored by Tengion, the company for autologous cell-seeded polyglycolide/polylactide matric scaffold (bankrupted in 2014), showed poor outcomes among all trials [40,41,42] with no bladder compliance or capacity improvement. When used as a conduit, all patients eventually needed explantation; as augmentation, severe complications such as bladder rupture, small bowel obstruction, and infections were reported.
Discussion
Regenerative medicine using tissue engineering technology has been given high expectations due to its rapid development with potential for revolutionary treatment strategies [46, 47]. Specifically, in the field of reconstructive urology, there is high demand for a tissue-engineered product to be used as urinary conduits or augment patches to eliminate the use of bowel as the replacement [48]. Reports from animal studies using tissue bioengineering for bladder substitutes seem to be promising [20, 49]; however, in our review of clinical studies to date, all have shown non-satisfactory results.
The clinical experience using biomaterials stand alone either natural source or synthetics have shown that native urothelial has the capability to regenerate; yet, the muscle fiber layers only develop into fibrotic tissue and leading to eventual contracture inability to function. Similarly, with scaffold seeded with autologous stem cells, adequate development of all layers may be seen in histological study; however, the functional capability as the bladder organ in toto was not achieved, considering that multiple aspect of its development was not taken into consideration. It is important to understand that the bladder biological function is not simply for storage. Behind its urothelial regenerative capability and frequent urine expulsion lies a complex and unique infrastructure. Hence, the complex function requires composite histology and anatomy (special urothelial lining, muscle and extracellular matrix ratio). It has the characteristic of impermeable lining with moderately dynamic muscular and richly vascularized organ with intricate innervation (Fig. 1) [3]. The fractional understanding of bladder with simplicity in functional assessment among the pre-clinical and clinical studies has led us to underestimate the bladder and assume rapid success in tissue engineering [49, 50].

Adopted and reprinted by permission from Springer Nature: lic number 4,553,430,369,670 Ajalloueian et al. [49]
Bladder structural anatomy and histologic characteristics.
A recent review by Adamowicz et al. summarizes the understanding of tissue engineering from pre-clinical experiments and gave important factors to consider for translational application of bladder tissue engineering include (a) generating a physiological mechanical property specific for the urinary system; (b) adequate pre-vascularity or capability for diffuse vascularization post-implantation to avoid necrosis of the graft; (c) antifibrosis and immunomodulating properties that avoid extensive local scarring; (d) neuronal network innervation of the graft with the capacity to generate/receive signal conduction; and an (e) ideal micro-environment to sustain healthy autologous cells with appropriate growth factors to ensure quality graft growth [11].
In principle, the biomaterial as scaffold matrix needs to be dynamic and yet to allow cells to grow into them [50]. Hence, they need to be permeable, but, when the scaffold or biomaterial is permeable, the regenerating cells from the native tissue will compete with urine seeping through the pores, causing fibrosis [51]. Therefore, it is a good strategy to consider to have the scaffold seeded with autologous cells prior to implantation, but, then, there should be a fast and effective blood vessels growth to sustain the viability of the seeded cells when implanted; otherwise, the cells will die and the pores will be leaking urine/then causes fibrosis [50,51,52]. A possible strategy that could work is to have the cell-seeded scaffolds implanted into a diverted bladder, while maintaining some robust method of nutrient delivery to the cell-seeded scaffold. Until the adequate angiogenesis occurred to supplement the cell-seeded scaffold with the necessary nutrients, then the bladder can be undiverted.
In general, the ideal engineered bladder needs to endure the urinary physiologic environment with the characteristics for supporting repeated storage and emptying of urine [53]. Addressing these complex and intricate processes requires more extensive research and advancements in technology; however, in the process of clinical studies, there need to have a both quality and safety of biomaterials in the human subjects [54]. To date, 3D-bioprinting, stem cell application, nanotechnology, and complex neuronal technologies are underway in pre-clinical phases [55,56,57,58,59,60], which should further advance the bladder tissue engineering experience.
