Abstract
Purpose
To report a multi-institutional experience on robotic radical nephroureterectomy (RNU) and segmental ureterectomy (SU) for upper tract urothelial carcinoma (UTUC).
Methods
Data were prospectively collected from patients with non-metastatic UTUC undergoing robotic SU or RNU at three referral centers between 2015 and 2018. Transperitoneal, single-docking robotic RNU followed established principles. Bladder cuff excision (BCE) was performed with robotic or open approach. Techniques for SU included: ureteral resection and primary uretero-ureterostomy; partial pyelectomy and modified pyeloplasty; ureteral resection with BCE and direct- or psoas hitch-ureteroneocystostomy. We retrospectively evaluated the technical feasibility, and peri-operative and oncologic outcomes after robotic RNU/SU.
Results
81 patients were included. No case required conversion to open surgery. Early major (Clavien–Dindo grade > 2) complications were reported in six (7.4%) patients (two after SU, four after RNU). Three patients experienced late major complications (one after SU, two after RNU). Median ΔeGFR at 3 months was − 1 ml/min/1.73 m2 after SU and − 15 ml/min/1.73 m2 after RNU. Positive surgical margins were recorded in five patients (one after SU, four after RNU). Median follow-up was 21 months and 22 months in the SU and RNU groups, respectively. Three (20%) patients had ipsilateral upper tract recurrence after SU, while five (7.5%) developed metastases after RNU. No case of port-site metastases or peritoneal carcinomatosis was reported. At last follow-up, 67 (82.7%) patients were alive without evidence of disease.
Conclusion
Robotic SU and RNU are technically feasible and achieved promising peri-operative and oncologic outcomes in selected patients with non-metastatic UTUC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Upper tract urothelial carcinoma (UTUC) is a relatively uncommon disease, accounting for 5–10% of urothelial cancers [1]. Contemporary treatment of UTUC should be individualized. While open radical nephroureterectomy (RNU) with bladder cuff excision (BCE) remains the gold standard for high-risk UTUC regardless of tumor location, kidney-sparing surgery (KSS) should be offered as primary treatment to patients with low-risk tumors and to selected patients with high-risk distal ureteral tumors [1,2,3,4]. Moreover, KSS may be considered on a case-by-case basis in patients with solitary kidney and/or impaired renal function, as long as oncologic outcomes are not jeopardized [1].
Over the past decade, minimally invasive approach for RNU and segmental ureterectomy (SU) has been increasingly reported at high-volume centers, with favorable peri-operative, functional and oncologic outcomes [5,6,7,8,9,10]. As such, in experienced hands, laparoscopic RNU is considered an alternative to open RNU [11]. Of note, while the oncological equivalence of laparoscopic and open RNU is likely in most cases, it could not be established in patients with locally advanced high-risk UTUC or when BCE was performed laparoscopically [12].
Recent population-based studies showed a significant increase in the use of robotic surgery for the management of UTUC [13, 14]; yet, despite the evolution of surgical techniques [10], data on robotic RNU and SU are still lacking [1, 4, 12].
In this study, our purpose was to report a multi-institutional experience with robotic RNU and SU for the treatment of selected patients with UTUC, focusing on peri-operative and oncologic outcomes.
Materials and methods
Patients and dataset
After obtaining Institutional Review Board Approval, we retrospectively reviewed a prospectively collected multi-institutional database of patients with non-metastatic (cN0M0) UTUC treated with robotic SU or RNU at three tertiary referral centers between January 2015 and September 2018.
All surgeons (n = 8) involved in the study had extensive experience in robotic urologic surgery (including pelvic, renal and reconstructive ureteral surgery).
Selection of treatment strategy (KSS vs RNU) and robotic surgical approach was done on a case-by-case basis after careful consideration of patient’s clinical characteristics, tumor features, surgeon’s preference and skills, as well as hospital resources.
Robotic SU was considered for ureteral UTUCs which could not be managed with a pure endoscopic approach but with no evidence of deep invasion of periureteric fat at pre-operative CT urography (stage cT3 or above).
Patients underwent diagnostic assessment of the lower and upper urinary tract (including urinary cytology, cystoscopy and abdomino-pelvic computed tomography [or magnetic resonance] urography).
