Abstract
Purpose
To assess whether real-time elastography-targeted biopsy (RTE-bx) is superior to the standard systematic transrectal ultrasound (TRUS)-guided biopsy in predicting subsequent prostate cancer (PCa) rates in patients with initially negative biopsy and to specifically reveal differences in the occurrence of high-grade (Gleason ≥ 4 + 3) PCa by comparing both biopsy methods.
Patients and methods
Overall, 630 patients had an initially negative prostate biopsy between 2007 and 2015, either RTE targeted (n = 213) or systematically (n = 417). Follow-up data, ascertained by a questionnaire, of patients receiving RTE-bx were compared to data of patients receiving systematic biopsy (sbx) using Mann–Whitney-U test and Chi-square test. We performed logistic regression analyses to assess any association with PCa or high-grade PCa occurrence.
Results
In total, 258 (41%) patients were diagnosed with PCa at repeat biopsy whereof 54 (8.6%) harboured high-grade PCa. PCa occurred in 95 (44.6%) patients with initially negative RTE-bx and in 163 (39.1%) patients with initially negative sbx (p = 0.003). 24 (11.3%) patients receiving RTE-bx and 30 (7.2%) patients receiving sbx were diagnosed with high-grade PCa (p = 0.095). Logistic regression analyses showed that patients with the initial RTE-bx vs. those with the initial sbx neither resulted in a significant higher risk for PCa occurrence (OR 1.35 [CI 0.87–2.1]; p = 0.2) nor for high-grade PCa occurrence (OR 1.52 [CI 0.66–3.35]; p = 0.3).
Conclusions
We found no statistically significant association of prior biopsy method to subsequent PCa or high-grade PCa occurrence. Referring to our analyses, RTE is not superior to sbx in predicting subsequent PCa rates and, therefore, not eligible to decide on repeat biopsy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer remains the most frequent malignant diagnosis in elderly men in the western world [1]. Still, prostate specific antigen (PSA)-testing and biopsy for detection of prostate cancer (PCa) is discussed controversially. On the one hand, early detection of PCa reduces morbidity and mortality, on the other hand extensive use of diagnostic goes along with a higher rate of overdiagnosis and overtreatment [2]. For definitive diagnosis, most urologists rely on systematic transrectal ultrasound (TRUS)-guided biopsy as the standard of care. However, specificity and sensitivity are nonsatisfying, leading to repeated biopsy if clinical suspicion for PCa persists [3]. This patient pool with initially negative biopsy is at significantly higher risk for insignificant disease, and still, rate of unfavourable pathological result is not negligible [4].
In consequence, better imaging modalities are required to avoid false-negative biopsy. Real-time elastography (RTE) is a promising candidate for PCa detection. In addition to the conventional B-mode imaging, RTE shows the ability to visualize PCa foci in real time. RTE leads to higher detection rates for PCa [5,6,7,8] and improves agreement between biopsy- and pathological-Gleason Score [9].
For further evidence of RTE’s clinical benefit compared to systematic biopsy (sbx), investigation of patients with initially negative biopsy will be of interest. Specifically, RTEs ability to correctly detect patients who are not harbouring PCa. We hypothesized that patients who had an initially negative RTE-targeted biopsy (RTE-bx) may less often harbour clinical significant PCa in repeat biopsies than those who initially underwent standard sbx with negative result. In fact, we expect that initially, negative RTE-bx is superior to initially negative sbx to predict subsequent PCa rates.
To test our hypotheses, patients with initially negative biopsy either performed RTE targeted or systematically were included in a follow-up. Clinical parameters, biopsy method, and results of further biopsies were collected. First, association between biopsy method and positive repeat biopsy was tested. Second, analyses were repeated to test for the occurrence of high-grade PCa.
Patients and methods
Participants
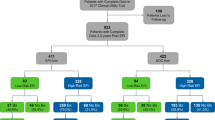
Between 2007 and 2015, 9695 patients underwent the initial TRUS-guided prostate biopsy in our institution, either performed RTE-targeted (n = 2037) or systematically (n = 7658). 3932 of these biopsies were assessed negative. Positive initial biopsy rate for RTE-bx was 51.4 and 61.6% for sbx. 19.4% of positive initial RTE-bx and in 23% of positive initial sbx contained high-grade PCa. We collected complete follow-up data from 713 patients with the initial negative biopsy and excluded patients (n = 83) with less than 6 months of follow-up and negative re-biopsy, leaving 630 patients for the study. Suspicious digital rectal examination (DRE) or an elevated PSA-level > 4 ng/ml indicated for prostate biopsy [10]. Prostate biopsy was performed either by RTE-bx (n = 213) or by sbx (n = 417). RTE-bx consisted of 4-core RTE-targeted biopsy in combination with 10-core systematic biopsy. Sbx included 12-core biopsy of the peripheral zone in a standardized fashion. All data were selected out of the institutional database (FileMaker pro 11, FileMaker, Inc.) used for prospective collection and storage of patients’ data. The study was approved by the local ethics board, and all subjects provided written informed consent.