Conclusion
The current clinical experience of bladder tissue engineering has not met a satisfactory outcome to replace enterocystoplasty. The main limitation was being due to the biomechanical characteristics of the urinary bladder and physiologic requirements to function optimally. Furthermore, issues regarding neovascularization and graft contraction due to immune-reaction or graft necrosis need to be addressed. Autologous cell-seeding and omental wrapping upon implantation seem to improve the clinical outcome; however, this is still not sufficient to endure long-term follow-up. While some of the clinical studies reported continence in a few patients, spontaneous voiding did not occur due to lack of neuronal networking as seen in a native bladder. Currently, technological advancements and scientific discoveries are underway to address these limitations and to advance bladder tissue engineering as a possible management option for patients needing a new bladder from oncologic, acquired, or congenital neurogenic and myogenic conditions.
References
Aitken KJ, Bägli DJ (2009) The bladder extracellular matrix. Part I: architecture, development and disease. Nat Rev Urol 6:596–611
Singh A, Bivalacqua TJ, Sopko N (2018) Urinary tissue engineering: challenges and opportunities. Sex Med Rev 6(1):35–44. https://doi.org/10.1016/j.sxmr.2017.08.004
Fry CH, Meng E, Young JS (2010) The physiological function of lower urinary tract smooth muscle. Auton Neurosci 154(1–2):3–13. https://doi.org/10.1016/j.autneu.2009.10.006
Stein R, Hohenfellner M, Pahernik S, Roth S, Thüroff JW, Rübben H (2012) Urinary diversion–approaches and consequences. Dtsch Arztebl Int 109(38):617–622. https://doi.org/10.3238/arztebl.2012.0617
Biers SM, Venn SN, Greenwell TJ (2012) The past, present and future of augmentation cystoplasty. BJU Int 109(9):1280–1293. https://doi.org/10.1111/j.1464-410X.2011.10650.x
Veeratterapillay R, Thorpe AC, Harding C (2013) Augmentation cystoplasty: contemporary indications, techniques and complications. Indian J Urol 29(4):322–327. https://doi.org/10.4103/0970-1591.120114
Huang Y, Pan X, Zhou Q, Huang H, Li L, Cui X, Wang G, Jizhong R, Yin L, Xu D, Hong Y (2015) Quality-of-life outcomes and unmet needs between ileal conduit and orthotopic ileal neobladder after radical cystectomy in a Chinese population: a 2-to-1 matched-pair analysis. BMC Urol 27(15):117. https://doi.org/10.1186/s12894-015-0113-7
Prcic A, Aganovic D, Hadziosmanovic O (2013) Impact of complications and bladder cancer stage on quality of life in patients with different types of urinary diversions. Med Arch 67(6):418–422. https://doi.org/10.5455/medarh.2013.67.418-422
Cho A, Lee SM, Noh JW, Choi DK, Lee Y, Cho ST, Kim KK, Lee YG, Lee YK (2017) Acid-base disorders after orthotopic bladder replacement: comparison of an ileal neobladder and an ileal conduit. Ren Fail 39(1):379–384. https://doi.org/10.1080/0886022X.2017.1287733
Serrano-Aroca Á, Vera-Donoso CD, Moreno-Manzano V (2018) Bioengineering approaches for bladder regeneration. Int J Mol Sci 19(6):1796. https://doi.org/10.3390/ijms19061796
Adamowicz J, Pokrywczynska M, Van Breda SV, Kloskowski T, Drewa T (2017) Concise review: tissue engineering of urinary bladder; we still have a long way to go? Stem Cells Transl Med 6(11):2033–2043. https://doi.org/10.1002/sctm.17-0101
Mahfouz W, Elsalmy S, Corcos J, Fayed AS (2013) Fundamentals of bladder tissue engineering. Afr J Urol 19(2):51–57