Diagnostic ureteroscopy with or without UTUC biopsy was performed in selected patients when it could significantly change the decision-making regarding RNU versus SU or regarding the choice of open vs robotic surgical approach.
Post-operative complications were reported according to the Clavien–Dindo grading system [15], in accordance with the European Association of Urology (EAU) Guidelines [16].
Tumor stage was assessed according to the 2017 TNM classification while tumor grade according to the 2004/2016 WHO classification [1].
Follow-up was performed in accordance to the EAU Guideline recommendations [1].
For reporting the study results, we followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement recommendations [17].
Surgical technique for robotic radical nephroureterectomy
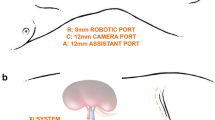
Patient positioning, port placement and technique for transperitoneal, single-docking robotic RNU followed established surgical principles [8, 18].
The ureter was clipped early during the procedure to prevent downward tumor spillage during kidney manipulation.
Adrenal-sparing robotic RNU was performed in a three- or four-arm configuration using the da Vinci Si or Xi robotic platform.
Lymph node dissection (LND) was performed in selected cases based on surgeon’s preference or intra-operative suspicion of lymph node metastases; overall, the LND template was not standardized [19].
In most cases, BCE was performed with either a pure robotic [8, 18] or open approach (via a midline open sagittal cystotomy). With the robotic approach, the detrusor muscle was dissected until the bladder mucosa; then, stay sutures were placed and a cystotomy was performed to remove the bladder cuff. The bladder was finally closed in two layers in a running fashion [8]. Trans-urethral resection of the intra-mural ureter or extravesical control of the distal (pre-mural) ureter with hem-o-lok clips (without BCE) was performed in highly selected patients.
Surgical technique for robotic segmental ureterectomy
A transperitoneal approach was chosen in all cases, as previously reported [5]. Patient positioning and port placement were tailored according to the UTUC location (i.e. flank position for UTUCs of the renal pelvis and upper/middle ureter; supine position for UTUCs of the distal ureter [21,22,23]). A three- or four-arm configuration was employed according to surgeon’s preference and UTUC characteristics.
Principles of robotic SU included: (a) atraumatic, “no touch” ureteral dissection; (b) identification of the limits of the ureteral tumor (with or without the use of concomitant ureteroscopy); (c) isolation of the affected ureteral segment to prevent tumor spillage [23]; (d) tumor resection with adequate (1–2 cm) safety margins.
Surgical techniques of ureteral reconstruction included: (a) primary tension-free uretero-ureterostomy on a JJ stent, for proximal/middle ureteral tumors and highly selected cases of lower ureteral tumors; (b) modified robotic pyeloplasty [20] on a JJ stent, for selected tumors of the renal pelvis managed with robotic partial pyelectomy; (c) ureteral reimplantation on a JJ stent with either direct- or psoas hitch-ureteroneocystostomy, for UTUCs of the distal ureter resected en bloc with an adequate bladder cuff [21,22,23].
Ipsilateral pelvic LND was performed for selected cases of distal ureteral tumors, according to surgeon’s preference and intra-operative findings.
Study endpoints and data analysis
The study endpoints included: (a) technical feasibility of robotic RNU and SU, defined as their successful completion without open conversion; (b) peri-operative and early (3 months) functional outcomes; (c) positive surgical margins rate and oncologic outcomes (including intravesical, local and distant recurrences).
Descriptive statistics were obtained reporting medians (and interquartile range, IQR) for continuous variables, while frequency and proportions for categorical variables, as appropriate.
Results
Characteristics of the study population
Overall, 81 patients were included in the study.
Pre-operative patient characteristics for the SU and RNU cohorts are shown in Table 1. Median patient age was 68 [interquartile range (IQR) 57–77] and 72 (IQR 64–77) years, respectively. In both groups, median BMI was 26. Median pre-operative eGFR was 69 ml/min/1.73 m2 (IQR 30–88) for the SU cohort and 61 ml/min/1.73 m2 (IQR: 53–80.5) for the RNU cohort.
Overall, 32 (48%) patients experienced previous abdominal surgery. Three patients in the SU group and one patient in the RNU group had a solitary kidney.
