Abstract
Background
68Ga-PSMA Positron Emission Tomography/Computerized Tomography (PET/CT) has shown promising results for the detection of recurrent prostate cancer (RPCa). However, the diagnostic value of this method is yet to be validated. The aim of this study was to determine the influence of clinical and biochemical variables on the detection rate of 68Ga-PSMA PET/CT in patients with RPCa.
Methods
This is a prospective study of 121 patients who underwent 68Ga-PSMA-PET/CT and conventional imaging (CI) for RPCa. Detection rates were analyzed and correlated with various clinical and biochemical variables such as Gleason score GS), androgen deprivation therapy (ADT), trigger PSA (tPSA), PSA doubling-time (PSAdt) and PSA velocity (PSAv).
Results
68Ga-PSMA-PET/CT showed at least one focus of pathological 68Ga-PSMA uptake in 92/121 (76%) of patients. Nodal metastases (in 47% of patients) were the most common site of recurrent disease followed by bones (36%) and prostate (32%). Out of 121 patients, 57 (47%) had only positive findings on PSMA scan verified by biopsy or follow-up. The majority of these lesion were located in the lymph nodes (31/57, 54,5%), which were below the detection limit of CT. Univariate analysis showed higher detection rate of PET/CT with increasing tPSA, PSAv and short PSAdt. Best cutoff for tPSA, PSAv and PSAdt was 0.5 ng/ml, 2.25 ng/ml/year and 8.65 months, respectively. The detection rate of PSMA-PET/CT was higher in patients with high grade tumors (GS > 7, 23.7% vs 76.3%) and in patients who were on ADT during of PSMA scan (76.3% vs 96%). In multiple logistic regression analysis, PSAdt and concurrent ADT were identified as predictors of positive 68Ga-PSMA-PET/CT.
Conclusion
68Ga-PSMA-PET/CT is useful for re-staging patients with RPCa and has improved performance compared with CI for disease detection. Detection rates are improved in patients on ADT and with short PSAdt.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the second leading cause of cancer death among men. Early detection and accurate re-staging of recurrent prostate cancer lesions are paramount to allow patients who are candidates for salvage therapy. In recent years, there have been great developments in diagnosis and treatment of PCa. One of the major developments occurred in molecular imaging involves detection of prostate-specific membrane antigen imaging (PSMA) with positron emission tomography (PET) which is overexpressed in prostate cancer cells. Radiolabeled prostate-specific membrane antigen ligand Glu-NH-CO–NH-Lys-(Ahx)-[68Ga (HBED-CC)], also known as 68Ga-PSMA-11, is the most popular radiotracer used for PET imaging and has been widely used for the diagnosis of primary and RPca [1,2,3]. Targeting the prostate-specific antigen on the surface of PCa cells allows imaging of the primary tumor and metastatic lesions. Despite successful studies showing the efficacy of 68Ga-PSMA PET/CT in patients with PCa, especially in patients with recurrence after definitive therapy, there is a still need for further studies to define the detection rates of 68Ga-PSMA PET in relation to PSA kinetics and clinical variables.
The purpose of this study is to investigate the diagnostic performance of 68Ga-PSMA PET/CT at different PSA kinetics and identify clinical and pathological variables for 68Ga-PSMA PET/CT positivity.
Materials and methods
Patients
We prospectively evaluated 121 patients with RPCa who underwent 68Ga-PSMA PET/CT between June 2014 and June 2016 at a tertiary academic institution. Patients who were previously diagnosed with prostate adenocarcinoma were referred for a PSMA PET scan due to rising PSA levels after therapy. Patients with a secondary malignant disease and poorly differentiated adenocarcinoma, neuroendocrine differentiation or small cell carcinoma were excluded. Detailed patient characteristics are summarized in Tables 1 and 2. Serum PSA levels at the time of the PET/CT scan (trigger PSA; tPSA), primary tumor Gleason score (GS), PSA doubling time (PSAdt) with PSA velocity were available in 119/121 (98.3%), 114/121 (94.2%), in 92/121 (76%) of patients, respectively. Patients’ castration status was determined by patients’ testosterone levels (< 50 ng/dl). This study was approved by local hospital ethics committee (Permission No: GO 14/484-17). All patients signed a written informed consent for performance of 68Ga-PSMA-11 PET/CT and anonymous publication of their data. All reported investigations were conducted in accordance with the Helsinki Declaration and with national regulations.
