Abstract
Purpose
To understand the longitudinal renal function trends in patients undergoing radical nephroureterectomy (RNU) and identify clinicopathologic characteristics associated with estimated glomerular filtration rate (eGFR) recovery.
Methods
147 patients were available for analysis. Longitudinal eGFR trends were assessed by plotting each patient’s eGFR measurements over time. The patient population was dichotomized using eGFR < 60 ml/min/1.73 m2 versus ≥ 60 ml/min/1.73 m2. Cumulative incidence and competing risk regression analysis were used to estimate recovery of postoperative eGFR to the preoperative level and identify clinicopathologic characteristics associated with eGFR recovery.
Results
Median age was 68.7 years and median preoperative eGFR was 55.9 ml/min/1.73 m2. 63.6% were male and 95.8% were white. The cumulative incidence of eGFR recovery was significantly higher in patients with baseline eGFR < 60 ml/min/1.73 m2 compared to those with baseline eGFR ≥ 60 ml/min/1.73 m2 (p = 0.01), with recovery rates at 2 years of 56.6% vs. 27.7%, respectively. Multivariable analysis revealed that preoperative hydronephrosis (HR 1.80) and preoperative eGFR < 60 ml/min/1.73 m2 (HR 1.87) were associated with increased chance of eGFR recovery.
Conclusion
Over half of patients with preoperative eGFR < 60 ml/min/1.73 m2 achieved eGFR recovery within the first 3 years after RNU, and hydronephrosis was a significant predictor of recovery. These findings should be considered when counseling patients regarding chronic kidney disease progression after RNU and timing of perioperative chemotherapy for high risk tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Upper tract urothelial carcinoma (UTUC) is a relatively rare malignancy and accounts for approximately 5% of all urothelial carcinomas [1, 2]. Consideration of renal function is particularly important for patients with UTUC, since many have either preexisting chronic kidney disease (CKD) or comorbidities associated with the development and progression of CKD such as older age, hypertension, diabetes, tobacco use, history of cardiovascular disease, and lower urinary tract obstruction [3]. Clinical practice guidelines recommend radical nephroureterectomy (RNU) for UTUC; however, doing so may lead to increased morbidity and mortality from CKD progression. Thus, patients with low risk tumors may be offered nephron sparing surgery with endoscopic ablation for ureteral tumors, percutaneous resection for renal pelvis tumors that are difficult to manage by flexible ureteroscopy [4, 5], or distal ureterectomy with reimplantation. For these patients, published results have shown that oncologic efficacy between RNU and nephron sparing surgery are comparable in only those with low-grade tumors but accurate grading and staging can still be problematic [6, 7]. Another consideration for patients with UTUC is the use of perioperative cisplatin-based chemotherapy. Since RNU removes functioning nephrons and results in renal function decline, chemotherapy given in the neoadjuvant setting is much more appealing. Two clinical trials (NCT01663285 and NCT01261728) are examining cancer-free survival and pathologic response after neoadjuvant gemcitabine and cisplatin followed by RNU. Full results from the studies are not available, but retrospective data show that adjuvant cisplatin-based chemotherapy after RNU for high-risk tumors may confer benefit with respect to disease-free survival and overall survival [8] but this is an option only for patients with adequate renal function after surgery [9]. Therefore, the ability to predict renal function recovery after RNU is important for counseling patients with respect to CKD progression and eligibility for perioperative and salvage chemotherapy.
Previous studies have identified risk factors associated with CKD progression or impaired renal function after surgery as defined by postoperative estimated glomerular filtration rate (eGFR) < 60 ml/min per 1.73 m2 [10,11,12,13,14,15]. Here, we examine the natural history of renal function recovery after RNU for UTUC in patients treated at a tertiary referral center. Since a widely used criterion for cisplatin eligibility is eGFR of ≥ 60 ml/min per 1.73 m2, we divided our cohort into two groups based on this cutoff. The goals of our study are to report the long-term eGFR trends after RNU and identify clinicopathologic characteristics associated with renal function recovery.
