Abstract
Purpose
To evaluate the effect of hexanic extract of Serenoa repens (HESr) on prostatic inflammation in patients with diagnosed prostatic inflammation.
Methods
Patients with prostatic inflammation histologically confirmed by TRUS prostatic biopsy were randomized either to receive HESr (320 mg/day) or no treatment. A second biopsy was performed 6 months later according to standard clinical practice. Inflammation was assessed by the Irani’s score and immunohistochemical staining using the CD3, CD4 and CD8 (for T-leucocytes), CD20 (for B-leucocytes) and CD163 (for macrophages) antibodies.
Results
Overall 97 patients were eligible for analysis. In the HESr group the mean inflammation grading and aggressiveness grading score significantly decreased from 1.55 and 1.55 at baseline to 0.79 (p = 0.001) and 0.87 (p = 0.001) at the second biopsy, respectively. In the control group the mean inflammation grading score was 1.44 at first biopsy and 1.23 at the second biopsy. The mean aggressiveness gradings core was 1.09 and 0.89, respectively. No statistical significance was found (p = 0.09 and p = 0.74).The mean decrease in all inflammation scores was statistically higher in the HESr patients compared to controls. The immunohistochemical staining showed a significant change in the expression of the analyzed antibodies for the HESr patients compared to the first biopsy. In the nontreatment group, no significant difference was found at the second biopsy. The change in expression of each antibody in the HESr group was statistical significant compared to control.
Conclusions
HESr seems to reduce prostatic inflammation in terms of histological and immunohistochemical parameters in this specific patients population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of prostatic inflammation in the development of benign prostatic hyperplasia (BPH) and cancer has attracted the attention of researchers during the last years [1, 2]. Prostatic inflammation is a common histopathological finding after a prostate biopsy or prostatectomy. One study has reported the presence of chronic inflammation in 43% of the cases after prostate biopsies while in the REDUCE trial, 77.4% of men with LUTS/BPH had chronic inflammation on biopsy [1, 3].
Prostatic inflammation could represent a treatment target for the management of male LUTS and available evidence support the rationale for investigating anti-inflammatory agents that may alter and improve the inflammatory environment in the prostate [4].
Serenoa repens is the most common plant extract used for the management of male LUTS. Many different extracts are available, but the hexanic lipidosterolic extract of Serenoa repens (HESr) has been extensively studied in both basic and clinical research. The proposed mechanisms of action of HESr include anti-inflammatory properties, anti-androgenic activity and anti-proliferative actions [5]. With regard to its anti-inflammatory effect, in vitro and in vivo studies have shown that HESr acts on the arachidonic acid cascade by inhibiting the production of prostaglandins and of 5-lipoxygenase metabolites of arachidonic acid; can modify inflammation status by decreasing infiltrates of B lymphocytes, IL-1b, and TNF-a, increasing the expression of anti-inflammatory genes and decreasing the expression of proinflammatory genes; and inhibit early steps of leukocyte infiltration by impeding monocyte and T-cell attraction and adherence [5, 6].
However, there is a lack of clinical studies investigating the effect of HESr on prostatic inflammation. Only two studies in patients have shown a reduction in inflammatory markers in prostatectomy specimens and urine stream [7, 8].
The objective of the present study was to evaluate the effect of HESr on prostatic inflammation based on histological and immunohistochemical examination in patients with diagnosed prostatic inflammation in transrectal ultrasound (TRUS) guided biopsy specimens.
Materials and methods
Patients with prostatic inflammation histologically confirmed by TRUS prostate biopsy were included in the study. Biopsies were performed due to elevated PSA, and/or positive digital rectal examination. Patients were randomized into two Groups: Group A received HESr 320 mg per day for 6 months while no therapy was given to Group B. The randomization has been based on http://www.randomizer.org (central randomization by computer). A second biopsy was performed according to standard clinical practice (persistent high PSA levels or positive DRE, or presence of atypical small acinar proliferation in the first biopsy) after discussion with the patients. Exclusion criteria included previous treatment with any plant extract or 5a-reductase inhibitors, history of pelvic radiotherapy, history of TURP or intravesical instillations for bladder carcinoma, and patients with an indwelling catheter. In addition, patients who have been found with prostate cancer at the second biopsy were excluded from the analysis of the study.