68Ga-PSMA PET-CT imaging
Radiopharmaceutical production
Patients were injected with 68Ga-labeled HBED-CC (68Ga-PSMA) that was synthesized as described previously [4]. The ligand was labelled with 68Ga from a 68Ge/68Ga radionuclide generator (Eckert & Ziegler) using a fully automated module (Modular Lab PharmTracer, Eckert & Ziegler Eurotope GmbH) and good manufacturing practice-grade disposable cassettes and reagent kits. The radiochemical purity of the final product was ≥ 98.
Patient preparation
Patients fasted for at least 3 h prior to injection. Oral contrast (Omnipaque 350 mg/50 ml) was given 1–2 h before PET/CT scan for optimal abdominopelvic imaging. Patients received a mean dose of 170 ± 32 MBq (range 111–222 MBq) of 68Ga-PSMA. Imaging was performed after 1 h. Patients were asked to void before imaging to reduce bladder activity.
Imaging protocol
All patients had PET scans from skull base to upper thigh with six to seven bed positions (3 min/bed) on a Discovery ST (GE Healthcare, Waukesha, WI, USA) PET/CT scanner. PET images were acquired in 3D mode in 128 × 128 matrix with a low dose CT (GE Smart mA noise index: 15 with 100–300 mA and 120 kV) that was used for attenuation correction. An iterative reconstruction algorithm (two iterations, twenty-one subsets) followed by a post-reconstruction smoothing Gaussian filter was used for image reconstruction.
Image analysis
Ga-68 PSMA PET/CT images were interpreted individually on GE Advantage workstation (GE Healthcare, Chicago, IL, USA) by two board certified nuclear medicine physicians who were blinded to patients’ history and clinical results. A PSMA positive lesion was defined as focal tracer uptake greater than normal or physiological local background activity. Foci of uptake were defined on a 3 point scale as 0: negative/below background, 1: equivocal and 2: positive/above background. If the findings were equivocal, the final decision was reached by consensus and recently proposed interpretation guidelines (Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE) [5]. Positive findings were validated by histological analysis in 10% of patients either after salvage surgery or biopsy of the lesion. In patients where histologic gold standard was absent, the best valuable comparator was defined based on the available current conventional imaging such as CT, Magnetic Resonance Imaging (MRI) or bone scintigraphy (BS), or clinical/radiological follow-up and repeat 68Ga-PSMA PET/CT. Progression of the metastatic lesions on follow-up imaging accompanied by an increase in PSA levels with/without treatment or regression of metastatic lesions with > 30% decline in PSA values after therapy was considered true positive.
Statistical analysis
Each lesion was classified as positive or negative. Positivity rates were calculated for 68Ga-PSMA PET/CT across PSA levels, PSAdt, PSAv and Gleason scores at diagnosis. Verification of results was done by histopathology, or follow-up based on PSA decline and disappearance/regression of abnormal lesions on follow-up scans. Descriptive statistics were used to summarize the data with mean, median and standard deviation. Multiple logistic regression analysis was performed to analyze multiple clinical and biochemical variables affecting 68Ga-PSMA PET/CT results. Chi-square, Fisher, t test, or Mann–Whitney U tests were used to evaluate the impact of ADT on detection rate. The association between PSMA PET positivity and multiple biochemical variables was investigated by multiple logistic regression analysis. Receiver operating characteristics (ROC) curve analyses were used to determine the optimal cutoff values with the highest discriminatory probability in predicting the of PET/CT positivity. Statistical significance was set at p < 0.05. IBM SPSS Statistics version 22.0 (Armonk, NY: IBM Corp.) was used to analyze the data.
Results
Among 121 patients with (RPCa) 68Ga-PSMA PET/CT showed at least one focus of pathological uptake in 92/121 (76%) of patients. Lymph nodes (present in 47.1% of all scans) (Fig. 1) were the most common site of recurrent disease followed by bones (36.4%) (Fig. 2) and prostate (32.2%). The distribution of dominant disease is summarized in Table 3.
