Abstract
Purpose
To report the etiology, presenting symptoms and outcomes of the different treatments performed in female patients with recurrent urethral stricture.
Materials and methods
Twenty-six patients with refractory LUTS were diagnosed with a urethral stricture. The symptoms, the treatment performed and the outcomes were prospectively recorded. Sixteen patients were treated with a urethroplasty using a buccal mucosal graft (BMG) in 14 cases (54 %) and a vaginal flap in 2 (8 %). Urethral dilatation, optical urethrotomy and meatoplasty were performed in 8 (31 %), 1 (3.8 %) and 1 (3.8 %) patients, respectively.
Results
Strictures were idiopathic in 11 patients (42 %). Previous urethral instrumentation and traumatic vaginal delivery were the commonest causes of urethral stricture (42 and 15 %, respectively). The most frequent symptoms were reduced flow (93 %), detrusor overactivity (50 %) and UTIs (42 %). The stricture was cured in 93 % of patients treated with a BMG urethroplasty and in all the patients in which a vaginal flap urethroplasty was performed. In the same group, the improvement in urethral pain was observed in the 67 and the 88 % of patients were cured from recurrent UTIs. All the patients treated with urethral dilatation needed further dilatations; hence, the cure of the stricture was achieved in none of them. Improvement in urethral pain, UTIs and detrusor activity was not recorded in the latter group.
Conclusion
Urethroplasty in its various forms has demonstrated in the present series the highest cure rate for the treatment of recurrent urethral stricture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urethral strictures in females are considered rare, and there is a lack of consensus regarding diagnosis and treatment. Up to 8 % of female patients with lower urinary tract symptoms (LUTS) are diagnosed with bladder outflow obstruction (BOO), and a urethral stricture is demonstrated in 4–13 % [1–4], i.e., 0.32–1 % of women with LUTS.
The presenting symptoms are variable and non-specific. Poor flow, urine dribbling, recurrent UTIs and urethral pain are commonly observed, but storage LUTS are not unusual [5]. Thorough investigation is essential for diagnosis, to plan appropriate treatment, and to exclude differential diagnoses such as malignancy, functional BOO and urethral diverticulum. Uroflowmetry with measurement of the post-void residual (PVR) represents the first level investigation, while urethroscopy and videourodynamics are performed to confirm the presence of a urethral stricture, its location and to exclude concomitant conditions of the lower urinary tract. An MRI of the pelvis may also be performed to assess for anatomical abnormalities of urethral or periurethral tissues or features suggestive of malignancy.
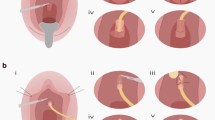
Several treatments have been reported and include dilatation, urethrotomy and meatoplasty for short or distal strictures. Patients undergoing urethral dilatation, typically the first-line treatment, require further intervention in more than 50 % of cases [4]. In those who have a history of recurrent stricture, a further intervention is required in 73 % [4]. In cases of severe or recurrent stricture, a urethroplasty is indicated and small series have reported success rates ranging between 80 and 100 % [6, 7]. There are different techniques of urethroplasty based on flaps and grafts. The vaginal flap urethroplasty is performed using a reverse U-shaped anterior flap that replaces the urethra at the level of the stricture as a ventral or dorsal inlay to restore an adequate caliber. A common graft used for female urethroplasty is the buccal mucosa. An adequate size graft is harvested from the inner cheek and is sutured to the healthy urethra proximally and distally to the stricture. In this case, the vascular supply is guaranteed by the spongiosa and further support can be achieved, harvesting a Martius fat pad that will represent the vascular bed for the graft.
In the present study, we report our experience with the management of severely symptomatic, urethral strictures in twenty-six female patients referred with refractory LUTS. We assessed the etiology, presenting symptoms and outcomes of the different treatments performed for this difficult group.