Median tumor diameter was 17 mm (IQR: 10–46) and 30 (IQR: 21–38), respectively. Most UTUCs in the SU group were located in the distal ureter, while in the RNU group in the renal pelvis or upper/middle ureter. Of note, robotic SU was employed in two patients with a single kidney and UTUC of the renal pelvis (Table 1).
Clinical T stage was < T3 in 100% and 45.5% of patients in the SU and RNU groups, respectively. Pre-operative ureteroscopy with UTUC biopsy was performed in 33.3% and 18.2% of patients, respectively.
Two patients underwent neoadjuvant cisplatin-based chemotherapy before RNU.
Surgical and peri-operative outcomes
Intra-operative and peri-operative outcomes for the SU and RNU cohorts are summarized in Table 2. No case required conversion to open surgery.
Median operative time and estimated blood loss were 140 min (IQR 110–220) and 180 cc (IQR:100–210) in the SU group while 195 min (IQR 180–270) and 200 cc (IQR 100–300) in the RNU group. Median length of hospitalization was 4 and 5 days, respectively.
In patients undergoing robotic RNU, BCE was performed in 57 (86.4%) patients, of which 30 with a pure robotic and 27 with an open approach.
For ureteral reconstruction after SU, primary uretero-ureterostomy was performed in five patients, modified pyeloplasty in two patients, direct ureteroneocystostomy in four patients and psoas hitch-ureteroneocystostomy in four patients. LND was performed in 25 (30.8%) patients.
Two patients experienced intra-operative complications during robotic RNU with LND (intra-operative bleeding, Table 2).
Overall, early (30-day) major (Clavien–Dindo grade > 2) surgical complications were reported in 6 (7.4%) patients. Of these, two were in the SU group and four in the RNU group (Table 2).
Median ΔeGFR at 3 months after SU and RNU was − 1 ml/min/1.73 m2 (IQR: − 5; − 10.5) and − 15 ml/min/1.73 m2 (IQR: − 20; − 7), respectively.
Three patients (one in the SU group and two in the RNU group) experienced late major surgical complications.
Histopathological and oncologic outcomes
At histopathological analysis, median tumor diameter was 20 mm (IQR 13–30) and 32 mm (IQR 25–50) in SU and RNU groups, respectively (Table 3).
Median number of lymph nodes removed were 10 (IQR 8–14) and 6 (IQR 3–13), respectively. Positive lymph nodes were found in eight (12.1%) patients in the RNU group while in no patients in the SU group.
Pathological stage in the SU group was T0 in two patients, T1 in three patients, T2 in seven patients and T3 in three patients. In the RNU group, pT stage was T1, T2, T3 and T4 in 33.3%, 22.7%, 39.5 and 4.5% of patients, respectively.
Tumor histotype was pure urothelial carcinoma in the majority of cases (100% and 89.4% of patients in the SU and RNU groups, respectively).
Positive surgical margins were recorded in one patient after SU while in four (6.0%) patients after RNU.
At a median follow-up of 21 months (IQR 14–38) and 22 months (IQR: 11–32), respectively, 4 (26.7%) patients in the SU group and 16 (24.2%) patients in the RNU group experienced intravesical recurrence. In these cases, treatment included trans-urethral resections, radical cystectomy or trimodal therapy (Table 3).
Three patients in the SU group experienced recurrence in the ipsilateral upper urinary tract; of these, two were treated with salvage open RNU.
Five (7.5%) patients developed metastatic disease after RNU and were treated with systemic therapy.
No case of port-site metastases or peritoneal/retroperitoneal carcinomatosis was reported.
At a median follow-up of 21 months and 22 months after robotic SU and robotic RNU, respectively, 12 (80%) and 55 (83%) patients were alive with no evidence of disease.
Discussion
Contemporary management of UTUC should be individualized [1], tailoring surgical strategy (RNU vs KSS) and approach (endoscopic vs open vs minimally invasive) to tumor’s risk and patient’s characteristics. Notably, despite open RNU with BCE is still the gold standard treatment of high-risk tumors [1], kidney-sparing approaches may be considered in selected clinical scenarios, given the similar oncological outcomes and the significantly better preservation of renal function [1,2,3,4].