Elastography and systematic biopsy principles
Two observers performed patients’ elastography examinations of the prostate using a Hitachi Preirus with a V53W endfire probe to aim transrectal biopsy at tumor suspicious areas. RTE visualizes the differences of prostate tissue’s stiffness in real time. Due to higher cell density of malignant tissue, PCa appears as stiffer tissue in elastography examination. By definition, tumor suspicious areas are displayed as reproducible dark blue areas on the video screen [11]. The RTE-bx comprises two cores out of the two most prominent suspicious areas. After targeted biopsy, a systematic sampled ten-core biopsy in the sagittal plane out of the peripheral zone is following.
Sbx as part of the RTE-bx as well as exclusive sbx was accomplished equally according to the EAU guidelines for detection of PCa [10]. After initially negative biopsy, a rising or persistently elevated PSA, suspicious DRE, atypical glands, or extensive high-grade prostatic intraepithelial neoplasia indicated for repeat biopsy [12]. Subsequent biopsies were conducted by German urologists in ambulatory care, whereof 93% adhere to prostate cancer guidelines [13]. Genitourinary pathologists separately analysed all biopsy cores. Origin of the core, length, percentage of tumor, Gleason grade, and sum of every single core is determined in every pathological examination.
Follow-up
Follow-up information was ascertained by a questionnaire sent by email. Phone interviews were conducted in patients, where no email address was available. Information about succeeding biopsies and cancer diagnosis including Gleason grade was collected between April 2015 and June 2016.
Statistical analyses
Medians and interquartile ranges (IQR) were reported for continuous variables. Frequencies and proportions were reported for categorical variables. The Mann–Whitney-U test and Chi-square test were used to compare medians and proportions, respectively. Uni- and multivariable analyses were performed using logistic regression analyses.
All statistical analyses were performed using the JMP software v9.0.2 (SAS Institute, Inc., Cary, NC, USA). Statistical significance was defined as p < 0.05.
Results
In Table 1, the patients’ characteristics and the outcome parameters are shown. Patient’s overall median age was 64 with 63 vs. 64 for RTE-bx vs. sbx (p = 0.8). The median number of cores was 10 for the overall cohort and 14 vs. 10 for RTE-bx vs. sbx (p < 0.001), the median prostate volume was 54 ml for the overall cohort and 56 ml for RTE-bx vs. 53 ml for sbx (p = 0.16), and the median PSA value was 6.6 ng/ml in the overall cohort and 7.4 vs. 6.3 ng/ml for RTE-bx vs. sbx (p = 0.002).
As an endpoint of interest, patients with PCa after initially negative biopsy are opposed to patients who live tumor free at the time of data collection (Table 1). In total, 258 patients were diagnosed with PCa at repeat biopsy (41%). PCa occurred in 44.6% of the patients who first underwent RTE-bx and in 39.1% of the patients who first underwent sbx (p = 0.003). 59% in the overall cohort stayed tumor free, subdivided into 55.4% in the RTE-bx cohort and 60.9% in the sbx cohort. In addition, Gleason ≥ 4 + 3 PCa is analysed separately: overall occurrence rate was 8.6, 11.3% in the RTE-bx cohort vs. 7.2% in the sbx cohort (p = 0.095). Median time of follow-up in case of patients harbouring PCa was 13.8 months for the overall cohort and 16.3 months for RTE-bx vs. 13.0 months for sbx (p = 0.72). Median time of follow-up if patients stayed tumor free for the overall cohort was 32.4 and 49.2 months for RTE-bx vs. 24.6 for sbx (p < 0.001).
In the second part, uni- and multivariable logistic regression analyses were performed. We examined several parameters to check if they are associated with the occurrence of PCa after initially negative biopsy. Our main interest was to assess whether RTE-bx or sbx is associated with a higher occurrence rate of first overall PCa and second Gleason ≥ 4 + 3 PCa. Results are shown in Table 2.