Endpoints of the study
The primary endpoint was the evaluation of change from baseline to end of study in total Irani’s score, histologic inflammation grading and aggressiveness grading subscores. The Irani’s score classifies prostatic inflammation on the basis of the extension of inflammatory cells and their effect on prostate tissue. A 4-point scale is used for inflammation (0-no inflammatory cells, 1-scattered inflammatory cell infiltrate, 2-nonconfluent lymphoid nodules and 3-large inflammatory areas with confluence of infiltrate) and aggressiveness (0-no contact between inflammatory cells and glandular epithelium; 1-contact between inflammatory cell infiltrate and glandular epithelium; 2-clear but limited, that is less than 25% of the examined material, glandular epithelium disruption, and 3-glandular epithelium disruption on more than 25% of the examined material) [9].
Secondary endpoints were changes from baseline to end of study in immunohistochemical staining for prostatic inflammation using the CD3, CD4, CD8, CD20, and CD163 antibodies.
The study has been approved by the Ethical Committee of the University Hospital of Larissa and registered in the Australian and New Zealand Clinical Trial Registry (ID: ACTRN12613000888763). Informed consent was obtained from all individual participants included in the study.
Pathology
Standard 12-core TRUS-guided biopsies were performed. The samples were fixed in 10% buffered formalin solution, embedded in paraffin blocks, and cut at 3-mm sections. The prostatic inflammation was estimated and graded according to the Irani’s score for both the histologic inflammation grading (extension of inflammatory cells, range 0–3) and aggressiveness (the effect of inflammatory cells on prostate tissue, range 0–3). Additionally, immunohistochemical evaluation was performed using antibodies specific for inflammatory cells: CD3 (rabbit polyclonal Ab A0452; antigen retrieval pH6; 1/300; Dako), CD4 (mouse monoclonal Ab clone 4B12; antigen retrieval EDTA pH8; 1/20; Novocastra-Menarini) and CD8 (mouse monoclonal Ab clone C8/144B; antigen retrieval pH9; 1/200; Dako) for T-leucocytes; CD20 (mouse monoclonal Ab clone L26; antigen retrieval pH6; 1/500; Dako) for B-leucocytes; CD163 (mouse monoclonal Ab clone 10D6; antigen retrieval pH6; 1/800; Novocastra-Menarini) for macrophages. The expression of each antibody in the prostate tissue has been graded from 0 to 3 (none = 0, mild = 1, moderate = 2, or marked = 3) based on the intensity and extent of tissue involvement.
The hematoxylin and eosin slides of the prostate biopsies and immunohistochemistry staining were reviewed independently by two pathologists who were not aware of which patients received therapy with HESr. In case of disagreement, a third analysis was performed and taken into account for the statistical analysis.
It was estimated that for a significant difference in inflammation score of 25%, a sample size of 50 patients per arm was required to reach a 90% power in our study. The statistical analysis, using SPSS v21.0, was based on the non-parametric Wilcoxon Test.
Results
Overall 110 men completed the study and finally 97 of them were eligible for analysis. Thirteen patients were excluded because prostatic cancer was found at the repeat biopsy. The HESr group included 49 patients (mean age 71.4 years, range 56–77), while the control group had 48 patients (mean age 68.7 years, range 58–74). The PSA values were 6,6 ng/ml (range 2.3–15.0) and 5.5 ng/ml (range: 2.3–13.5) for groups A and B, respectively. Table 1 shows the baseline characteristics of both groups.
Primary endpoint
The mean inflammation grading score for the HESr group was 1.55 (median 2.0) at first biopsy and decreased to 0.79 (median 1.0) after therapy. The mean aggressiveness grading score was 1.55 (median 2.0) and 0.87 (median 1.0) at the first and second biopsy, respectively. The difference in both scores was statistically significant (p = 0.001 for both comparisons). In the control group the mean inflammation grading score was 1.44 (median 1.0) at first biopsy, and 1.23 (median 1.0) at the second biopsy. The mean aggressiveness grading score for this Group was 1.09 (median 1.0), and 0.89 (median 1.0) at first and second biopsy, respectively. No statistically significant difference was found between those scores (p = 0.09 and p = 0.74, respectively). There was a statistically significant decrease in total Irani’s score for the HESr Group (p = 0.001), but in the control group a slight but not significant decrease was found (p = 0.52) (Table 2).
When the two groups were compared, the mean decrease in all inflammation scores (histologic inflammation grading, aggressiveness grading and total Irani’s score) was statistically higher in the HESr group (Table 2).
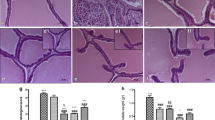
Figures 1 and 2 present biopsy specimens of the same patient before and 6 months after treatment with HESr. A decrease in prostatic inflammation is documented.