Methods
We retrospectively reviewed our prospectively gathered outpatient and videocystometrogram (VCMG) databases (N = 535) for all female patients referred to our center with refractory LUTS during the time period January 1, 2007, and December 31, 2014. A detailed clinical history was gathered to highlight the presenting symptoms and the possible causes for the stricture. In particular, the presence of urethral pain, recurrent UTIs, overactive bladder (OAB) symptoms, stress urinary incontinence (SUI) and reduced flow were documented. The preoperative evaluation protocol performed in our institution (also for the patients diagnosed elsewhere) included physical examination, a flow rate with PVR and a cystourethroscopy in all patients. A VCMG was performed for all 22 patients requesting urethroplasty (85 %) but not for those requesting continued endoscopic management. An MRI of the pelvis was also performed in 14 (53 %) of these patients who had worrisome physical findings suggestive of possible malignancy. The VCMG was performed with a filling rate of 50 ml/min of contrast solution through a 6-Fr dual lumen catheter. Fluoroscopy images were recorded every 100 ml of bladder filling and during the voiding phase. Urethrographic evidence of urethral ballooning proximal to a segment of narrow urethra and a detrusor pressure at Qmax (pDetQmax) > 2 × Qmax were considered diagnostic for a urethral obstruction (Solomon-Greenwell pressure flow nomogram [8] (Fig. 1). A diagnosis of stricture was only made in those patients with elevated VCMG pressure flow and radiographic evidence of BOO on voiding study (if possible) combined with cystoscopic evidence of an anatomical stricture.
Direct visualization with cystourethroscopy was performed in all patients in our institution. Patients with VCMG pressure flow and radiographic evidence of BOO without evidence of anatomical stricture went on to have urethral pressure profilometry (N = 46, 8.5 %) to assess for functional sphincteric BOO. Dynamic urethral pressure profile (UPP) was performed using a 9-Fr catheter withdrawn along the urethra with a mechanical arm at a rate of 1 mm/sec using a perfusion rate of 50 ml/min, and the maximal urethral closure pressure (MUCP) was calculated from the average of three consecutive measurements. A value of MUCP = 92 patient age in years was considered within the normal range [9].
In our practice, patients with newly diagnosed urethral stricture are offered a urethral dilatation or a urethrotomy in the first instance. For patients with a recurrent stricture, urethral dilation or urethrotomy with post-procedure weekly intermittent self-dilatation (ISD) or a repair with meatoplasty for distal strictures or a urethroplasty with buccal mucosal graft (BMG) or vaginal flap are offered.
The outcomes of urethral stricture treatment have been classified according to the treatment received. Uroflowmetry and PVR were performed in all patients postoperatively. All urethroplasty patients were requested to have VCMG at 3 months post-surgery to assess for urodynamic relief of obstruction, but only 10 (38 %) agreed to this. Success was defined as resolution of bladder outlet obstruction (BOO) as determined by: resolution of voiding symptoms with no requirement for further urethral dilatations or intermittent self-catheterization, improvement in flow rate and post-void residual and resolution of BOO on VCMG when available. Secondary outcome measures were cure of pain and OAB symptoms, improvement in frequency of UTI’s and development of new-onset SUI.
Results
Twenty-six (4.9 %) female patients referred with LUTS were diagnosed with a urethral stricture in this 8-year period (mean age 45.8 years; range 30–71). Urethral stricture was diagnosed in another institution in twenty patients (77 %): Nineteen (73 %) failed previous urethral dilatations/urethrotomies, and in one patient (4 %) a previous V–Y plasty was not successful. Thirteen patients (50 %) were performing intermittent self-dilatation (ISD) on a regular basis prior to being treated in our department.
Previous urethral instrumentation including traumatic catheterization was the most common cause of urethral stricture (n = 11; 42 %) followed by traumatic vaginal delivery in 4 patients (15 %). Strictures were idiopathic in 11 patients (42 %). The preoperative symptoms are reported in Table 1. The most frequent complaint was a significantly reduced flow (93 %), but recurrent UTIs, urethral pain and obstruction induced OAB were not uncommon.
The urethral stricture was identified with cystoscopy in all patients. Reduced flow was confirmed with uroflowmetry in all 26 patients (average Qmax = 9.5 ml/sec, range 7–32 ml/sec). The VCMG was performed in the 22 patients considering urethroplasty and was diagnostic of BOO in all: A pDetQmax > 2 x Qmax was reported for every patient (average pDetQmax = 51 ± 21 cmH20; average Qmax = 10 ± 6 cmH20), while proximal ballooning to the stricture was evident in the 18 patients (84 %) able to void in a standing position. The remaining four patients were only able to void in a sitting position, and screening of the voiding phase was not possible. Preoperative detrusor overactivity (DO) and SUI were urodynamically diagnosed in 6 (23 %) and 2 (8 %) patients, respectively. A pelvic MRI was performed in 14 patients for the indications described. No tumors or other pelvic abnormalities were noted. Interestingly, the MRI was able to identify a urethral stricture in 86 % of these cases (n = 12).