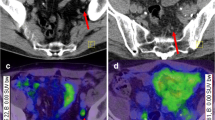
A 70-year-old patient with prostate cancer (GS: 7). He had multimodality therapy which included radical prostatectomy, adjuvant radiation therapy and ADT. 10 years after definitive therapy, he was referred to 68Ga-PSMA PET/CT (tPSA: 1.3 ng/ml, PSAdt: 6 months, PSAv: 1 ng/ml/y). PET/CT showed a 5 mm lymph node compatible with metastatic disease in left iliac region with SUVmax of 3 (black arrows on PSMA PET, white arrows on CT and fusion images)
A 75-year-old PCa patient with a GS of 9 (4 + 5). He had biochemical PSA relapse after external beam radiation therapy after 2 years with tPSA, doubling time and PSA velocity of 3 ng/ml, 7 months and 1.6 ng/ml/year, respectively. 68Ga-PSMA PET/CT showed bilobar recurrence in the prostate gland (a; black arrow heads) and an isolated intramedullary left femur metastases with no corresponding CT abnormality (a; arrows). Bone scan was normal (b)
CT, BS and multi-parametric MRI were available in 100, 30 and 16% of patients, respectively. In 64 (53%), findings were concordant between PET/CT and conventional imaging. Out of 121 patients, 57 (47%) had only positive findings on PSMA scan verified by biopsy or follow-up. The majority of these lesions were located in the lymph nodes (31/57, 54.5%), followed by bones (17/57, 30%) and prostate bed (2/57, 3.5%). In seven patients, metastatic uptake was found both in lymph nodes and bones (12%). Size of the lymph nodes detected only by PSMA scan was significantly smaller than the lymph nodes detected by conventional imaging (7.5 ± 4, range 4–12 versus 14.8 ± 7.5, range 9–45 mm, p < 0.01).
Correlation of Ga-68 PSMA PET/CT with clinical parameters
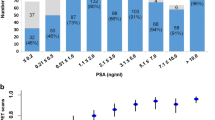
The detection rate of PSMA PET/CT was significantly higher in patients with high (> 7) GS, trigger PSA, PSAv and short PSAdt (Table 4). On ROC analysis, the optimal cutoff points were ≥ 0.5 ng/ml for trigger PSA, ≤ 8.65 months for PSAdt and ≥ 2.25 ng/ml/year for PSAv (Table 5).
The detection rates of 68Ga-PSMA PET/CT were further analyzed according to the thresholds commonly used in the literature [6, 7]. On subgroup analyses, detection rate was 87.8% for a tPSA ≥ 2 ng/ml, compared to 43.3% for < 1 ng/ml. Figure 3 summarizes PSMA positivity at various cutoff levels.
Effect of ADT on PSMA PET/CT
Since 79 of 121 patients were on ADT during PET/CT, these patients were analyzed separately. Compared with hormone naive patients, PSMA scan had significantly higher detection rate in these patients. (76.3% vs 96%) (Tables 6, 7) (p: 0.012).
Finally, multiple logistic regression analysis showed two statistically significant variables; PSAdt and concurrent ADT as predictors of PET/CT positivity (Table 8).
Discussion
68Ga PSMA PET/CT is a promising technique for the detection of RPCa for optimal treatment planning. Our study showed that detection rate is influenced by several clinical, biochemical and pathological parameters.
We were able to find recurrent disease in 76% (92/121) of our patients with PSMA PET scan. This is slightly lower than previous studies reported by Eiber et al. [6] (89.5%) and Afshar-Oromieh et al. [3] (82.8%) but higher than Ceci et al. [8] (74.2%). The possible explanation for this variation may be attributable to the difference in patient cohorts. Eiber et al. [6] investigated patients with biochemical recurrence after radical prostatectomy and only 8% of their cohort received radiation therapy and 28% had ADT. Afshar-Oromieh et al. [3] had a more heterogeneous group of patients with surgery (%70), radiation therapy (primary or post-surgery) or both with/without ADT. Ceci et al. [8] studied patients with RP (62.9%), RP plus adjuvant RT (17.1%), EBRT (7%) with or without ADT. As opposed to the other cohorts, we included patients who had undergone chemotherapy and had a higher percentage of patients on ADT. The extent of disease was also different among studies. Our patients and cohorts of Eiber et al. [6] and Afshar-Oromieh et al. [3] had more advanced disease than those studied by Ceci et al. [8].
In our study, univariate analysis showed higher detection rate of PET/CT with high trigger PSA, PSAv, and short PSAdt. The best cutoff for triger PSA, PSAv and PSAdt were 0.5 ng/ml, 8.65 months and 2.25 ng/ml/year, respectively. Eiber et al. also found that the detection rates correlated positively with increasing PSA and PSAv; however, no significant correlation was found with PSAdt [6]. Afshar-Oromieh et al. [7] also found increasing detection rates at high PSA levels. In a prostate cancer patients referred for re-staging, Ceci et al. found that PSA levels and PSAdt were significantly different between PET-positive and PET-negative patients. In their study, ROC analysis showed that PSAdt 6.5 months and PSA 0.83 ng/ml were the optimal cutoff values [8]. Short doubling time and high PSA velocity appear to be the results of rapidly growing tumor. Discrepancies found among studies may be explained by the heterogeneity of patient groups.