Age occurred as a risk factor for PCa at repeat biopsy (Odds ratio (OR) 1.04 [95%-confidence interval (CI) 1.01–1.07]; p value = 0.005). In contrary, prostate volume was associated with a lower risk of PCa occurrence (OR 0.98 [CI 0.97–0.99]; p < 0.001). PSA value did not emerge as independent risk factor (OR 1.02 [CI 1–1.05]; p = 0.2). Finally, patients who received RTE-bx vs. those receiving sbx did not result in a significant higher risk for PCa occurrence (OR 1.35 [CI 0.87–2.1]; p = 0.2).
In the next step, we separately analysed the same parameters for any association with the occurrence of a Gleason ≥ 4 + 3 PCa. Occurrence rate of Gleason ≥ 4 + 3 PCa in the overall cohort was 7.2%. Age occurred as a risk factor for Gleason ≥ 4 + 3 PCa at repeat biopsy (OR 1.11 [CI 1.04–1.18]; p value < 0.001). In contrary, prostate volume was associated with a lower risk of PCa occurrence (OR 0.97 [CI 0.96–0.99]; p = 0.003). PSA value did not emerge as independent risk factor (OR 1.02 [CI 0.99–1.05]; p = 0.1). Finally, patients who received RTE-bx vs. those receiving sbx did not result in a significant higher risk for PCa occurrence (OR 1.52 [CI 0.66–3.35]; p = 0.3).
Discussion
Definite diagnosis of PCa mainly relies on prostate biopsy. To date, an initially negative biopsy cannot guarantee the absence of PCa [14]. In consequence, efforts of developing better imaging modalities rest on the aim to enhance predictive accuracy of biopsies. Predictive value of being cancer free, if biopsy was negative, ought to be as high as possible. False-negative results at biopsy should be reduced to a minimum. At re-biopsy, PCa detection rates are as high as 30% [15]. Nonetheless, a careful indication for repeat biopsy is of importance to minimize potential unfavourable consequences such as complications from biopsy and inefficiently handling financial and human resources.
The objective of this study was to disclose possible differences in the PCa occurrence rates between patients with initially negative RTE-targeted biopsy and patients with initially negative sbx. The previous studies showed several benefits of RTE-targeted biopsy over sbx alone. For example, agreement of biopsy- and pathological-Gleason Score was significantly higher for RTE-targeted biopsy vs. sbx alone [9]. The question that remained from recent studies was if RTE-targeted biopsy might also show potential benefit to keep PCa occurrence rates after initially negative biopsy low. Therefore, we examined follow-up data for any association with the occurrence of PCa and separately to the occurrence of unfavourable PCa variants (Gleason ≥ 4 + 3).
The current study reveals some interesting findings regarding the value of having an initially negative prostate biopsy. First, when comparing negative RTE-targeted biopsies to negative systematic biopsies, there is a statistically significant difference in the occurrence of consecutive PCa diagnosis (44 vs. 39%, p = 0.003). However, after adjustment for potential confounders, no significant association between repeat RTE-targeted vs. sbx and the occurrence of PCa or Gleason pattern ≥ 4 + 3 variants revealed. Taken together, none of the two biopsy methods is associated with higher rates of subsequent PCa occurrence.
Several studies report on PCa detection rates of RTE-targeted biopsies. Most of them showed promising results in comparison with sbx [7, 16]. Particularly, detection rates for Gleason score greater than 7 increased when using RTE [3, 17]. In contrast, some studies reported limited reliability of RTE prediction [18, 19]. To the best of our knowledge, this is the first study specifically analysing the follow-up data of patients with initially negative biopsies to compare the groups of RTE-targeted biopsies vs. systematic biopsies.
Our results substantiate the findings of Schiffmann et al. [18] that there is a proportionate high rate of PCa diagnosis at repeat RTE-bx. Schiffmann et al. compared clinical characteristics between men at first and repeat RTE-targeted biopsy. They found out that reliability of negative RTE was higher in patients who underwent repeat biopsy than patients who underwent first biopsy. A false-negative rate of 27% in patients at first biopsy was reported. Schiffmann et al. ascertained that there is no selection in Gleason score at false-negative results. In our cohort, PCa occurrence rate is 41%; however, rate of high-risk PCa occurrence is only 8.6%.