Secondary endpoints
The immunohistochemical staining showed a significant change in the expression of CD3, CD4 and CD8 (for T-leucocytes), CD20 (for B-leucocytes) and CD163 (for macrophages) antibodies after 6 months of treatment with HEsr compared to the expression at first biopsy. As it was expected in the non-treatment group, no significant difference in the expression of the specific antibodies was found at the second biopsy (Table 3). The immunohistochemical results between the two groups were compared. The change in expression of each antibody in the HESr patients reached statistical significance compared to controls (Table 3).
Interestingly, in the control group there was an increase in the second biopsy expression of CD3, CD4, CD8, CD20 and CD163 in 14 (29.1%), 10 (20.8%), 10 (20.8%), 11 (22.9%) and 11 (22.9%) patients, respectively. On the other hand, only one patient (2.04%) treated with HEsr had an increase in CD3 expression. All the other patients remained stable or had a lower expression of the antibodies.
Discussion
In this randomized double blinded trial, the effect of HESr on prostatic inflammation in patients was investigated using specific histological and immunohistochemical criteria. To our knowledge, this is the first and only study in humans who underwent two prostatic biopsies (baseline and end of study).
In Irani’s score, the histologic inflammation grading subscore reflects the extension of inflammatory cells while the histologic aggressiveness subscore grades the effect that the inflammatory cells produce on prostate tissue [9]. Our results showed that treatment with HESr resulted in significant decrease in all scores (histologic grading, aggressiveness grading and total score) at the second biopsy. The difference in inflammation improvement was significant for the HESr group when compared with the control group. These data show for the first time that treatment with HESr reduces both extension and aggressiveness of inflammation in the same patient.
In the literature, there are scarce data and only indirect comparisons can be made due to the differences in the study designs and populations. A multicenter, open label, pilot study included 35 patients who underwent prostatic surgery (TURP or adenectomy) [7]. Patients (n = 16) who had received HESr 3 months before surgery compared with patients (n = 19) without any treatment for 3 weeks before surgery. Tissue samples were analyzed for infiltrates and inflammatory markers (CD-3, CD-20, and CD-68). In the HESr group a significant decrease of B lymphocytes (36.24% reduction of CD20+ cells) and large and significant reductions in the levels of IL-1b and tumor necrosis factor (TNF)-a were reported [7]. Another RCT involved 206 patients who received treatment with HESr 320 mg/day or tamsulosin 0.4 mg for 3 months, to assess the anti-inflammatory properties of HESr [8]. Urine stream taken after direct rectal examination was analyzed for MCP1/CCL2, interferon gamma-induced protein 10/C-X-C motif chemokine 10 and macrophage migration inhibitory factor (MIF). All the markers were found significantly reduced in the HESr group [8].
CD3, CD4, CD8 and CD20 antibodies are commonly used for the characterization of immune cells in tissue samples while CD163 is expressed on most subpopulations of mature tissue macrophages. Robert et al. studied prostatic inflammation on tissue microarrays after standard coloration and immunohistochemistry [10]. The majority of patients had inflammatory cells infiltrating BPH tissues: T-lymphocytes markers (CD3+ cells), B-lymphocytes markers (CD20+) and macrophages markers (CD163+) were found in 81, 52 and 82% of the BPH specimens, respectively. In our study, we evaluated the change in the expression of these immunohistochemical markers and a significant decrease in grade of all the examined markers was recorded. In the study of Vela-Navarrete, a reduction of B lymphocytes was found but no significant difference in the number of T-lymphocytes and macrophages was observed in patients treated with HESr prior to surgery [7]. However, in the latter study, treatment was given for 3 months compared with the 6-month administration of HESr in our trial and the study population was different.
Another interesting finding was that the inflammation score was upgraded in 25% patients (12 out 48) in the control group while only 6.1% (3 out of 49) of the HESr patients had a higher Irani’s score at the second biopsy. Similarly, there was an increase in the intensity of the antibodies in the patients who remained without therapy (for CD3 29.1%, CD4 20.8%, CD8 20.8%, CD20 22.9% and CD163 22.9% of the patients, respectively) while only one patient treated with HESr had an increase in CD3 expression at the second biopsy. These results suggest that inflammation seems to progress over time and confirm the anti-inflammatory properties of HESr.
