Abstract
Purpose
Recently, prostate-specific membrane antigen-radioguided surgery (PSMA-RGS) has been introduced as a promising new and individual treatment concept in patients with localised recurrent prostate cancer (PC). In the following, we want to review our experience with PSMA-RGS in patients with localised biochemical recurrent PC.
Methods
A non-systematic review of the literature was carried out with focus on technical and logistical aspects of PSMA-RGS. Furthermore, published data on intraoperative detection of metastatic lesions compared to preoperative PSMA-PET and postoperative histopathology, postoperative complications as well as oncological follow-up data are summarized. Finally, relevant aspects on prerequisites for PSMA-RGS, patient selection, and the potential benefit of additional salvage radiotherapy or potential future applications of robotic PSMA-RGS with drop-in γ-probes are discussed.
Results
First results show that PSMA-RGS is very sensitive and specific in tracking suspicious lesions intraoperatively. Prerequisite for patient selection and localisation of tumour recurrence is a positive Ga-HBED-CC PSMA positron-emission tomography (PET) scan with preferably only singular soft tissue or lymph node recurrence after primary treatment. Furthermore, PSMA-RGS has the potential to positively influence oncological outcome.
Conclusions
PSMA-RGS seems to be of high value in patients with localised PC recurrence for exact localisation and resection of oftentimes small metastatic lesions using intraoperative and ex vivo γ-probe measurements. However, patient identification on the basis of Ga-HBED-CC-PSMA PET imaging as well as clinical parameters is crucial to obtain satisfactory results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Prostate cancer (PC) is the most frequent tumour in men worldwide and accounts for the third most cause for cancer-associated death in men [1]. After radical prostatectomy (RP) or radiotherapy (RT) for primary treatment of PC up to 50% of all patients develop tumour recurrence as determined by rising prostate-specific antigen (PSA) values [2]. Therefore, early and sensitive diagnosis of recurrence is crucial for patient counselling and treatment selection.
Several studies have shown that morphological imaging procedures like computed tomography or magnetic resonance imaging as well as functional positron emission tomography (PET) imaging using radiolabelled choline-derivatives are of limited used in the detection of recurrent disease and often underestimate the extent of metastatic spread [3]. The introduction of 68Ga-HBED-CC- prostate-specific membrane antigen (PSMA) and 68Ga-PSMA-I&T based PET hybrid imaging several years ago has revolutionised imaging of PC with significantly improved detection rates in comparison to tracers like 18F-choline in patients with recurrent PC after radical prostatectomy, especially at low PSA values [4,5,6,7,8,9,10,11,12,13]. With the advent of PSMA-ligand PET and the visualisation of even small soft tissue metastases salvage surgery became of increasing interest in recurrent PC. However, intraoperative detection is challenging due to inconspicuous morphology and/or atypical localization of small tissue recurrence. Above, resection might be complicated due to fibrotic changes after previous surgery and/or RT.
PSMA-radioguided surgery (PSMA-RGS)
In April 2014, PSMA-RGS was introduced at the Technical University of Munich in cooperation between the Institute of Pharmaceutical Radiochemistry, Department of Nuclear Medicine and the Department of Urology, as an individual treatment concept [14, 15]. PSMA-RGS uses the preoperative intravenous (i.v.) administration of radioactively labelled PSMA-ligands method to facilitate the intraoperative detection of small and atypically located metastatic PC lesion using a γ-probe. At first, 111Indium-labelled ligands (111In-PSMA-I&T) were used with i.v. injection of mean 150 MBq (range 86-298 MBq) followed by 111In-PSMA-RGS [14]. Later on, the protocol was adapted to a more broadly available 99mTechnetium-labelled PSMA-ligand (99mTc-PSMA-I&S) with i.v. injection of mean 570 MBq (range 221–857 MBq) [16]. After injection of the radioactively labelled PSMA-ligand combined single photon emission computed tomography/computer tomography (SPECT/CT) is performed to validate correct physiological tracer uptake and appropriate tracer uptake of tumour recurrence. Intraoperatively, suspicious lesions are localised and identified using a γ-probe with acoustic feedback (e.g. Crystal Probe, Crystal Photonics) [15, 17]. Resected tissue specimens are measured ex vivo again (rating positive or negative in comparison to background) to validate successful resection immediately. A count rate at least double the background reference is judged as positive.
