Abstract
Background
IDH1 mutations are oncogenic through induction of DNA damage and genome instability. They are of therapeutic interest because they confer increased sensitivity to radiation and cytotoxic therapy and hold potential for vaccination therapy.
Methods
In this study, we analyzed more than 17,000 primary prostate cancer tissues with a mutation-specific antibody for the IDH1R132H mutation.
Results
IDH1 mutation-specific staining was found in 42 of 15,531 (0.3%) interpretable cancers. IDH1 mutation was associated with higher preoperative PSA and Gleason grade (p < 0.05, each) but was unrelated to PSA recurrence. A comparison with other molecular tumor features available from earlier studies revealed that TMPRSS2-ERG fusion as well as deletion of PTEN, 5q21, 6q15, and 3p13 was less frequent in IDH1-mutated than in non-mutated cancer. Increased lethality of genetically instable, “aberration-rich” cancer cells in the presence of IDH1 mutations could possibly explain this observation. Heterogeneity analysis revealed a homogeneous mutation in only 1 of 16 IDH1-mutated cancers. This high degree of heterogeneity may profoundly limit therapeutic targeting of IDH1 mutations in prostate cancer.
Conclusions
The data show that 0.3% of prostate cancers have an IDH1R132H mutation and that these are mostly heterogeneous. Once specific anti-IDH1 therapy becomes reality, only a very small group of prostate cancer patients may benefit from such a treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isocitrate dehydrogenase 1 (IDH1) is an enzyme involved in the citrate cycle. IDH1 catalyzes the conversion of isocitrate to α-ketoglutarate (α-KG) with release of nicotinamide adenine dinucleotide phosphate (NADPH), a key molecule for energy production and an essential reducing factor required for cellular defense mechanisms against oxidative damage [1]. IDH1 has gained considerable interest in 2008, when a specific hotspot mutation Arg132His (IDH1R132H) was discovered in human glioblastoma [1]. Subsequent studies revealed that this IDH1 mutation occurs most frequently in particular subtypes of brain cancer, including > 80% of low-grade glioma and secondary glioblastoma [2]. IDH1 mutations are believed to represent early and potentially cancer-initiating events in these subtypes [3], and have been linked to other specific genetic alterations including deletion of chromosome 1p/19p and p53 mutation [4]. The IDH1R132H mutation results in a neo-enzymatic function leading to the synthesis of d-2-hydroxyglutarate (2-HG) instead of α-KG, a process that consumes NADPH instead of synthesizing it [5]. High levels of 2-HG are believed to exert oncogenic functions in at least two ways, i.e., modification of epigenetic control through inhibition of α-KG-dependent histone- and DNA-demethylases [6, 7], as well as induction of DNA damage and genome instability as a consequence of lowered cellular NADPH levels [8].
The different function of mutant IDH1 may hold promise for novel therapeutic approaches in several ways: The association of IDH1 mutations with DNA hypermethylation raises the possibility that hypomethylating agents may be effective against IDH-mutated cancers [8]. Moreover, the hotspot nature of mutant IDH1 makes it a highly promising candidate for novel immunotherapy and vaccination strategies. In addition, cell line models of glioblastoma suggest that IDH1-mutated cells with low NADPH levels are sensitive to irradiation and chemotherapy [9], which might explain the prolonged survival of patients with IDH1-mutated glioblastoma [10]. Accordingly, the IDH1 mutation status is now routinely assessed in brain tumors.
IDH1 mutations are not limited to brain cancer, but do also occur in 50–70% of malignant chondrosarcomas [11], 5–15% of acute myeloid leukemias [12] and at least occasionally in melanoma [13] thyroid cancer [14], breast [15] and prostate cancer [16]. In a recent study performing next generation sequencing on 453 prostate cancers, IDH1 mutations were found in 1% of tumors. Because of the absence of other key molecular features in IDH1-mutated cancers, IDH1 mutation was suggested to define a molecular subgroup [17]. Based on the observation in brain cancer, it is tempting to speculate that IDH1 mutations could identify prostate cancers with increased response rate to radiotherapy. To learn more about the prevalence of IDH1 mutations, their role in tumor initiation and progression, and possible association to molecularly defined subsets of the disease, we employed an IDH1 mutation-specific commercial antibody to stain our prostate cancer tissue microarray containing more than 17,000 samples.