Relating our results to the findings of Ganzer et al. [8], our findings cannot confirm their hypothesis that the initial RTE-bx in comparison with the initial sbx reduces the number of unnecessary biopsies following. Ganzer et al. compared PCa findings in 4 core RTE-bx to following tenfold sbx in the initial and repeat biopsies. Study results showed that the likelihood to detect PCa increases significantly, when biopsy is performed RTE targeted. At repeated biopsy, cancer detection rate was 55.2% summed up for both groups, which is even higher than our study investigated.
Regarding the question of the clinical benefit of RTE utilization, the findings in our current study add some useful information to the results of one of our previous RTE studies [9]. Specificity gain for RTE combined biopsy to distinguish between postoperatively favourable and unfavourable PCa, as well as significantly decreasing false-positive rate for PCa cannot be reflected in PCa rates after initially negative biopsy. Our results show that RTE-bx is not superior to sbx in predicting PCa rates after initially negative biopsy. In addition, no significant difference in the subsequent occurrence of PCa variants Gleason ≥ 4 + 3 was revealed, whereas recent studies reported that elastography works best in higher Gleason tumors [3, 20].
There are mainly two possible reasons, why repeat biopsy could be positive for PCa. First, a sampling error might be causal. This means that the initial biopsy has missed malignant tissue and the patient was already harbouring PCa at the first biopsy. Second, the patient did not harbour PCa at the timepoint of the initial biopsy, but developed PCa later on.
Some studies refer to handling negative biopsy and persisting suspicion for PCa. By following 1995 patients for 10 years, Ploussard et al. [21] analysed clinical parameters which are associated with the risk of repeat biopsy. Detection rate for PCa after negative biopsy (7%) was reported to be low. Chun et al. [15] developed a predictive nomogram for repeat biopsy with the intend to simplify decision for repeat biopsy and prediction for PCa rates after initially negative biopsy. Predictor variables used in the nomogram, namely, patient age, DRE, PSA, percent-free PSA, sampling density, and the number of previous negative biopsy sessions, lead to 76% predictive accuracy. By the current study, it is shown that the initial biopsy method is not reliable for further decision to repeat biopsy or to predict subsequent PCa rates.
To handle high rates of repeat biopsies needed for PCa diagnosis, magnetic resonance imaging (MRI) represents an alternative imaging modality for targeting prostate biopsies. It specifically improves PCa detection of tumor located in the anterior or transitional zone [22]. When comparing RTE-targeted biopsies to MRI-targeted biopsies, Junker et al. [23] reported on similar detection rates for RTE and MRI and a significantly higher detection rate of low risk tumors for MRI. In addition, RTE is the more cost-effective technique, which is non-invasive and can be performed under real time. Nam et al. [24] investigated the role of MRI in patients with no prior biopsy. Negative predictive value for MRI score as predictor for cancer was reported to be 85.7% for normal PSA and 66.7% for PSA > 4 ng/ml.
For further elucidation of how RTE can improve targeted biopsy, a European multicentre study with standardized biopsy protocol and RTE equipment has been launched in 2013.
The present study is not without limitations. First, the retrospective character of this study as well as the lack of randomization between RTE-bx and sbx patients represent the most important drawbacks. Second, negative biopsies were performed in our institution, whereas repeat biopsies were also performed by urologists in ambulatory care. Potentially different standards in biopsy settings and performance might have biased the results. Finally, we did not compare equivalent follow-up duration, which might influence on PCa occurrence rate. Follow-up of tumor-free RTE-bx patients (49.2 months) is significantly longer than tumor-free sbx patients’ follow-up (24.6 months) (p < 0.001). PCa diagnosis rates after a comparable follow-up time might be higher for sbx when follow-up time extends. However, median time to PCa diagnosis in patients with initially negative sbx is 13 months. Therefore, we consider it likely, that, typically, PCa would have already been diagnosed in a follow-up period of 24.6 months.
In conclusion, elastography is not eligible to predict PCa rates in case of initially negative biopsy. No significant association of either RTE-bx or sbx to the subsequent occurrence of PCa could be revealed. The clinical benefit of RTE resulting from incremental PCa detection rates could not be reflected. Our findings show that medical attendance after initially negative biopsy remains mandatory due to high PCa occurrence rates in the follow-up.