111In-PSMA-I&T SPECT/CT for preoperative localization of tumour recurrence
In a study of our group comparing preoperative 111In-PSMA-I&T SPECT/CT with preoperative 68Ga-HBED-CC-PSMA PET scan in 22 patients with early biochemical recurrence, 111In-PSMA I&T SPECT/CT detected only 14 out of 29 PET-positive lesions (48.3%) with no additional lesions identified with 111In-PSMA I&T SPECT/CT [18]. A total number of 15 PET-positive lesions were not detectable in 111In-PSMA I&T SPECT/CT, neither prospectively nor retrospectively in knowledge of the PET results. There was a weak trend toward a higher detectability in 111In-PSMA I&T SPECT/CT regarding lesion size and initial PSA level, although this was not statistically significant most probably due to the small number of patients analysed. One major reason for the observed lower sensitivity of 111In-PSMA I&T SPECT/CT compared with 68Ga-PSMA HBED-CC PET has contributed to the principal technical differences between PET and SPECT imaging leading to a highly superior spatial resolution of PET. Therefore, if available, PSMA-ligand PET should be preferred to PSMA SPECT/CT for diagnostic purposes as well as to confirm indication for PSMA-RGS.
99mTc-PSMA-I&S ligands for RGS
The routine clinical application of 111In-PSMA-I&T for PSMA-RGS is limited by its suboptimal nuclear properties, high costs and restricted availability of 111InCl3. To meet the clinical need for a widely available and more cost-effective alternative with less radiation exposure to patients and medical personnel, the PSMA-I&T-based tracer concept was adapted to a 99mTc-labelled PSMA agent (99mTc-PSMA-I&S) for PSMA-RGS [19]. Therefore, in our department, 111In-labelled PSMA-I&T has been completely replaced by 99mTc-labelled PSMA-I&S. First experiences with 99mTc-PSMA-I&S ligands and subsequent RGS indicate its equal performance compared to 111In-PSMA-I&T as a probe for PSMA-RGS. As with 111In-PSMA-I&T, 99mTc-PSMA-I&S can be used as a SPECT imaging agent and might be considered as alternative PC imaging technique in institutions without access to PET. In the following, recently published data of PSMA-RGS with both ligands combined are summarised [20].
Intraoperative detection of soft tissue metastases with PSMA-RGS
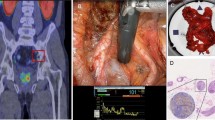
So far, 111In- or 99mTc-based PSMA-RGS has been performed in 63 patients with biochemical recurrent PC (median PSA: 1.29 ng/ml, range 0.19–13.9) after primary treatment (n = 61 RP, n = 1 curative intended RT, n = 1 high-intensity focused ultrasound) at our university hospital. All patients presented with a positive 68Ga-HBED-CC PSMA PET scan before PSMA-RGS proving singular or sporadic soft tissue or lymph node recurrence. Additional preoperative SPECT/CT of the pelvis was performed in all patients after administration of 111In-PSMA-I&T or 99mTc-PSMA-I&S to prove appropriate tracer uptake of tumour recurrence. A representative case of a patient with recurrent PC treated with PSMA-RGS is shown in Fig. 1.
A 67-year old patient with biochemical recurrent PC (PSA 0.39 ng/ml) after radical prostatectomy (pT3b pN1 (2/12) cM0 R0, Gleason score 4 + 3, iPSA 15.4 ng/ml) 6 years ago and salvage radiation therapy 3 years ago. During PSMA-RGS the lesion identified on 68Ga-HBED-CC-PSMA PET/CT was rated as strongly positive by ex vivo acoustic measurement and corresponded to a PSMA-positive lymph node metastasis. a Axial CT shows a small, morphologically unsuspicious lymph node in the right internal region (red arrow). b 68Ga-HBED-CC-PSMA PET/CT shows a distinct signal of this lymph node highly suspicious for metastasis. c Especially in cases of small lymph nodes and location within the depths of the pelvis guidance by γ-probe is helpful for reliable identification. d Ex vivo measurements confirm resection of the PSMA-labelled lymph node
Direct comparison of preoperative 68Ga-HBED-CC-PSMA PET, intraoperative ex vivo measurements and histopathology was available in 60 patients. In all 60 patients, positive lesions were found by intraoperative γ-probe measurements during PSMA-RGS verified by positive histology as well. In three patients, however, suspicious lesions on preoperative 68Ga-HBED-CC-PSMA PET could not be detected during PSMA-RGS. Compared to 68Ga-HBED-CC-PSMA PET, PSMA-RGS was able to detect additional suspicious lesions in 7/60 patients (11.7%), verified by positive histopathology as well, suggesting that intraoperative measurements with PSMA-RGS is more sensitive than preoperative 68Ga-HBED-CC-PSMA PET, most probably due to reduced lesion-to-detector distance. In 14 of our 60 patients (23.3%) histology showed additional metastatic specimens in comparison to 68Ga-HBED-CC-PSMA PET suggesting that histologic evaluation is still more sensitive than preoperative 68Ga-HBED-CC-PSMA PET or measurements during PSMA-RGS. These findings demonstrate that PSMA-RGS is a valid method to track suspicious lesions and potentially identify additional lesions compared to PSMA-PET. However, microscopic lesions still might be missed which underlines the importance of complete resection of tissue adjacent to the intraoperatively detected lesions. This is concordant to a recently published study of Jilg et al. who reported a necessary short diameter of LNM to be ≥ 2.3 and ≥ 4.5 mm to reach a detection rate of 50 and 90% in 68Ga-HBED-CC-PSMA PET [21]. In three patients false positive lesions (positive with γ-probe and unobtrusive in histopathology) were detected. However, it has to be noted that most of them showed only a low to moderate activity count rate when measured with the γ-probe.