Materials and methods
Patients
Radical prostatectomy specimens were available from 17,747 consecutive patients, operated at the Department of Urology and the Martini Clinic of the University Medical Center Hamburg-Eppendorf between 1992 and 2014. The specimens were analyzed as described before [18]. Histopathological data were retrieved from the patients’ record, including tumor stage, Gleason grade, nodal and resection margin status. In addition to the classical Gleason categories, “quantitative” Gleason grading was performed as detailed in [19]. Follow-up was available for 12,579 patients with a median follow-up of 48 months (range 1–276 months). Postoperative prostate-specific antigen (PSA) level of 0.2 ng/ml and higher was defined as PSA recurrence. The tissue microarray (TMA) had a spot size of 0.6 mm and contained various internal controls (e.g., normal prostate). The attached molecular database contained results on ERG expression, ERG break apart fluorescence in situ hybridization (FISH) analysis, deletion status of 5q21 (CHD1), 6q15 (MAP3K7), 10q23 (PTEN) and 3p13 (FOXP1).
Immunohistochemistry
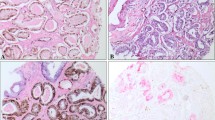
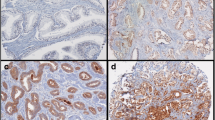
Freshly cut TMA sections were stained on 1 day and in one experiment. Slides were deparaffinized and exposed to heat-induced antigen retrieval for 5 min at 121 °C in Tris–EDTA-citrate buffer pH 7.8. The mouse monoclonal antibody DIA H09 (Dianova, Hamburg, Germany; dilution 1:20) specific for IDH1R132H was applied at 37 °C for 60 min. Bound antibody was visualized with the EnVision Kit (Dako, Glostrup, Denmark). The IDH1R132H specific antibody typically stained the cytoplasm in all (100%) tumor cells of a positive tissue spot (Fig. 1).
Statistics
JMP 12.0 software (SAS Institute Inc., NC, USA) was used. Contingency tables were calculated to study association between IDH1R132H expression and clinico-pathological variables, and p values were obtained with the Chi square (likelihood) test. Kaplan–Meier curves were generated using biochemical (PSA) recurrence as the clinical endpoint and the log-rank test was used for p values.
Results
IDH1R132H staining
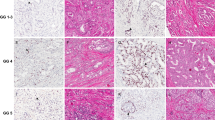
A total of 15,531 (87%) of tumor samples were interpretable in our TMA analysis (Table 1). Reason for 2221 (13%) non-informative cases was lack of tissue sample or absence of unequivocal cancer tissue in the TMA spot. Normal prostate glands or stromal tissue did not show any IDH1R132H staining under the selected experimental conditions. Also the vast majority of cancers lacked positive staining. Only 42/15,531 (0.3%) tumors showed IDH1R132H staining. Staining was clear cut, involved 100% of cancer cells and was of moderate to strong intensity (Fig. 1). In order to study whether staining was homogeneous in IDH1R132H positive cancer, we selected 16 IDH1R132H positive cancers and analyzed either conventional large section (5 patients) or 0.6 mm tissue spots (11 patients) from all tumor containing tissue blocks. 16/16 tumors showed heterogeneous staining with the presence of both IDH1R132H positive and negative tumor areas.
Association with tumor phenotype and patient outcome
The relationship between IDH1R132H staining and prostate cancer phenotype is shown in Table 1. Positive IDH1R132H staining was statistically linked to high preoperative PSA levels (p = 0.0232) and—due to its absence in Gleason 3 + 3 = 6 cancers—also to high Gleason grade (p = 0.0128). Follow-up data were available for 12,579 patients with interpretable IDH1R132H staining on the TMA. IDH1R132H staining was unrelated to PSA recurrence (Fig. 2).
Association with TMPRSS2:ERG fusion status and chromosomal deletions
Data on TMPRSS2:ERG fusion status obtained by FISH were available from 6333 and by immunohistochemistry from 9464 tumors with evaluable IDH1R132H staining. Data on both ERG FISH and IHC were available from 6090 cancers, and an identical result (ERG IHC positive and break by FISH or ERG IHC negative and missing break by FISH) was found in 5804 of 6090 (95.3%) cancers. IDH1R132H positive cancers were less often ERG positive than IDH1R132H negative cancers. This was valid for both FISH and IHC evaluation of the ERG status (p < 0,05 each; Fig. 3). The comparison of IDH1R132H staining with PTEN, 5q21, 6q15, and 3p13 deletions revealed that every deletion was less common in IDH1R132H positive cancers. However, these differences did not reach statistical significance (Fig. 4).