Methods
After Institutional Review Board approval, we queried our institutional kidney cancer database at Memorial Sloan Kettering Cancer Center and identified 147 patients who underwent RNU from 2006 to 2013 for analysis. We excluded 3 patients with unknown race and 26 patients who received platinum chemotherapy. RNU was performed using an open technique in 46 patients and either laparoscopic or robotic technique in 72 patients. The presence of hydronephrosis prior to RNU was identified using either ultrasound or axial imaging and characterized as mild, moderate, or severe; however, for univariable and multivariable analysis, hydronephrosis was dichotomized as absent or present.
eGFR was calculated using the CKD-Epidemiology Collaboration [16] formula as follows:
where S cr is serum creatinine in mg/dL, κ is 0.7 for females and 0.9 for males, α is − 0.329 for females and − 0.411 for males, min indicates the minimum of S Cr/κ or 1, and max indicates the maximum of S Cr/κ or 1. Serum creatinine levels were obtained prior to surgery and at all postoperative visits. The frequency of follow-up visits was risk adapted, and those with high-risk tumors (> pT1) had more frequent visits. In general, follow-up visits occurred every 3–6 months.
The trajectory of each patient’s eGFR was plotted from the preoperative visit to up to 3 years after RNU to examine trends in individual patient trajectories of eGFR. The population was then dichotomized into patients with eGFR ≥ 60 ml/min per 1.73 m2 those with preoperative eGFR of < 60 ml/min per 1.73 m2. Locally weighted scatterplot smoothing was used to examine the overall trends in eGFR trajectory over time according to preoperative eGFR. Associations between clinicopathologic variables and preoperative eGFR were analyzed using Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
The primary endpoint of this study was postoperative recovery of eGFR to preoperative levels within a 5% margin of error [17]. The cumulative incidence of eGFR recovery was estimated, with patients censored if they did not achieve eGFR recovery by the date of their last eGFR measurement or at 36 months, whichever occurred first, and with death from any cause treated as a competing event. Gray’s test was used for between group comparisons, and competing risks regression was used for multivariable analysis.
We considered a p value of < 0.05 to represent a statistically significant difference. All analyses were conducted using R software, version 3.1.0 (R Core Development Team, Vienna, Austria) including the ‘cmprsk’ and ‘survival’ packages.
Results
118 patients with upper tract urothelial carcinoma who underwent RNU were available for analysis. Three patients (2.5%) had a preoperative eGFR of > 90 ml/min per 1.73 m2, 43 (36.4%) had a preoperative eGFR between 60 and 89 ml/min per 1.73 m2, 68 (57.6%) had a preoperative eGFR between 30 and 59 ml/min per 1.73 m2, 3 (2.5%) had a preoperative eGFR between 15 and 29 ml/min per 1.73 m2, and 1 (0.8%) had a preoperative eGFR of < 15 ml/min per 1.73 m2. We dichotomized the patient population into two groups. The high eGFR group (preoperative CKD stages 1 and 2) had preoperative eGFR of ≥ 60 ml/min per 1.73 m2 (n = 46, 39.0%) and the low eGFR group (preoperative CKD stages 3–5) had preoperative eGFR of < 60 ml/min per 1.73 m2 (n = 72, 61.0%). The median preoperative eGFR was 55.9 (interquartile range (IQR): 45.8, 69.8) ml/min per 1.73 m2 for the entire cohort, 74.7 (IQR: 66.0, 80.4) ml/min per 1.73 m2 for the high eGFR group, and 47.8 (IQR: 39.3, 54.3) ml/min per 1.73 m2 for the low eGFR group. Other relevant clinicopathologic characteristics are shown in Table 1. The median patient age was 68.7 years. 63.6% patients were male, and 95.8% patients were white. 55.9% had hypertension, 16.1% were diabetic, 18.6% had coronary artery disease, and 64.4% were smokers. Patients in the low eGFR group were significantly older (72.2 vs 64.0 years, p < 0.001), had a significantly higher BMI (29.2 vs. 27.0 kg/m2, p = 0.044), and were more likely to have hypertension (65.3% vs. 41.3%, p = 0.014).