El-Assmy A, Hafez AT, El-Sherbiny MT, El-Hamid MA, Mohsen T, Nour EM, Bazeed M (2004) Use of single layer small intestinal submucosa for long segment ureteral replacement: a pilot study. J Urol 171(5):1939–1942
Wezel F, Southgate J, Thomas DFM (2011) Regenerative medicine and urology. BJU Int 108:1046–1065. https://doi.org/10.1111/1464-410X.2011.10206.x
Chung SY, Krivorov NP, Rausei V, Thomas L, Frantzen M, Landsittel D, Kang YM, Chon CH, Ng CS, Fuchs GJ (2005) Bladder reconstitution with bone marrow derived stem cells seeded on small intestinal submucosa improves morphological and molecular composition. J Urol 174(1):353–359
Zou Q, Fu Q (2018) Tissue engineering for urinary tract reconstruction and repair: progress and prospect in China. Asian J Urol 5(2):57–68. https://doi.org/10.1016/j.ajur.2017.06.010
Jayo MJ, Jain D, Wagner BJ, Bertram TA (2008) Early cellular and stromal responses in regeneration versus repair of a mammalian bladder using autologous cell and biodegradable scaffold technologies. J Urol 180:392–397
Xu Y, Sun DC, Wei ZT, Hong BF, Yang Y (2014) Experimental study on transplantation of autologous minced muscle with human umbilical cord mesenchymal stem cells for urethral reconstruction. Eur Rev Med Pharmacol Sci 18:3412–3419
Alberti C (2016) Whyever bladder tissue engineering clinical applications still remain unusual even though many intriguing technological advances have been reached? G Chir 37(1):6–12
Sloff M, Simaioforidis V, de Vries R, Oosterwijk E, Feitz W (2014) Tissue engineering of the bladder–reality or myth? A systematic review. J Urol 192(4):1035–1042. https://doi.org/10.1016/j.juro.2014.03.116
Bohne AW, Urwiller KL (1957) Experience with urinary bladder regeneration. J Urol 77(5):725–732
Portilla Sanchez R, Blanco FL, Santamarina A, Casals RJ, Mata J, Kaufman A (1958) Vesical regeneration in the human after total cystectomy and implantation of a plastic mould. Br J Urol 30:180–188
Tsulukidze A, Murvanidze D, Dvali R, Ivaschenko G (1964) Formation of a bladder by a plastic shell after total cystectomy. Br J Urol 36:102–105
Tsuji I, Kuroda K, Fujieda J, Shiraishi Y, Kassai T, Shida H (1963) A clinical and experimental study on cystoplasty not using the intestine. J Urol 89:214–225
Tsuji I, Kuroda K, Fujieda J, Shiraishi Y, Kunishima K (1967) Clinical experiences of bladder reconstruction using preserved bladder and gelatin sponge bladder in the case of bladder cancer. J Urol 98:91–92
Orikasa S, Tsuji I (1970) Enlargement of contracted bladder by use of gelatin sponge bladder. J Urol 104:107–110
Taguchi H, Ishizuka E, Saito K (1977) Cystoplasty by regeneration of the bladder. J Urol 118:752–756
Fujita K (1978) The use of resin-sprayed thin paper for urinary bladder regeneration. Invest Urol 15:355–357
Schmiedt E, Carl P, Staehler G, Wanner K (1974) Subtotal substitution of urinary bladder by a complete human dura of the skull cap (author’s transl). Urologe A 13(5):228–231
Kelami A (1975) Duroplasty of the urinary bladder—results after two to six years. Eur Urol 1:178–181
Günther M, Pietruschka U, Siegel W, Erdmann T (1979) Use of the durain the surgical treatment of bladder cancer. Z Urol Nephrol 72:473–482
Selli C, Carcangiu ML, Carini M (1986) Bladder carcinoma arising from regenerated urothelium over lyophilized dura patch. Urology 27(1):53–55
Kakimoto S, Sakai H, Kubota S, Kondo A, Kishikawa M (1989) Partial cystectomy for bladder carcinoma: lyophilized human duraas a bladder wall substitute. Nihon Hinyokika Gakkai Zasshi 80:22–27