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics 2016. CA Cancer J Clin 66(1):7–30. https://doi.org/10.3322/caac.21332
Auvinen A, Moss SM, Tammela TL, Taari K, Roobol MJ, Schroder FH, Bangma CH, Carlsson S, Aus G, Zappa M, Puliti D, Denis LJ, Nelen V, Kwiatkowski M, Randazzo M, Paez A, Lujan M, Hugosson J (2016) Absolute effect of prostate cancer screening: balance of benefits and harms by center within the European Randomized Study of Prostate Cancer Screening. Clin Cancer Res 22(1):243–249. https://doi.org/10.1158/1078-0432.CCR-15-0941
Salomon G, Kollerman J, Thederan I, Chun FK, Budaus L, Schlomm T, Isbarn H, Heinzer H, Huland H, Graefen M (2008) Evaluation of prostate cancer detection with ultrasound real-time elastography: a comparison with step section pathological analysis after radical prostatectomy. Eur Urol 54(6):1354–1362. https://doi.org/10.1016/j.eururo.2008.02.035
Resnick MJ, Lee DJ, Magerfleisch L, Vanarsdalen KN, Tomaszewski JE, Wein AJ, Malkowicz SB, Guzzo TJ (2011) Repeat prostate biopsy and the incremental risk of clinically insignificant prostate cancer. Urology 77(3):548–552. https://doi.org/10.1016/j.urology.2010.08.063
Salomon G, Drews N, Autier P, Beckmann A, Heinzer H, Hansen J, Michl U, Schlomm T, Haese A, Steuber T, Graefen M, Becker A (2014) Incremental detection rate of prostate cancer by real-time elastography targeted biopsies in combination with a conventional 10-core biopsy in 1024 consecutive patients. BJU Int 113(4):548–553. https://doi.org/10.1111/bju.12517
Zhang Y, Tang J, Li YM, Fei X, He EH, Li QY, Shi HY (2012) The contribution of strain patterns in characterization of prostate peripheral zone lesions at transrectal ultrasonography. Acta Radiol 53(1):119–126. https://doi.org/10.1258/ar.2011.110504
Aigner F, Pallwein L, Junker D, Schafer G, Mikuz G, Pedross F, Mitterberger MJ, Jaschke W, Halpern EJ, Frauscher F (2010) Value of real-time elastography targeted biopsy for prostate cancer detection in men with prostate specific antigen 1.25 ng/ml or greater and 4.00 ng/ml or less. J Urol 184(3):913–917. https://doi.org/10.1016/j.juro.2010.05.026
Ganzer R, Brandtner A, Wieland WF, Fritsche HM (2012) Prospective blinded comparison of real-time sonoelastography targeted versus randomised biopsy of the prostate in the primary and re-biopsy setting. World J Urol 30(2):219–223. https://doi.org/10.1007/s00345-011-0679-y
Boehm K, Tennstedt P, Beyer B, Schiffmann J, Beckmann A, Michl U, Beyersdorff D, Budaus L, Graefen M, Karakiewicz PI, Salomon G (2016) Additional elastography-targeted biopsy improves the agreement between biopsy Gleason grade and Gleason grade at radical prostatectomy. World J Urol 34(6):805–810. https://doi.org/10.1007/s00345-015-1714-1
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, Matveev VB, Moldovan PC, van den Bergh RC, Van den Broeck T, van der Poel HG, van der Kwast TH, Rouviere O, Schoots IG, Wiegel T, Cornford P (2016) EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. https://doi.org/10.1016/j.eururo.2016.08.003
Konig K, Scheipers U, Pesavento A, Lorenz A, Ermert H, Senge T (2005) Initial experiences with real-time elastography guided biopsies of the prostate. J Urol 174(1):115–117. https://doi.org/10.1097/01.ju.0000162043.72294.4a
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N, European Association of U (2014) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65(1):124–137. https://doi.org/10.1016/j.eururo.2013.09.046
Frohner M, Khan C, Koch R, Schorr SG, Wirth M (2014) Implementation of the S3 prostate cancer guideline in daily clinical practice: results of a survey among urologists. Urologe A 53(10):1500–1503. https://doi.org/10.1007/s00120-014-3518-0
Benecchi L, Pieri AM, Melissari M, Potenzoni M, Pastizzaro CD (2008) A novel nomogram to predict the probability of prostate cancer on repeat biopsy. J Urol 180(1):146–149. https://doi.org/10.1016/j.juro.2008.03.043