In addition, laparoscopic and robotic RNU and SU have been shown to mirror the principles of open surgery, adding the advantages of minimally invasive approaches and ensuring oncologic efficacy [5,6,7,8,9,10, 18, 21, 23,24,25,26].
In this context, as robotic surgery is being increasingly performed for UTUC worldwide [13, 14, 26], our multi-institutional experience provides novel data confirming feasibility and safety of robotic RNU and robotic SU for carefully selected patients with non-metastatic UTUC.
The main finding of our study is that robotic RNU and SU were technically feasible and accurately duplicated the established open techniques, adhering to strict oncologic principles (i.e. no-touch surgery [1], avoidance of tumor spillage [12], BCE [27] and LND [19]). In particular, thanks to the magnified 3D vision and the EndoWrist technology, robotic surgery allowed to improve technical finesse of specific steps of RNU, including distal ureteral dissection and BCE (without need of patient repositioning or re-docking [10]), watertight bladder closure and LND [8,9,10, 18, 28]. Similarly, robotic technology allowed precise ureteral resections and reconstructions (with adequate specimens for definitive histopathological analysis) adapting surgical technique to UTUC location and length of ureteral defect [5, 7, 21, 23] (Table 2) while ensuring proper LND and respect of all oncologic principles [1]. Of note, robotic SU and ureteroneocystostomy for distal ureteral tumors did not preclude subsequent surveillance ureteroscopies, as previously reported [5]. Notably, previous abdominal surgery and body habitus did not prevent a minimally invasive approach for RNU or SU in our series (Table 1). Moreover, no case required conversion to open surgery.
It is important to note that selection criteria for robotic surgery in our study were not standardized. Indeed, the choice to proceed with robotic RNU or SU (rather than endoscopic laser ablation) was undertaken on a case-by-case basis, balancing patient-related factors (good performance status and no contraindications for minimally invasive surgery), tumor-related factors (no bulky pelvicalyceal tumor and no invasion beyond the muscolaris propria into the periureteric fat in case of ureteral tumors) as well as surgeon-related factors (experience, skills and confidence with robotic approach in this setting).
A second key finding of our study is that robotic RNU and SU achieved favorable peri-operative outcomes in terms of operative time, blood loss, hospital stay and complication profile (Table 2), as reported by previous robotic series [6,7,8,9,10, 18, 21, 23]. Of note, the overall rate of major (Clavien–Dindo grade > 2) complications at last follow-up in our series was 11%, which might be partly related to the surgeon’s learning curve for robotic RNU and SU. As such, the morbidity profile of robotic RNU and SU requires further investigation.
In addition, as expected [3], robotic SU lead to a significantly better preservation of post-operative renal function compared to robotic RNU (Table 2), highlighting the potential benefit of KSS in selected (i.e. older and/or comorbid) patients with ureteral UTUCs.
Finally, to the best of our knowledge, this is one of the largest series so far on robotic management of UTUC in literature [7, 8]. Of note, despite the limited length of follow-up, oncological outcomes after robotic RNU and SU in our series were promising and comparable to those of previously published series [7, 8, 21, 24, 25].
In particular, negative surgical margins were achieved in the vast majority of cases and we did not record any case of port-site metastases or peritoneal carcinomatosis. Moreover, although the median follow-up in our study was relatively short (21 and 22 months after robotic SU and robotic RNU, respectively), more than 80% of patients were alive without evidence of disease at last follow-up (Table 3).
As previously reported [27], intravesical recurrence was detected in a non-negligible proportion of patients after both RNU and SU. Also, it is important to highlight that three patients developed recurrence within the ipsilateral upper urinary tract after robotic SU (of which two were treated with salvage open RNU). These findings reinforce the importance of close surveillance with urinary cytology, cross-sectional imaging, cystoscopy and ureteroscopy after surgery for UTUC, particularly after SU.
Our study is not devoid of limitations. First, this was a retrospective evaluation of a cohort of highly selected patients with non-metastatic UTUC treated at referral academic centers by highly experienced surgeons. In particular, the selection criteria for robotic surgery in our study were based on patient-, tumor- and surgeon-related factors which may be not entirely generalizable. Moreover, the criteria for performing pre-operative ureteroscopy (with or without UTUC biopsy) were not standardized across included centers.