Romero Pérez P, Lobato Encinas J, Megía Carrigos J, Pelluch AA, Mira LA (1990) Partial parietal cystectomy and cystoplasty using alyophilized human dura mater patch as an alternative in pallia-tive surgery for bladder cancer. Arch Esp Urol. 43:867–875
Arikan N, Ozdiler E, Yaman O, Go¨gu¨ s O (1995) Augmentation duracystoplasty in neurogenic bladder dysfunction. Int J Urol 2:172–175
Moon SJ, Kim DH, Jo JK, Chung JH, Lee JY, Park SY, Kim YT, Park HK, Choi HY, Moon HS (2011) Bladder reconstruction using bovine pericardium in a case of enterovesical fistula. Korean J Urol 52:150–153
Barski D, Gerullis H, Ecke T, Varga G, Boros M, Pintelon I, Timmermans JP, Winter A, Bagner JW, Otto T (2015) Repair of a vesico-vaginal fistula with amniotic membrane—step 1 of the IDEAL recommendations of surgical innovation. Cent Eur J Urol 68(4):459–461. https://doi.org/10.5173/ceju.2015.683
Mansson W, Harzmann R (1988) Clinical experience with an alloplasticstoma prosthesis (Biocarbon) for urinary conduits and cutaneousureterostomy. Scand J Urol Nephrol 22:223–226
Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB (2006) Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367:1241–1246
Joseph DB, Borer JG, De Filippo RE et al (2014) Autologous cell seeded biodegradable scaffold for augmentation cystoplasty: phase II study in children and adolescents with spina bifida. J Urol 191:1389–1394
Bivalacqua T, Steinberg G, Smith N, Joice G, Sopko N, Lerner S, Bochner B, Lee C, Rivera E, Jain D, Bertram T, Salem W, Schoenberg M (2018) Final results of a phase 1 clinical trial evaluating the use of a tissue engineered neo-urinary conduit using adipose derived smooth muscle cells for urinary reconstruction. J Urol 199(4S):e578
Tengion. NCT00512148. An open label multicenter study of augmentation cystoplasty using an autologous neo-bladder construct in subjects with neurogenic bladder following spinal cord injury. https://clinicaltrials.gov/ct2/show/NCT00512148. Accessed 20 Mar 2019
Caione P, Boldrini R, Salerno A, Nappo SG (2012) Bladder augmentation using acellular collagen biomatrix: a pilot experience in exstrophic patients. Pediatr Surg Int 28:421–428
Schaefer M, Kaiser A, Stehr M, Beyer HJ (2013) Bladder augmentation with small intestinal submucosa leads to unsatisfactory long-term results. J Pediatr Urol 9:878–883
Zhang F, Liao L (2014) Tissue engineered cystoplasty augmentation for treatment of neurogenic bladder using small intestinal submucosa: an exploratory study. J Urol 192:544–550
Del Gaudio C, Baiguera S, Ajalloueian F, Bianco A, Macchiarini P (2014) Are synthetic scaffolds suitable for the development of clinical tissue-engineered tubular organs? J Biomed Mater Res A 102(7):2427–2447. https://doi.org/10.1002/jbm.a.34883
Smolar J, Horst M, Sulser T, Eberli D (2018) Bladder regeneration through stem cell therapy. Expert Opin Biol Ther 18(5):525–544. https://doi.org/10.1080/14712598.2018.1439013
Serrano-Aroca Á, Vera-Donoso CD, Moreno-Manzano V (2018) Bioengineering approaches for bladder regeneration. Int J Mol Sci. https://doi.org/10.3390/ijms19061796
Ajalloueian F, Lemon G, Hilborn J, Chronakis IS, Fossum M (2018) Bladder biomechanics and the use of scaffolds for regenerative medicine in the urinary bladder. Nat Rev Urol 15(3):155–174. https://doi.org/10.1038/nrurol.2018.5
Farhat WA, Yeger H (2008) Does mechanical stimulation have any role in urinary bladder tissue engineering? World J Urol 26(4):301–305. https://doi.org/10.1007/s00345-008-0318-4
Farhat W, Chen J, Erdeljan P, Shemtov O, Courtman D, Khoury A, Yeger H (2003) Porosity of porcine bladder acellular matrix: impact of ACM thickness. J Biomed Mater Res A 67(3):970–974