Oncological follow-up after PSMA-RGS
In 24 patients (38.1%) complications related to the salvage surgery procedure were observed. However, no specific complication related to the PSMA-RGS procedure and no adverse reaction related to tracer injection were observed. In most patients the complications were regarded as mild (grade 1 as defined by Clavien Dindo classification) such as temporary incontinence, temporary paralytic bladder with prolonged micturition or postoperative lymphedema. Six patients (9.5%) presented with high-grade complications (grade 3b according to Clavien-Dindo classification, e.g. injury of the ureter, perforation of the rectum) and further interventions were required. However, these complication rates are in concordance with results presented in literature [19]. The higher complication rates of salvage surgery procedures compared to the primary surgery setting are attributed not only to fibrotic changes after previous surgery and RT, but also reflects the often challenging location of recurrent PC lesions.
Follow-up information was available in 59/63 patients. A PSA reduction > 50% was observed in 46/59 (78%) patients and a reduction > 90% in 30/59 (51%) patients without additional treatments. In 38/59 (67%) patients a complete biochemical response with a PSA drop below 0.2 ng/ml was observed. Of these 38 patients, 17 patients (45%) are free of biochemical recurrence and without further therapy after a median follow-up of 12.3 (range 6.7–31.9) months. Duration of treatment-free survival as well as biochemical-free survival after PSMA-RGS is shown in Fig. 2.
Prerequisites and appropriate patient selection for PSMA-RGS
First of all, a positive 68Ga-HBED-CC PSMA PET scan with preferably only singular soft tissue or lymph node recurrence after primary treatment is required. Here, standardisation of PET imaging protocols as well as interdisciplinary (urology, nuclear medicine and radiology) side-by-side analysis of 68Ga-HBED-CC PSMA PET can help to improve and standardise its evaluation and interpretation. A study of Rausch et al. has recently identified significant variations in FDG-PET/CT protocol parameters and system performance among different PET/CT users [22]. Subsequently, efforts need to be put in place to further standardise imaging protocols. At a minimum clinical PET/CT operations should ensure compliance with existing guidelines. Therefore, the recently published joint first EANM and SNMMI procedure guidelines for PC imaging can offer assistance to improve the quality of 68Ga-HBED-CC PSMA PET examinations [23].
Although PSMA-RGS seems to reduce tumour burden effectively reflected by the high drop of postoperative PSA level and might, therefore, delay additional systemic treatment, it represents still an individual and not guideline-conform treatment concept without proven benefit on overall surveillance or cancer-specific survival. Therefore, the morbidity and the potential surgical complications of PSMA-RGS have to be weighted against the potential delay of systemic treatment. Individual assessment of each patient prior to PSMA-RGS with critical discussion with each patient is mandatory. Recently, several factors identifying suitable candidates for salvage lymphadenectomy have been defined in order to achieve acceptable cancer control rates and to avoid unnecessary morbidity including a preoperative PSA value < 4 ng/ml, a primary Gleason score < 8 and metastases limited to the pelvis [24, 25]. In a study with patients undergoing secondary lymphadenectomy, it was observed that the 5-year clinical recurrence-free survival was higher for patients with preoperative PSA values below 4 ng/ml compared to patients with a PSA ≥ 4 ng/ml (48 versus 13%) [25, 26]. Further, 5-year clinical recurrence-free survival was lower for patients with positive LN in the retroperitoneum compared to patients revealing positive LN only in the pelvis (11 vs 53%; p < 0.001) [25, 26].