Another factor that positively influenced the detection rate in our study was high Gleason score which represents aggressive tumor [9]. It is known that PSMA expression is increased in PCa with high Gleason score [10]. We found that patients with high GS had a higher likelihood of positive PSMA scans. This finding is also supported by Eiber et al. [6] but not reported by Verburg et al. [11] and Afshar-Oromieh et al. [7, 12]. In addition to the patient selection, discrepancies may also be explained by the fact that most of the Gleason scores were recorded at the initial diagnosis and tumor biology might have changed in the interval period.
Additionally, we were able to demonstrate the relation between PSMA PET detection rate and ADT. Patients who were on ADT during PSMA scan had higher test positivity. This finding is in concordance with Afshar-Oromieh et al. [7]; however, Eiber et al. [6] found no significant difference in detection efficacy in relation to ADT. Although there is still an ongoing discussion regarding the impact of ADT on scan results, there are several explanations for our findings. Watt et al. reported that folate hydrolase 1 (FOLH1) gene encodes PSMA protein and PSMA expression is inhibited in the existence of androgens [13, 14]. Evans et al. reported the upregulation of PSMA expression as a result of ADT in a PCa animal model. They found that ADT either by castration of mice or via treatment with enzalutamide increases 64Cu-J591 radiolabeled antibody uptake in xenografts of AR-positive and hormone-sensitive PCa cell lines [15]. Hope et al. also confirmed increased PSMA uptake (1.5- to 2.0-fold) in the LNCaPAR xenograft mouse model after treatment with both orchiectomy and apalutamide (ARN-509), which is a nonsteroidal competitive AR inhibitor. Additionally, the authors imaged one patient with castration-sensitive prostate cancer before and 4 weeks after treatment with androgen deprivation therapy who had a sevenfold increase in PSMA uptake [16]. In a very recent clinical pilot study using PSMA-targeted SPECT scans, Vallabhajosula et al. [17] monitored metastatic CRPC patients undergoing ADT and found that 2 weeks after therapy by either abiraterone or enzalutamide, 99 m Tc-MIP-1404 PSMA scans showed more lesions or higher uptake compared to baseline. However, after 12 weeks, there was a considerable reduction of the PSMA-ligand uptake in the lesions as a response to therapy [18]. Hence, it appears as long-term ADT may decrease tumor size and 68Ga PSMA uptake. Denmeade et al. recently examined cell lines in different states of androgen deprivation and discovered that PSMA activity in prostate cancer cell lines increased, as cells became more androgen independent [19]. So, patients with recurrent disease despite ADT may have tumor cell lines with higher PSMA expression. Therefore, multiple factors may have contributed to the high detection rate in patients on ADT in our study such as induced PSMA expression after ADT or hormone resistance. The correlation between ADT and PSMA uptake needs to be validated by more studies in different cell lines and clinical situations.
Out of 121 patients, 57 (47%) patients had only positive findings on PSMA scan verified by biopsy or follow-up. The evaluation of small lymph nodes is challenging in RPCa and PSMA scan is especially useful for the detection of small lymph nodes which remains under the size threshold of CT. Similarly, Eiber et al. [6] reported superiority of Ga 68 PSMA PET mainly in lymph nodes (38% of patients), followed by bones in 11%. We were also able to detect 24 patients with bone metastases which were negative on BS or CT. The most striking finding regarding the bone lesions detected only by PSMA scan was the absence of sclerosis on CT. Although BS detects osteoblastic activity in areas of bone metastases, early intramedullary lesions with no or mild bone reactivity activity can be easily missed. Superiority of Ga-68 PSMA PET compared to BS was also reported by Pyka et al. [20] and there is an ongoing debate on added value of BS in the PSMA PET era.
The limitations of this study are: 1-Lack of histopathological proof in some patients, which could not be obtained due to practical and ethical reasons. As used by several authors, validation of scan results was conducted by follow-up. 2-Heterogeneity of patients with different previous therapies may also affect the relation between PSMA PET detection rate and PSA kinetics.