Discussion
The results of this study demonstrate that IDH1 mutations occur in prostate cancer but at very low frequency, and that they are usually limited to cancer subpopulations. A positive IDH1R132H staining was found in only 42 of 15,531 (0.3%) analyzable prostate cancers in this study. The applied antibody “IDH1R132H clone H09” is a well-established mutation-specific antibody that has shown 94–100% sensitivity and 100% specificity for detection of IDH1R132H mutations [20]. The clear-cut distinction of positive cancers—which were always strongly stained—from negative cancers, which did not show the slightest staining, argues for the quality of the reagent used in this study and the validity of our method. Our findings argue for IDH1R132H mutation rates well below 0.5% in prostate cancer. This fits perfectly with next generation sequencing data generated within The Cancer Genome Atlas (TCGA) and the International Cancer Genome Consortium (ICGC) studies [21]. These groups found IDH1 codon 132 mutations in 11 of 1843 (0.6%) prostate cancers. However, only seven of these 11 mutations were Arg132His, while the remaining four cases had other amino acid substitutions (Arg132Cys and Arg132Gly). These mutations have the same effect as Arg132His but are not identified by the antibody IDH1R132H clone H09 [22, 23]. Higher IDH1 mutation rates had earlier been suggested from 2 studies analyzing cohorts of 118 and 75 cancers. One of these studies employed the same antibody on a TMA and identified positivity in 3 of 118 (2.5%) prostate cancers [24]. The other study used single-strand conformation polymorphism (SSCP) analysis to find a mutation in 2 of 75 (2.7%) prostate cancers from Korean patients [16]. We do not feel that the two previous studies suggesting IDH1 mutation rates greater than 2% really contradict our data as the number of patients analyzed in these studies is too low to assess events occurring in the 0.5–3% range. It cannot be excluded, however, that Korean patients have a higher rate of IDH1 mutations than the mostly Caucasian population analyzed by the ICGC/TCGA consortium and us. There are various examples of ethnic differences in cancer biology. For example, HER2 amplification occurs markedly more frequent in Korean or Arabian than in Caucasian breast cancer patients. Overall, the available data demonstrate that, at least in Caucasian patients, the frequency of IDH1 mutations is about 0.5% in prostate cancer. Our findings in > 15,000 patients further show that IDH1R132H mutations are not strongly linked to a particular tumor phenotype or patient outcome in untreated patients. Of interest, IDH1R132H mutations were predominantly seen in cancers lacking ERG rearrangements, and IDH1R132H positive cancers tended to have lower deletion rates. These data fit well with the findings of the TCGA consortium suggesting that IDH1 mutation defines a distinct molecular subtype of prostate cancers. Based on the comprehensive analysis of 333 prostate cancers, the consortium identified seven distinct molecular subtypes, four of which were characterized by gene fusions involving members of the ETS family of transcription factors (ERG, ETV1, ETV4, and FLI1), and three of which were defined by mutations of the SPOP, FOXA1, and IDH1 genes [17].
The reason for a paucity of molecular aberrations in IDH1-mutated cancers is not obvious. It may, however, be possible that impaired repair efficacy in IDH1-mutated cells may harm particularly such tumor cells that have already acquired a certain degree of genetic instability as indicated by translocations and deletions. In such cases, spontaneous IDH1 mutation may potentiate the risk for accumulating very high—and eventually lethal—numbers of genetic defects. It is thus possible that IDH1 mutations are generally limited to genetically more stable tumor subsets. Of note, these speculations are based on only very few observations.
The detection of IDH1 mutations has potential clinical relevance. Preclinical studies demonstrated that inhibition of mutant IDH1/2 can impair cell growth and promote differentiation in IDH1-mutated glioma and acute myeloid leukemia (AML) cells [25], decrease intracellular 2-HG levels and reverse DNA and histone hypermethylation [26]. At present, numerous clinical studies are recruiting patients with many different cancer types in order to evaluate several drugs targeting mutant IDH1 protein (NCT02746081, NCT02632708, NCT02074839, NCT02073994). Given the high quality of mutation-specific diagnostic antibodies, one could imagine that this mutation results in a highly immunogenic epitope. Diverse vaccines against mutant IDH1/2 have indeed been developed and some of them showed activity in sarcoma and glioma models [27, 28]. In addition, several studies reported better response to chemo- and radiotherapy in IDH1-mutated gliomas [29], possibly as a consequence of altered oxidative stress responses [30]. The high rate of heterogeneity observed for IDH1 mutations may, however, limit therapeutic targeting of these mutations in prostate cancer. It appears very likely that a potential drug or vaccination effect largely depends on whether the entire tumor or only a fraction is IDH1 positive. The analysis of multiple blocks of our prostate cancers for which a positive immunostaining had been detected revealed that all except one IDH1-positive cancers had a heterogeneous mutation status suggesting that IDH1 mutation typically occurs late during tumor progression. Finding one case with homogenous IDH1 mutation, however, indicates that IDH1 mutation can also occur in early stages of the disease—and that anti-IDH1 therapy may be applicable in a very small subset of patients.
In summary, our data show that about 0.3% of prostate cancers have an IDH1R132H mutation and that these are mostly heterogeneous. Once specific anti-IDH1 therapy becomes reality, only a very small group of prostate cancer patients may benefit from such a treatment.