Second, due to the small sample size, we could not compare the outcomes of robotic RNU and robotic SU in a meaningful way. In addition, we could not evaluate the impact of adjuvant bladder instillations on intravesicle recurrence rate. Third, we did not evaluate the cost-effectiveness of robotic surgery compared to standard laparoscopy or open surgery. Fourth, length of follow-up was limited.
Acknowledged these limitations, our multi-institutional experience adds novel data confirming feasibility and safety of robotic surgery for selected UTUCs.
There is a need for multicenter prospective studies with longer follow-up and with common inclusion criteria and endpoints [29]. These studies should evaluate the long-term oncologic efficacy of elective robotic RNU and SU for non-metastatic UTUC; compare peri-operative and oncologic outcomes after open vs robotic surgery for UTUC and define standardized criteria to select the patients most likely to benefit from minimally invasive approaches.
Conclusion
Our multi-institutional experience confirmed feasibility and safety of robotic RNU and SU for the treatment of selected patients with non-metastatic UTUC at referral academic centers.
Adhering to strict oncological principles, robotic RNU and SU duplicated the principles of open surgery and achieved promising peri-operative and oncologic outcomes.
Further studies with longer follow-up are needed to confirm the oncologic safety of these techniques and to define indications and limits of robotic surgery for UTUC.
References
Rouprêt M, Babjuk M, Burger M et al (2018) European Association of Urology (EAU) guidelines on upper tract urothelial carcinoma (UTUC). EAU Guidelines. Edn. Presented at the EAU Annual Congress Copenhagen 2018. ISBN 978-94-92671-01-1. Available at http://uroweb.org/guideline/upper-urinary-tract-urothelial-cell-carcinoma/
Seisen T, Peyronnet B, Dominguez-Escrig JL et al (2016) Oncologic outcomes of kidney-sparing surgery versus radical nephroureterectomy for upper tract urothelial carcinoma: a systematic review by the EAU non-muscle invasive bladder cancer guidelines panel. Eur Urol 70(6):1052–1068
Fang D, Seisen T, Yang K et al (2016) A systematic review and meta-analysis of oncological and renal function outcomes obtained after segmental ureterectomy versus radical nephroureterectomy for upper tract urothelial carcinoma. Eur J Surg Oncol 42(11):1625–1635
Mullen E, Ahmed K, Challacombe B (2017) Systematic review of open versus laparoscopic versus robot-assisted nephroureterectomy. Rev Urol 19(1):32–43
Rouprêt M, Harmon JD, Sanderson KM et al (2007) Laparoscopic distal ureterectomy and anastomosis for management of low-risk upper urinary tract transitional cell carcinoma: preliminary results. BJU Int 99(3):623–627
McClain PD, Mufarrij PW, Hemal AK (2012) Robot-assisted reconstructive surgery for ureteral malignancy: analysis of efficacy and oncologic outcomes. J Endourol 26(12):1614–1617
Krane LS, Hemal AK (2012) Surgeon-controlled robotic ureteral surgery. Curr Opin Urol 22(1):70–77
Hemal A, Stansel I, Babber P, Patel M (2011) Robotic-assisted nephroureterectomy and bladder cuff excision without intraoperative repositioning. Urology 78:357–364
Zargar H, Krishnan J, Autorino R et al (2014) Robotic nephroureterectomy: a simplified approach requiring no patient repositioning or robot redocking. Eur Urol 66:769–777
Pathak RA, Hemal AK (2018) Techniques and outcomes of robot-assisted nephroureterectomy for upper tract urothelial carcinoma. Eur Urol Focus 4(5):657–661
Gakis G, Schubert T, Alemozaffar M et al (2017) Update of the ICUD-SIU consultation on upper tract urothelial carcinoma 2016: treatment of localized high-risk disease. World J Urol 35(3):327–335
Peyronnet B, Seisen T, Dominguez-Escrig JL et al (2017) Oncological outcomes of laparoscopic nephroureterectomy versus open radical nephroureterectomy for upper tract urothelial carcinoma: an European Association of Urology guidelines systematic review. Eur Urol Focus. https://doi.org/10.1016/j.euf.2017.10.003 (Epub ahead of print)