Conclusion
68Ga-PSMA PET/CT has contributed additional value over CI for re-staging in patients with RPCa. The two most relevant predictors for test positivity are PSAdt and concurrent ADT. Further studies in different patient groups are warranted.
References
Eder M, Neels O, Muller M et al (2014) Novel preclinical and radiopharmaceutical aspects of [68 Ga]Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate cancer. Pharmaceuticals (Basel) 7(7):779–796
Afshar-Oromieh A, Zechmann CM, Malcher A et al (2014) Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 41(1):11–20
Afshar-Oromieh A, Avtzi E, Giesel FL et al (2015) The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 42(2):197–209
Minamimoto R, Hancock S, Schneider B et al (2016) Pilot comparison of (6)(8)Ga-RM2 PET and (6)(8)Ga-PSMA-11 PET in patients with biochemically recurrent prostate cancer. J Nucl Med 57(4):557–562
Eiber M, Herrmann K, Calais J et al (2018) Prostate cancer molecular imaging standardized evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med 59(3):469–478
Eiber M, Maurer T, Souvatzoglou M et al (2015) Evaluation of hybrid (6)(8)Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med 56(5):668–674
Afshar-Oromieh A, Malcher A, Eder M et al (2013) PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging 40(4):486–495
Ceci F, Uprimny C, Nilica B et al (2015) (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging 42(8):1284–1294
Wright GL, Haley C Jr, Beckett ML et al (1995) Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol 1(1):18–28
Marchal C, Redondo M, Padilla M et al (2004) Expression of prostate specific membrane antigen (PSMA) in prostatic adenocarcinoma and prostatic intraepithelial neoplasia. Histol Histopathol 19(3):715–718
Verburg FA, Pfister D, Drude NI, Mottaghy FM, Behrendt F (2017) PSA levels, PSA doubling time, Gleason score and prior therapy cannot predict measured uptake of [68Ga]PSMA-HBED-CC lesion uptake in recurrent/metastatic prostate cancer. Nuklearmedizin 56(6):225–232
Afshar-Oromieh A, Holland-Letz T, Giesel FL et al (2017) Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging 44(8):1258–1268
Good D, Schwarzenberger P, Eastham JA et al (1999) Cloning and characterization of the prostate-specific membrane antigen promoter. J Cell Biochem 74(3):395–405
Watt F, Martorana A, Brookes DE et al (2001) A tissue-specific enhancer of the prostate-specific membrane antigen gene, FOLH1. Genomics 73(3):243–254
Evans MJ, Smith-Jones PM, Wongvipat J et al (2011) Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci USA 108(23):9578–9582
Hope TA, Truillet C, Ehman EC et al (2017) 68Ga-PSMA-11 PET imaging of response to androgen receptor inhibition: first human experience. J Nucl Med 58(1):81–84
Vallabhajosula S, Jhanwar Y, Tagawa S et al (2016) 99 m Tc-MIP-1404 planar and SPECT scan: imaging biomarker of androgen receptor (AR) signaling and prostate specific membrane antigen (PSMA) expression. J Nucl Med 57:1541
Schlenkhoff CD, Gaertner F, Essler M, Hauser S, Ahmadzadehfar H (2016) 68Ga-labeled anti-prostate-specific membrane antigen peptide as marker for androgen deprivation therapy response in prostate cancer. Clin Nucl Med 41(5):423–425
Denmeade SR, Sokoll LJ, Dalrymple S et al (2003) Dissociation between androgen responsiveness for malignant growth vs. expression of prostate specific differentiation markers PSA, hK2, and PSMA in human prostate cancer models. Prostate 54(4):249–257
Pyka T, Okamoto S, Dahlbender M et al (2016) Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging 43(12):2114–2121
Author information
Authors and Affiliations
Contributions
ENA protocol/project development, data collection, data analysis, manuscript writing/editing. MT protocol/project development, data collection or management, data analysis, manuscript writing. FA data collection, manuscript editing. CYB data collection, manuscript editing. DEB data collection, manuscript editing. EK manuscript editing, statistics. HO data collection, manuscript editing. MC protocol/project development, data management, data analysis, manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Akdemir, E.N., Tuncel, M., Akyol, F. et al. 68Ga-labelled PSMA ligand HBED-CC PET/CT imaging in patients with recurrent prostate cancer. World J Urol 37, 813–821 (2019). https://doi.org/10.1007/s00345-018-2460-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2460-y