References
Parsons DW, Jones S, Zhang X et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812
Yan H, Parsons DW, Jin G et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773
Watanabe T, Nobusawa S, Kleihues P et al (2009) IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol 174:1149–1153
Eckel-Passow JE, Lachance DH, Molinaro AM et al (2015) Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372:2499–2508
Dang L, White DW, Gross S et al (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462:739–744
Chowdhury R, Yeoh KK, Tian YM et al (2011) The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep 12:463–469
Xu W, Yang H, Liu Y et al (2011) Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 19:17–30
Dang L, Yen K, Attar EC (2016) IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol 27:599–608
Bleeker FE, Atai NA, Lamba S et al (2010) The prognostic IDH1(R132) mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol 119:487–494
Glas M, Bahr O, Felsberg J et al (2011) NOA-05 phase 2 trial of procarbazine and lomustine therapy in gliomatosis cerebri. Ann Neurol 70:445–453
Amary MF, Bacsi K, Maggiani F et al (2011) IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol 224:334–343
Rakheja D, Konoplev S, Medeiros LJ et al (2012) IDH mutations in acute myeloid leukemia. Hum Pathol 43:1541–1551
Shibata T, Kokubu A, Miyamoto M et al (2011) Mutant IDH1 confers an in vivo growth in a melanoma cell line with BRAF mutation. Am J Pathol 178:1395–1402
Murugan AK, Bojdani E, Xing M (2010) Identification and functional characterization of isocitrate dehydrogenase 1 (IDH1) mutations in thyroid cancer. Biochem Biophys Res Commun 393:555–559
Fathi AT, Sadrzadeh H, Comander AH et al (2014) Isocitrate dehydrogenase 1 (IDH1) mutation in breast adenocarcinoma is associated with elevated levels of serum and urine 2-hydroxyglutarate. Oncologist 19:602–607
Kang MR, Kim MS, Oh JE et al (2009) Mutational analysis of IDH1 codon 132 in glioblastomas and other common cancers. Int J Cancer 125:353–355
Cancer Genome Atlas Research N (2015) The molecular taxonomy of primary prostate cancer. Cell 163:1011–1025
Schlomm T, Iwers L, Kirstein P et al (2008) Clinical significance of p53 alterations in surgically treated prostate cancers. Mod Pathol 21:1371–1378
Sauter G, Steurer S, Clauditz TS et al (2016) Clinical utility of quantitative gleason grading in prostate biopsies and prostatectomy specimens. Eur Urol 69:592–598
Capper D, Zentgraf H, Balss J et al (2009) Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol 118:599–601
Gao J, Aksoy BA, Dogrusoz U et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1
Bogdanovic E (2015) IDH1, lipid metabolism and cancer: shedding new light on old ideas. Biochim Biophys Acta 1850:1781–1785
Dang L, White DW, Gross S et al (2010) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 465:966
Mauzo SH, Lee M, Petros J et al (2014) Immunohistochemical demonstration of isocitrate dehydrogenase 1 (IDH1) mutation in a small subset of prostatic carcinomas. Appl Immunohistochem Mol Morphol 22:284–287
Rohle D, Popovici-Muller J, Palaskas N et al (2013) An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 340:626–630
Kernytsky A, Wang F, Hansen E et al (2015) IDH2 mutation-induced histone and DNA hypermethylation is progressively reversed by small-molecule inhibition. Blood 125:296–303
Schumacher T, Bunse L, Pusch S et al (2014) A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 512:324–327
Pellegatta S, Valletta L, Corbetta C et al (2015) Effective immuno-targeting of the IDH1 mutation R132H in a murine model of intracranial glioma. Acta Neuropathol Commun 3:4
Houillier C, Wang X, Kaloshi G et al (2010) IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology 75:1560–1566
Mohrenz IV, Antonietti P, Pusch S et al (2013) Isocitrate dehydrogenase 1 mutant R132H sensitizes glioma cells to BCNU-induced oxidative stress and cell death. Apoptosis 18:1416–1425
Author information
Authors and Affiliations
Contributions
RS, GS, and TS developed the project; AH, MB, MK, CMK, SS, AL, AA, CW, EN, CG, FB, SM, WW, FJ, TSC, TK and MCT collected data; AH, CHM, MK, RS and CS did data analysis; AH, MK, RS, GS and CS wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethics approval
The study was approved by the ethics committee Ärztekammer Hamburg (WF-049/09 and PV3652). The work has been carried out in compliance with the Helsinki Declaration.
Informed consent
According to local laws (HmbKHG, §12,1) informed consent was not necessary.
Rights and permissions
About this article
Cite this article
Hinsch, A., Brolund, M., Hube-Magg, C. et al. Immunohistochemically detected IDH1R132H mutation is rare and mostly heterogeneous in prostate cancer. World J Urol 36, 877–882 (2018). https://doi.org/10.1007/s00345-018-2225-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2225-7