Correspondingly, all of our 17 patients presenting without biochemical recurrence (PSA < 0.2 ng/ml) so far showed a preoperative PSA < 4 ng/ml (median 1.14 ng/ml, range 0.28–3.29) and no extra-pelvic tumour manifestation. Interestingly, 6 of those 17 patients presented with tumour manifestation in more than one localization (e.g. lymph node metastases adjacent to the external iliac artery on both sides). Therefore, constraining salvage surgery procedures only to patients with singular metastases within the pelvis might not be necessary in the era of PSMA-PET imaging. Jilg et al. found a primary Gleason score of 8–10 to be an independent predictor of clinical progression [27]. However, 7 of our 17 recurrence-free patients presented with an initial Gleason score of 8 or 9. Thus, further studies are necessary to confirm relevant clinical parameters for appropriate patient selection.
Adjuvant radiotherapy after PSMA-RGS?
So far, it remains uncertain whether patients can profit from additional adjuvant RT after PSMA-RGS. Rischke et al. evaluated retrospectively the use of adjuvant RT after salvage lymphadenectomy versus salvage lymphadenectomy alone in patients with recurrent PC restricted to pelvic/retroperitoneal lymph node metastases after primary therapy. Adjuvant RT after salvage lymphadenectomy resulted in overall significantly improved 5-year relapse-free survival compared to patients who received salvage lymphadenectomy alone (5-year relapse-free rate 70.7 versus 26.3%) [28]. However, in this study salvage surgery was based on conventional imaging or choline-based PET examinations. Thus, currently, it remains unclear which parameters should trigger adjuvant radiation therapy.
Future vision of robotic PSMA-RGS with drop-in γ-probe
Currently PSMA-RGS procedures at our institution are performed in an open surgical fashion. Reasons for this are oftentimes challenging intraoperative conditions precluding the use of available laparoscopic γ-probes with limited manoeuverability and the lack of γ-probes for robotic intervention. Recently, van Oosterom et al. described a drop-in γ-probe to be inserted via a trocar that can be picked up and manoeuvered by a robotic grasper [29]. However, evaluation of this medical device for intraoperative use is warranted to determine its usability for complex PSMA-RGS procedures requiring subtle and extensive resections.
In summary, PSMA-RGS proved to be of high value for intraoperative detection and identification of small soft tissue metastases. In patients with biochemical recurrent PC, salvage surgery using PSMA-RGS has the potential to delay further disease progression and the need for further systemic treatment (e.g. ADT, chemotherapy). However, identification of suitable patients on the basis of PSMA-PET as well as clinical parameters is crucial to obtain satisfactory results.
References
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64(1):9–29
Boorjian SA, Thompson RH, Tollefson MK, Rangel LJ, Bergstralh EJ, Blute ML et al (2011) Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur Urol 59(6):893–899
Umbehr MH, Muntener M, Hany T, Sulser T, Bachmann LM (2013) The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol 64(1):106–117
Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG et al (2014) Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 41(1):11–20
Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B et al (2015) Evaluation of hybrid (6)(8)Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med 56(5):668–674
Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q et al (2015) Prospective comparison of 18F-fluoromethylcholine versus 68 Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med 56(8):1185–1190
Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M et al (2015) The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 42(2):197–209
Weineisen M, Simecek J, Schottelius M, Schwaiger M, Wester HJ (2014) Synthesis and preclinical evaluation of DOTAGA-conjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer. EJNMMI Res 4(1):63
Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S et al (2015) 68 Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med 56(8):1169–1176
Herrmann K, Bluemel C, Weineisen M, Schottelius M, Wester HJ, Czernin J et al (2015) Biodistribution and radiation dosimetry for a probe targeting prostate-specific membrane antigen for imaging and therapy. J Nucl Med 56(6):855–861
Eder M, Schafer M, Bauder-Wust U, Hull WE, Wangler C, Mier W et al (2012) 68 Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem 23(4):688–697
Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG et al (2016) Sensitivity, specificity, and predictors of positive 68 Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol 70(6):926–937