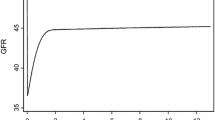
There were a total of 1962 eGFR values available for analysis, and patients had between 2 and 115 measurements over the 36-month follow-up period (median = 11). Each patient’s longitudinal postoperative eGFR values are plotted in Fig. 1. Patients in both groups had declining eGFR after surgery that later began to recover. Over a median follow-up period of 10.8 months (minimum = 0.03, maximum = 36), 56 (47.4%) of patients experienced complete renal function recovery to their preoperative eGFR, and 8 (6.7%) died without renal function recovery. Fifteen patients in the high eGFR group and 41 patients in the low eGFR group had complete renal function recovery. The median time to renal function recovery was 31.6 months (95% confidence interval (CI) 21.4, not reached (NR)). In the low eGFR group, median time to renal function recovery was 1.6 months (95% CI 0.1, NR) whereas in the high eGFR group, median time to renal function recovery was 35.9 months (95% CI 28.5, NR). The cumulative incidence of complete renal function recovery was significantly higher in the low as compared to the high eGFR group (Fig. 2) (p = 0.010). Estimates of the cumulative incidence of renal function recovery in the low and high eGFR groups were 53.1 and 19.8%, respectively, at 12 months and 56.6 and 27.7%, respectively, at 24 months. There was no significant difference in death without renal function recovery between the two groups (p = 0.738).
Longitudinal trends of eGFR values after radical nephroureterectomy. Individual patient trajectories of postoperative eGFR were plotted for patients with preoperative eGFR ≥ 60 ml/min per 1.73 m2 (green dotted lines) and patients with postoperative eGFR < 60 ml/min per 1.73 m2 (blue dotted lines). Locally weighted scatterplot smooths by preoperative eGFR are shown with solid lines
Cumulative incidence of eGFR recovery after radical nephroureterectomy. The solid black line indicates patients with preoperative eGFR ≥ 60 ml/min per 1.73 m2 and the dotted black line indicates patients with preoperative eGFR < 60 ml/min per 1.73 m2. Gray lines indicate cumulative incidences of death in the two groups
To examine differences in association between clinicopathologic variables and renal function recovery by eGFR group, we first tested for interaction effects in competing risks regression models. The only significant interaction effect found was between gender and eGFR group (p = 0.022), such that males in the high eGFR group have significantly increased chance of renal function recovery, whereas there was no difference between males and females in the low eGFR group. Since no other factor differed by preoperative eGFR group, data were analyzed overall. We performed univariable competing risks regression analysis on clinicopathologic factors associated with complete renal function recovery, and the results are presented in Table 2. Higher body mass index (BMI) (p = 0.048), larger tumor size (p = 0.005), open versus laparoscopic or robotic surgery (p = 0.035), presence of hydronephrosis (p = 0.001), and preoperative eGFR < 60 versus ≥ 60 (p = 0.012) were all associated with increased chance of eGFR recovery. Factors incorporated in multivariable analysis were determined through a combination of clinical and statistical considerations. On multivariable analysis, only hydronephrosis (HR 1.8; 95% CI 1.05–3.11, p = 0.034) and preoperative eGFR of < 60 ml/min per 1.73 m2 (HR 1.87; 95% CI 1.02–3.45, p = 0.044) were significantly associated with increased risk of renal function recovery (Table 3).
Discussion
Cancer control and preservation of renal function are the primary goals of UTUC management. Factors that can make preservation of renal function particularly challenging in this group of patients include high prevalence of CKD, comorbidities associated with the development and progression of CKD, and need for RNU for high-risk tumors. Moreover, in some areas of the world, environmental toxins such as aristolochic acid can function both as a nephrotoxin and as an etiologic agent for UTUC [18]. Use of perioperative cisplatin-based chemotherapy is also an important consideration for patients with high-risk tumors. Retrospective data suggest a benefit for both neoadjuvant [19,20,21] and adjuvant [8, 22] chemotherapy; but due to the high prevalence of CKD in this population, approximately half of those who have preoperative eGFR of > 60 ml/min per 1.73 m2 become cisplatin-ineligible after RNU [9, 23, 24]. Thus, it is generally accepted that cisplatin is best given prior to RNU for practical reasons. The ability to predict renal function recovery and estimate the timeframe in which we expect it to occur would be valuable for counseling patients with respect to CKD progression and cisplatin eligibility after RNU.