Two recent systematic reviews assessed the efficacy and safety of the HESr [11, 12]. The first systematic review analyzed data only from RCTs and the second data from RCTs and observational studies. Both studies found that HESr was effective for increasing urinary flow in men with prostatic enlargement compared with placebo while urinary symptoms and nocturia were also improved. They also confirmed that HESr had a comparable to tamsulosin and short-term finasteride in symptoms improvement [11, 12]. No correlation between clinical improvement and prostatic inflammation or any other parameter could be investigated due to the study designs.
The main limitation of our study is that participants belong to a specific group of patients with elevated serum PSA or/and positive direct rectal examination, to be eligible for a prostate biopsy. In addition, only patients with confirmed inflammation at the first biopsy were included. However, our study contributes to the understanding of the action of HESr in clinical setting and urges the need for a larger trial to assess if and how this improvement in prostatic inflammation can be translated into clinical practice. Future research directions should focus on which baseline characteristics can predict treatment response and if patients with the higher decrease in prostatic inflammation also experience the larger clinical benefit with HESr treatment in terms of LUTS relief.
Conclusion
The present study showed that after a 6-month treatment, HESr seems to reduce prostatic inflammation in terms of histological and immunohistochemical parameters in patients who underwent two biopsies due to elevated PSA and/or suspicious DRE. Further investigation is needed to translate this finding into clinical practice.
References
Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS (2008) The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol 54(6):1379–1384
De Nunzio C, Kramer G, Marberger M et al (2011) The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol 60:106–117
Di Silverio F, Gentile V, De Matteis A et al (2003) Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol 43:164–175
Samarinas M, Gacci M, de la Taille A, Gravas S (2018) Prostatic inflammation: a potential treatment target for male LUTS due to benign prostatic obstruction. Prostate Cancer Prostatic Dis. https://doi.org/10.1038/s41391-018-0039-8
Buck AC (2004) Is there a scientific basis for the therapeutic effects of Serenoa repens in benign prostatic hyperplasia? Mechanisms of action. J Urol 172:1792–1799
De la Taille A (2013) Therapeutic approach: the importance of controlling prostatic inflammation. Eur Urol Suppl 12:116–122
Vela Navarrete R, Garcia Cardoso JV, Barat A, Manzarbeitia F, López Farré A (2003) BPH and inflammation: pharmacological effects of permixon on histological and molecular inflammatory markers. Results of a double blind pilot clinical assay. Eur Urol 44:549–555
Latil A, Pétrissans MT, Rouquet J et al (2015) Effects of hexanic extract of Serenoa repens (Permixon® 160 mg) on inflammation biomarkers in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate 75(16):1857–1867
Irani J, Levillain P, Goujon JM, Bon D, Dore B, Aubert J (1997) Inflammation in benign prostatic hyperplasia: correlation with prostate specific antigen value. J Urol 157(4):1301–1303
Robert G, Descazeaud A, Nicolaïew N, Terry S, Sirab N, Vacherot F, Maillé P, Allory Y, de la Taille A (2009) Inflammation in benign prostatic hyperplasia: a 282 patients’ immunohistochemical analysis. Prostate 69(16):1774–1780
Novara G, Giannarini G, Alcaraz A et al (2016) Efficacy and safety of hexanic lipidosterolic extract of Serenoa repens (Permixon) in the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: systematic review and meta-analysis of randomized controlled trials. Eur Urol Focus 2(5):553–561
Vela-Navarrete R, Alcaraz A, Rodríguez-Antolín A et al (2018) Efficacy and safety of a hexanic extract of Serenoa repens (Permixon®) for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH): systematic review and meta-analysis of randomized controlled trials and observational studies. BJU Int. https://doi.org/10.1111/bju.14362 (in press)
Author information
Authors and Affiliations
Contributions
SG: protocol and project development, data collection, data analysis, manuscript editing. MS: data collection, data analysis, manuscript writing. KZ: data collection and management. AK: data collection. GK: data collection and management, manuscript editing. VT: data collection. MM: data collection and management, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
Stavros Gravas has received speaker honoraria from Astellas, Pierre Fabre Medicament and consultancy honoraria from Astellas and GSK. Michael Samarinas, Konstantina Zacharouli, Anastasios Karatzas, Georgios Koukoulis, Vasileios Tzortzis, Michael Melekos declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Gravas, S., Samarinas, M., Zacharouli, K. et al. The effect of hexanic extract of Serenoa repens on prostatic inflammation: results from a randomized biopsy study. World J Urol 37, 539–544 (2019). https://doi.org/10.1007/s00345-018-2409-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2409-1