Chun FK, Briganti A, Graefen M, Porter C, Montorsi F, Haese A, Scattoni V, Borden L, Steuber T, Salonia A, Schlomm T, Latchemsetty K, Walz J, Kim J, Eichelberg C, Currlin E, Ahyai SA, Erbersdobler A, Valiquette L, Heinzer H, Rigatti P, Huland H, Karakiewicz PI (2007) Development and external validation of an extended repeat biopsy nomogram. J Urol 177(2):510–515. https://doi.org/10.1016/j.juro.2006.09.025
Pallwein L, Mitterberger M, Struve P, Horninger W, Aigner F, Bartsch G, Gradl J, Schurich M, Pedross F, Frauscher F (2007) Comparison of sonoelastography guided biopsy with systematic biopsy: impact on prostate cancer detection. Eur Radiol 17(9):2278–2285. https://doi.org/10.1007/s00330-007-0606-1
Pallwein L, Mitterberger M, Pinggera G, Aigner F, Pedross F, Gradl J, Pelzer A, Bartsch G, Frauscher F (2008) Sonoelastography of the prostate: comparison with systematic biopsy findings in 492 patients. Eur J Radiol 65(2):304–310. https://doi.org/10.1016/j.ejrad.2007.03.032
Schiffmann J, Grindei M, Tian Z, Yassin DJ, Steinwender T, Leyh-Bannurah SR, Randazzo M, Kwiatkowski M, Karakiewicz PI, Hammerer P, Manka L (2016) Limitations of elastography based prostate biopsy. J Urol 195(6):1731–1736. https://doi.org/10.1016/j.juro.2015.12.086
Taverna G, Magnoni P, Giusti G, Seveso M, Benetti A, Hurle R, Colombo P, Minuti F, Grizzi F, Graziotti P (2013) Impact of real-time elastography versus systematic prostate biopsy method on cancer detection rate in men with a serum prostate-specific antigen between 2.5 and 10 ng/mL. ISRN Oncol 2013:584672. https://doi.org/10.1155/2013/584672
Zhu Y, Chen Y, Qi T, Jiang J, Qi J, Yu Y, Yao X, Guan W (2014) Prostate cancer detection with real-time elastography using a bi-plane transducer: comparison with step section radical prostatectomy pathology. World J Urol 32(2):329–333. https://doi.org/10.1007/s00345-012-0922-1
Ploussard G, Nicolaiew N, Marchand C, Terry S, Allory Y, Vacherot F, Abbou CC, Salomon L, de la Taille A (2013) Risk of repeat biopsy and prostate cancer detection after an initial extended negative biopsy: longitudinal follow-up from a prospective trial. BJU Int 111(6):988–996. https://doi.org/10.1111/j.1464-410X.2012.11607.x
Salami SS, Ben-Levi E, Yaskiv O, Ryniker L, Turkbey B, Kavoussi LR, Villani R, Rastinehad AR (2015) In patients with a previous negative prostate biopsy and a suspicious lesion on magnetic resonance imaging, is a 12-core biopsy still necessary in addition to a targeted biopsy? BJU Int 115(4):562–570. https://doi.org/10.1111/bju.12938
Junker D, Schafer G, Kobel C, Kremser C, Bektic J, Jaschke W, Aigner F (2014) Comparison of real-time elastography and multiparametric MRI for prostate cancer detection: a whole-mount step-section analysis. AJR Am J Roentgenol 202(3):W263–W269. https://doi.org/10.2214/AJR.13.11061
Nam RK, Wallis CJ, Stojcic-Bendavid J, Milot L, Sherman C, Sugar L, Haider MA (2016) A pilot study to evaluate the role of magnetic resonance imaging for prostate cancer screening in the general population. J Urol 196(2):361–366. https://doi.org/10.1016/j.juro.2016.01.114
Author information
Authors and Affiliations
Contributions
Protocol/project development: KB, JK, GS, and PD. Data collection or management: GS and PT. Data analysis: KB, PT, and JK. Manuscript writing/editing: JK, KB, GS, AH, MG, and DT.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the local ethics board, and all subjects provided written informed consent.
Rights and permissions
About this article
Cite this article
Kratzenberg, J., Salomon, G., Tennstedt, P. et al. Prostate cancer rates in patients with initially negative elastography-targeted biopsy vs. systematic biopsy. World J Urol 36, 623–628 (2018). https://doi.org/10.1007/s00345-018-2178-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2178-x