We performed this study to better understand the longitudinal eGFR trends in patients who undergo RNU for UTUC. Since the most common renal function requirement for cisplatin eligibility is eGFR of ≥ 60 ml/min per 1.73 m2, we divided our cohort in two groups. The high eGFR group had a preoperative eGFR of ≥ 60 ml/min per 1.73 m2, whereas the low eGFR group had a preoperative eGFR of < 60 ml/min per 1.73 m2. Over the study period, the cumulative incidence of complete renal function recovery was significantly lower in high eGFR group with only 19.8% at 12 months and 27.7% at 24 months. These data suggest that, if a patient with a high-risk UTUC has a borderline preoperative eGFR (~ 60 ml/min per 1.73 m2), the opportunity to receive cisplatin may only be available in the neoadjuvant setting since there is a high likelihood that renal function may not recover sufficiently for the patient to be cisplatin-eligible in the adjuvant setting. Moreover, those who actually experience renal function recovery may not do so for years after RNU, making salvage cisplatin-based chemotherapy an unlikely option if disease progression occurs.
In contrast to patients in the high eGFR group, those in the low eGFR group were more likely to experience complete renal function recovery at 12 (53.1%) and 24 (56.6%) months. The underlying biological mechanisms responsible for this difference are unclear, but it is possible that prior to definitive surgical intervention, contralateral kidney compensation has begun and hence facilitating this renal functional recovery. We have also observed this phenomenon in our cohort of patients who underwent radical nephrectomy for renal cell carcinoma [17]. Our findings have important implications with respect to surgical management of UTUC and CKD progression. Conservative management of UTUC consisting of endoscopic ablation, percutaneous resection, or segmental resection is typically offered electively for those with low-risk tumors and imperatively for those with solitary kidney, bilateral UTUC, or severe or end stage CKD at risk for dialysis if RNU is performed. When deciding the appropriate management strategy for patients with moderate to severe CKD, strong consideration should be given to the fact that a significant fraction will achieve renal function recovery after RNU and potentially avoid dialysis.
Of the clinicopathologic factors available for analysis, only hydronephrosis and preoperative eGFR of < 60 ml/min per 1.73 m2 were associated with return to preoperative eGFR. There are mixed results in the literature regarding effect of hydronephrosis on renal function recovery after RNU. Rodriguez Faba et al. published a retrospective analysis on 546 patients who underwent RNU and found that preoperative hydronephrosis was a significant predictor for a postoperative eGFR < 60 ml/min per 1.73 m2 with OR = 10.34 [13]. Similarly, Hashimoto et al. found that hydronephrosis was a negative predictor for postoperative eGFR in 110 patients who underwent RNU [14]. However, our data agree with other reports showing hydronephrosis is actually associated with decreased risk of renal function decline after RNU [10, 25]. One possible mechanism that explains this finding is that hydronephrosis and obstruction result in a poorly functioning kidney such that the contralateral kidney has already functionally adapted to contribute to most of the observed renal function. Therefore, surgical extirpation of the hydronephrotic kidney leads to a higher probability of renal function recovery. In patients without hydronephrosis, a higher percentage of functional nephrons are being removed by RNU, resulting in a decreased likelihood of renal function recovery. This finding is particularly important in patients with preoperative eGFR of ≥ 60 ml/min per 1.73 m2, since those with hydronephrosis will be more likely to be cisplatin-eligible after RNU. We do not routinely perform renal scintigraphy to calculate differential function prior to performing RNU and acknowledge that data from such studies could shed light on the unexpected effect of hydronephrosis on renal function recovery.
Our study is limited by the relatively small sample size and its retrospective nature. Since UTUC is a relatively uncommon malignancy, it would be necessary to validate our findings in a multi-institutional database of patients undergoing RNU. We also did not have detailed information regarding medical comorbidities that can potentially affect renal function such as severity of hypertension, diabetes, and cardiovascular disease. Non-random drop-out may also have occurred which may be a potential source of bias. Additionally, median follow-up time for this population was 10.8 months; however, median time to eGFR recovery was 31.6 months. A prospective study with longer median follow-up will be needed to confirm time to eGFR recovery.