Tinay I, Gelpi-Hammerschmidt F, Leow JJ et al (2016) Trends in utilisation, perioperative outcomes, and costs of nephroureterectomies in the management of upper tract urothelial carcinoma: a 10-year population-based analysis. BJU Int 117:954–960
Rodriguez JF, Packiam VT, Boysen WR et al (2017) Utilization and outcomes of nephroureterectomy for upper tract urothelial carcinoma by surgical approach. J Endourol 31(7):661–665
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Mitropoulos D, Artibani W, Graefen M et al (2012) Reporting and grading of complications after urologic surgical procedures: an ad hoc EAU guidelines panel assessment and recommendations. Eur Urol 61:341–349
von Elm E, Altman DG, Egger M et al (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457
Pugh J, Parekattil S, Willis D et al (2013) Perioperative outcomes of robot-assisted nephroureterectomy for upper urinary tract urothelial carcinoma: a multi-institutional series. BJU Int 112(4):E295–E300
Campi R, Minervini A, Mari A et al (2017) Anatomical templates of lymph node dissection for upper tract urothelial carcinoma: a systematic review of the literature. Expert Rev Anticancer Ther 17(3):235–246
Mari A, Sforza S, Morselli S et al (2018) Surgical outcome of 100 consecutive robot-assisted pyeloplasty cases with no drainage placement for ureteropelvic junction obstruction. Int J Urol 25(7):700–701
Musch M, Hohenhorst L, Pailliart A et al (2013) Robot-assisted reconstructive surgery of the distal ureter: single institution experience in 16 patients. BJU Int 111(5):773–783
Nakada SY, Hsu THS (2012) Management of upper urinary tract obstruction. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (eds) Campbell-Walsh urology, vol 2, 10th edn. Saunders, Philadelphia, pp 1122–1168
Glinianski M, Guru KA, Zimmerman G et al (2009) Robot-assisted ureterectomy and ureteral reconstruction for urothelial carcinoma. J Endourol 23(1):97–100
Aboumohamed AA, Krane LS, Hemal AK (2015) Oncologic outcomes following robot-assisted laparoscopic nephroureterectomy with bladder cuff excision for upper tract urothelial carcinoma. J Urol 194(6):1561–1566
Lim SK, Shin TY, Kim KH et al (2013) Intermediate-term outcomes of robot-assisted laparoscopic nephroureterectomy in upper urinary tract urothelial carcinoma. Clin Genitourin Cancer 11(4):515–521
Trudeau V, Gandaglia G, Shiffmann J et al (2014) Robot-assisted versus laparoscopic nephroureterectomy for upper-tract urothelial cancer: a population-based assessment of costs and perioperative outcomes. Can Urol Assoc J 8(9–10):E695–E701
Xylinas E, Rink M, Cha EK et al (2014) Impact of distal ureter management on oncologic outcomes following radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol 65:210–217
Lenis A, Donin N, Faiena I et al (2018) Role of surgical approach on lymph node dissection yield and survival in patients with upper tract urothelial carcinoma. Urol Oncol 36:e1–e9
Kamat AM, Sylvester RJ, Böhle A et al (2016) Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: recommendations from the International Bladder Cancer Group. J Clin Oncol 34:1935–1944
Author information
Authors and Affiliations
Contributions
RC: project concept and design, drafting of the manuscript. JC: data collection, drafting of the manuscript. FS: data collection, critical revision of the manuscript. TS: critical revision of the manuscript. RT: data collection, data analysis. DA: data collection. NM: data collection. AG: data collection. AM: critical revision of the manuscript. FP: critical revision of the manuscript. SS: critical revision of the manuscript. AM: critical revision of the manuscript. MR: project concept and design, critical revision of the manuscript, supervision
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Campi, R., Cotte, J., Sessa, F. et al. Robotic radical nephroureterectomy and segmental ureterectomy for upper tract urothelial carcinoma: a multi-institutional experience. World J Urol 37, 2303–2311 (2019). https://doi.org/10.1007/s00345-019-02790-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-02790-y