Loai Y, Yeger H, Coz C, Antoon R, Islam SS, Moore K, Farhat WA (2010) Bladder tissue engineering: tissue regeneration and neovascularization of HA-VEGF-incorporated bladder acellular constructs in mouse and porcine animal models. J Biomed Mater Res A 94(4):1205–1215. https://doi.org/10.1002/jbm.a.32777
Horst M, Madduri S, Gobet R, Sulser T, Milleret V, Hall H, Atala A, Eberli D (2013) Engineering functional bladder tissues. J Tissue Eng Regen Med 7(7):515–522. https://doi.org/10.1002/term.547
Oerlemans AJ, Feitz WF, van Leeuwen E, Dekkers WJ (2013) Regenerative urology clinical trials: an ethical assessment of road blocks and solutions. Tissue Eng Part B Rev 19(1):41–47. https://doi.org/10.1089/ten.TEB.2012.0136
Ling Q, Wang T, Yu X, Wang SG, Ye ZQ, Liu JH, Yang SW, Zhu XB, Yu J (2017) UC-VEGF-SMC three dimensional (3d) nano scaffolds exhibits good repair function in bladder damage. J Biomed Nanotechnol 13(3):313–323
Bishop ES, Mostafa S, Pakvasa M, Luu HH, Lee MJ, Wolf JM, Ameer GA, He TC, Reid RR (2017) 3-D bioprinting technologies in tissue engineering and regenerative medicine: current and future trends. Genes Dis 4(4):185–195. https://doi.org/10.1016/j.gendis.2017.10.002
Pi Q, Maharjan S, Yan X, Liu X, Singh B, van Genderen AM, Robledo-Padilla F, Parra-Saldivar R, Hu N, Jia W, Xu C, Kang J, Hassan S, Cheng H, Hou X, Khademhosseini A, Zhang YS (2018) Digitally tunable microfluidic bioprinting of multilayered cannular tissues. Adv Mater 30(43):e1706913. https://doi.org/10.1002/adma.201706913
Alarçin E, Guan X, Kashaf SS, Elbaradie K, Yang H, Jang HL, Khademhosseini A (2016) Recreating composition, structure, functionalities of tissues at nanoscale for regenerative medicine. Regen Med 11(8):849–858
Yudintceva NM, Nashchekina YA, Blinova MI, Orlova NV, Muraviov AN, Vinogradova TI, Sheykhov MG, Shapkova EY, Emeljannikov DV, Yablonskii PK, Samusenko IA, Mikhrina AL, Pakhomov AV, Shevtsov MA (2016) Experimental bladder regeneration using a poly-l-lactide/silk fibroin scaffold seeded with nanoparticle-labeled allogenic bone marrow stromal cells. Int J Nanomed 11:4521–4533 (eCollection 2016)
Moe AA, Suryana M, Marcy G et al (2012) Microarray with micro- and nanotopographies enables identification of the optimal topography for directing the differentiation of primary murine neural progenitor cells. Small 8:3050–3061
Acknowledgements
The author group would like to acknowledge the kind assistance of the library staffs from Edward E. Brickell Medical Sciences Library of Eastern Virginal Medical School in providing technical support and retrieving the full-text articles for the literature review of this paper.
Author information
Authors and Affiliations
Contributions
MEC: project development, data Collection, data analysis, and manuscript writing. WAF: data collection, data analysis, and manuscript writing/editing. JMM: data analysis and manuscript writing/editing. KAM: project development, data collection, and manuscript writing/editing.
Corresponding author
Ethics declarations
Conflicts of interest
Authors have nothing to disclose.
Research involving human participants and/or animals
Not applicable for current article.
Informed consent
Not applicable for current article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chua, M.E., Farhat, W.A., Ming, J.M. et al. Review of clinical experience on biomaterials and tissue engineering of urinary bladder. World J Urol 38, 2081–2093 (2020). https://doi.org/10.1007/s00345-019-02833-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-02833-4