Conclusion
In patients undergoing RNU, those with preoperative eGFR < 60 ml/min per 1.73 m2 are more likely to achieve long-term renal function recovery compared with those with preoperative eGFR of ≥ 60 ml/min per 1.73 m2. Hydronephrosis is a significant predictor for renal function recovery and should be considered when counseling patients regarding CKD progression and the timing of perioperative chemotherapy for high-risk tumors. Future studies should include preoperative renal scintigraphy data to determine whether the contralateral kidney has already functionally compensated for the hydronephrotic kidney.
References
Munoz JJ, Ellison LM (2000) Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol 164(5):1523–1525
Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics. CA Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21387
Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI (2014) KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63(5):713–735. https://doi.org/10.1053/j.ajkd.2014.01.416
Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, Cowan NC, Bohle A, Van Rhijn BW, Kaasinen E, Palou J, Shariat SF (2015) European association of urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol 68(5):868–879. https://doi.org/10.1016/j.eururo.2015.06.044
Network NCC Bladder Cancer Version 1.2017—December 21, 2016. https://www.nccn.org/professionals/physician_gls/PDF/bladder.pdf. Accessed Jan 16, 2017
Simhan J, Smaldone MC, Egleston BL, Canter D, Sterious SN, Corcoran AT, Ginzburg S, Uzzo RG, Kutikov A (2014) Nephron-sparing management vs radical nephroureterectomy for low- or moderate-grade, low-stage upper tract urothelial carcinoma. BJU Int 114(2):216–220. https://doi.org/10.1111/bju.12341
Seisen T, Peyronnet B, Dominguez-Escrig JL, Bruins HM, Yuan CY, Babjuk M, Bohle A, Burger M, Comperat EM, Cowan NC, Kaasinen E, Palou J, van Rhijn BW, Sylvester RJ, Zigeuner R, Shariat SF, Roupret M (2016) Oncologic outcomes of kidney-sparing surgery versus radical nephroureterectomy for upper tract urothelial carcinoma: a systematic review by the EAU non-muscle invasive bladder cancer guidelines panel. Eur Urol 70(6):1052–1068. https://doi.org/10.1016/j.eururo.2016.07.014
Leow JJ, Martin-Doyle W, Fay AP, Choueiri TK, Chang SL, Bellmunt J (2014) A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol 66(3):529–541. https://doi.org/10.1016/j.eururo.2014.03.003
Kaag MG, O’Malley RL, O’Malley P, Godoy G, Chen M, Smaldone MC, Hrebinko RL, Raman JD, Bochner B, Dalbagni G, Stifelman MD, Taneja SS, Huang WC (2010) Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol 58(4):581–587. https://doi.org/10.1016/j.eururo.2010.06.029
Hoshino K, Kikuchi E, Tanaka N, Akita H, Ito Y, Miyajima A, Jinzaki M, Oya M (2012) Preoperative hydronephrosis: independent predictor for changes in renal function following nephroureterectomy. Jpn J Clin Oncol 42(3):202–207. https://doi.org/10.1093/jjco/hyr199
Kaag M, Trost L, Thompson RH, Favaretto R, Elliott V, Shariat SF, Maschino A, Vertosick E, Raman JD, Dalbagni G (2014) Preoperative predictors of renal function decline after radical nephroureterectomy for upper tract urothelial carcinoma. BJU Int 114(5):674–679. https://doi.org/10.1111/bju.12597
Raman JD, Lin YK, Kaag M, Atkinson T, Crispen P, Wille M, Smith N, Hockenberry M, Guzzo T, Peyronnet B, Bensalah K, Simhan J, Kutikov A, Cha E, Herman M, Scherr D, Shariat SF, Boorjian SA (2014) High rates of advanced disease, complications, and decline of renal function after radical nephroureterectomy. Urol Oncol 32(1):47 e49-14. https://doi.org/10.1016/j.urolonc.2013.06.015