Meredith G, Wong D, Yaxley J, Coughlin G, Thompson L, Kua B et al (2016) The use of 68 Ga-PSMA PET CT in men with biochemical recurrence after definitive treatment of acinar prostate cancer. BJU Int 118(Suppl 3):49–55
Schottelius M, Wirtz M, Eiber M, Maurer T, Wester HJ (2015) [(111)In]PSMA-I&T: expanding the spectrum of PSMA-I&T applications towards SPECT and radioguided surgery. EJNMMI Res 5(1):68
Maurer T, Weirich G, Schottelius M, Weineisen M, Frisch B, Okur A et al (2015) Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur Urol 68(3):530–534
Robu S, Schottelius M, Eiber M, Maurer T, Gschwend J, Schwaiger M et al (2017) Preclinical evaluation and first patient application of 99mTc-PSMA-I&S for SPECT imaging and radioguided surgery in prostate cancer. J Nucl Med 58(2):235–242
Maurer T, Schwamborn K, Schottelius M, Wester HJ, Schwaiger M, Gschwend JE et al (2016) PSMA theranostics using PET and subsequent radioguided surgery in recurrent prostate cancer. Clin Genitourin Cancer 14(5):e549–e552
Rauscher I, Maurer T, Souvatzoglou M, Beer AJ, Vag T, Wirtz M et al (2016) Intrapatient comparison of 111In-PSMA I&T SPECT/CT and hybrid 68 Ga-HBED-CC PSMA PET in patients with early recurrent prostate cancer. Clin Nucl Med 41(9):e397–e402
Robu S, Schottelius M, Eiber M, Maurer T, Gschwend J, Schwaiger M et al (2016) Preclinical evaluation and first patient application of 99mTc-PSMA-I&S for SPECT imaging and radioguided surgery in prostate cancer. J Nucl Med 58(2):235–242
Horn T, Rauscher I, Eiber M, Gschwend JE, Maurer T (2017) PSMA-radioguided surgery in localised recurrent prostate cancer. Der Urologe Ausg A 56(11):1417–1423
Jilg CA, Drendel V, Rischke HC, Beck T, Vach W, Schaal K et al (2017) Diagnostic accuracy of Ga-68-HBED-CC-PSMA-ligand-PET/CT before salvage lymph node dissection for recurrent prostate cancer. Theranostics 7(6):1770–1780
Rausch I, Bergmann H, Geist B, Schaffarich M, Hirtl A, Hacker M et al (2014) Variation of system performance, quality control standards and adherence to international FDG-PET/CT imaging guidelines. A national survey of PET/CT operations in Austria. Nuklearmedizin 53(6):242–248
Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S et al (2017) 68 Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging 44(6):1014–1024
Rauscher I, Duwel C, Wirtz M, Schottelius M, Wester HJ, Schwamborn K et al (2017) Value of 111In-prostate-specific membrane antigen (PSMA)-radioguided surgery for salvage lymphadenectomy in recurrent prostate cancer: correlation with histopathology and clinical follow-up. BJU Int 120(1):40–47
Rigatti P, Suardi N, Briganti A, Da Pozzo LF, Tutolo M, Villa L et al (2011) Pelvic/retroperitoneal salvage lymph node dissection for patients treated with radical prostatectomy with biochemical recurrence and nodal recurrence detected by [11C]choline positron emission tomography/computed tomography. Eur Urol 60(5):935–943
Suardi N, Gandaglia G, Gallina A, Di Trapani E, Scattoni V, Vizziello D et al (2015) Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: results of a single-institution series with a minimum follow-up of 5 years. Eur Urol 67(2):299–309
Jilg CA, Rischke HC, Reske SN, Henne K, Grosu AL, Weber W et al (2012) Salvage lymph node dissection with adjuvant radiotherapy for nodal recurrence of prostate cancer. J Urol 188(6):2190–2197
Rischke HC, Schultze-Seemann W, Wieser G, Kronig M, Drendel V, Stegmaier P et al (2015) Adjuvant radiotherapy after salvage lymph node dissection because of nodal relapse of prostate cancer versus salvage lymph node dissection only. Strahlentherapie Onkologie 191(4):310–320
van Oosterom MN, Simon H, Mengus L, Welling MM, van der Poel HG, van den Berg NS et al (2016) Revolutionizing (robot-assisted) laparoscopic gamma tracing using a drop-in gamma probe technology. Am J Nucl Med Mol Imaging 6(1):1–17
Author information
Authors and Affiliations
Contributions
I Rauscher: Protocol/project development, Data collection and management, Data analysis, Manuscript writing and editing. T Horn: Data collection and management, Data analysis, Manuscript writing and editing. M Eiber: Protocol/project development, Data analysis, Manuscript writing and editing. JE Gschwend: Protocol/project development, Manuscript writing and editing. T Maurer: Protocol/project development, Data collection and management, Data analysis, Manuscript writing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest or competing interests.
Research involving human participants
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed written consent was obtained from all individual participants included in this study.
Rights and permissions
About this article
Cite this article
Rauscher, I., Horn, T., Eiber, M. et al. Novel technology of molecular radio-guidance for lymph node dissection in recurrent prostate cancer by PSMA-ligands. World J Urol 36, 603–608 (2018). https://doi.org/10.1007/s00345-018-2200-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2200-3