Rodriguez Faba O, Palou J, Breda A, Maroto P, Fernandez Gomez JM, Wong A, Villavicencio H (2014) Predictive factors for impaired renal function following nephroureterectomy in upper urinary tract urothelial cell carcinoma. Urol Int 92(2):169–173. https://doi.org/10.1159/000353652
Hashimoto T, Ohno Y, Nakashima J, Gondo T, Nakagami Y, Namiki K, Horiguchi Y, Yoshioka K, Ohori M, Tachibana M (2015) Prediction of renal function after nephroureterectomy in patients with upper tract urothelial carcinoma. Jpn J Clin Oncol 45(11):1064–1068. https://doi.org/10.1093/jjco/hyv136
Singla N, Gayed BA, Bagrodia A, Krabbe LM, Palazzi KL, Mirheydar H, Harrow B, Jacobs C, Youssef R, Darwish O, Sagalowsky A, Lotan Y, Derweesh I, Margulis V (2015) Multi-institutional analysis of renal function outcomes following radical nephroureterectomy and partial ureterectomy for upper tract urothelial carcinoma. Urol Oncol 33(6):268 e261–267. https://doi.org/10.1016/j.urolonc.2015.03.006
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Zabor EC, Furberg H, Mashni J, Lee B, Jaimes EA, Russo P (2016) Factors associated with recovery of renal function following radical nephrectomy for kidney neoplasms. Clin J Am Soc Nephrol 11(1):101–107. https://doi.org/10.2215/CJN.04070415
Lai MN, Wang SM, Chen PC, Chen YY, Wang JD (2010) Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J Natl Cancer Inst 102(3):179–186. https://doi.org/10.1093/jnci/djp467
Matin SF, Margulis V, Kamat A, Wood CG, Grossman HB, Brown GA, Dinney CP, Millikan R, Siefker-Radtke AO (2010) Incidence of downstaging and complete remission after neoadjuvant chemotherapy for high-risk upper tract transitional cell carcinoma. Cancer 116(13):3127–3134. https://doi.org/10.1002/cncr.25050
Porten S, Siefker-Radtke AO, Xiao L, Margulis V, Kamat AM, Wood CG, Jonasch E, Dinney CP, Matin SF (2014) Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer 120(12):1794–1799. https://doi.org/10.1002/cncr.28655
Aziz A, Dobruch J, Hendricksen K, Kluth LA, Necchi A, Noon A, Rink M, Roghmann F, Seiler R, Gontero P, Kassouf W, Shariat SF, Xylinas E, Young Academic Urologists Urothelial Carcinoma Group of the European Association of U (2017) Perioperative chemotherapy in upper tract urothelial carcinoma: a comprehensive review. World J Urol. https://doi.org/10.1007/s00345-016-1995-z
Seisen T, Krasnow RE, Bellmunt J, Rouprêt M, Leow JJ, Lipsitz SR, Vetterlein MW, Preston MA, Hanna N, Kibel AS, Sun M (2017) Effectiveness of adjuvant chemotherapy after radical nephroureterectomy for locally advanced and/or positive regional lymph node upper tract urothelial carcinoma. J Clin Oncology 35(8):852–860
Lane BR, Smith AK, Larson BT, Gong MC, Campbell SC, Raghavan D, Dreicer R, Hansel DE, Stephenson AJ (2010) Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer 116(12):2967–2973. https://doi.org/10.1002/cncr.25043
Xylinas E, Rink M, Margulis V, Clozel T, Lee RK, Comploj E, Novara G, Raman JD, Lotan Y, Weizer A, Roupret M, Pycha A, Scherr DS, Seitz C, Ficarra V, Trinh QD, Karakiewicz PI, Montorsi F, Zerbib M, Shariat SF, Collaboration U (2013) Impact of renal function on eligibility for chemotherapy and survival in patients who have undergone radical nephro-ureterectomy. BJU Int 112(4):453–461. https://doi.org/10.1111/j.1464-410X.2012.11649.x
Singla N, Hutchinson R, Haddad A, Sagalowsky A, Lotan Y, Margulis V (2016) Preoperative hydronephrosis is associated with less decline in renal function after radical nephroureterectomy for upper tract urothelial carcinoma. Can J Urol 23(4):8334–8341
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, B.H., Zabor, E.C., Tennenbaum, D. et al. Renal function recovery after radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol 36, 257–263 (2018). https://doi.org/10.1007/s00345-017-2139-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-017-2139-9