Eight patients (31 %) decided to opt for a cystoscopy and urethral dilatation (6 new presentation and 2 recurrent strictures); 1 (3.8 %) chose a urethrotomy (recurrent stricture); and 1 (3.8 %) with a recurrent distal stricture chose a meatoplasty. Sixteen patients with recurrent stricture were treated with a urethroplasty using a buccal mucosal graft (BMG) in 14 cases (54 %) and a vaginal flap in 2 (8 %).
The treatment outcomes have been gathered after a mean follow-up of 35.6 months (range 15–96), and the results are reported in Table 2.
The most satisfactory results have been achieved with an open urethroplasty. Both patients in whom a vaginal flap was used have had no recurrence of their stricture to date. Their postoperative uroflowmetry showed an average Qmax of 18.5 ml/sec (range 12–25) and an average PVR of 15.5 ml (range 0–31). In the same group, pain and OAB symptoms resolved after surgery and neither patient needed further urethral dilatation, ISD or developed SUI.
For the 14 patients treated with a BMG urethroplasty, there has been no evidence of recurrent stricture in 93 % of patients and 67 % reported a resolution of urethral pain. Postoperative uroflowmetry showed an average Qmax of 16.2 ml/sec (range 6–37) and an average PVR of 58 ml (range 0–170). Cure of recurrent UTIs was achieved in 88 % of the patients complaining of this symptom before surgery, and only 1 patient (8 %) required a further urethral dilatation for a recurrent obstructive stricture occurring 26 months post-surgery. New-onset mild SUI was diagnosed in 3 patients (23 %) but did not require further intervention and settled with pelvic floor muscle training (PFMT).
Of the 8 patients who had a urethral dilatation under our care, resolution of urethral pain was not achieved in any. A temporary improvement was seen with uroflowmetry in 4 patients (50 %) (average Qmax 20 ml/sec range 10–38, average PVR 20 ml range 0–56), but all the patients needed further dilatations (50 %) or regular intermittent self-dilatations (63 %), and for this reason their stricture has been considered to have recurred.
Discussion
Urethral strictures in females are considered rare, but there is an increasing interest in this condition. The incidence of BOO is reported to be 2.7–8 % in female patients with voiding LUTS [1]. Excluding concomitant conditions, such as cystocele or functional obstruction, BOO can be caused by a urethral stricture in 4–13 % of female patients [2, 3], i.e., urethral stricture may be the cause of BOO in 0.1–1.0 % of female patients. In the present series, we report a higher incidence, with 4.9 % of all female patient, presenting with LUTS having a urethral stricture, which is likely related to the fact that our institute represents a high-volume tertiary referral center with experience in the surgical management of female urethral strictures. In this time period, NHS England HES data indicate that urethral stricture was the main diagnosis in 54,302 finished consultant episodes (admissions for treatment) and that these patients underwent 63, 345 treatments for their stricture during this time period (on average 1.2 procedures per person). These treatments were: 62,069 urethral dilatations (98 %), 1175 urethrotomies (1.9 %) and 380 urethroplasty (0.16 %) [10].
There remains a lack of consensus regarding the diagnosis of female urethral stricture, with definitions varying between different authors. Calibration of the urethra represents the most common criteria, but significant differences among studies are evident. Branner [11] reported that a calibration < 20 Fr can be considered pathologic and should be treated, while Smith et al. [12] in a series of 7 female patients treated with urethral dilatations and ISD defined a urethral stricture as a fixed anatomical narrowing between the bladder neck and distal urethra of <14 F preventing catheterization. Osman et al. [4] in a recent systematic review described the urethral stricture as ‘a symptomatic anatomical narrowing of the female urethra based on direct visualization and/or radiological evidence and/or urethral calibration with the exclusion of other competing etiologies.’ In our study, the diagnosis was based on clinical, urodynamic and cystoscopic findings, while a pelvic MRI was performed in selected cases to exclude other conditions causing the obstruction. The VCMG was demonstrated to be a reliable investigation showing the pathognomonic urethral ballooning proximal to a segment of narrow urethra in 82 % (100 % of all patient who were able to void while standing screening). This ballooning was associated with pDetQmax > 2 x Qmax in all the patients who had this investigation. A cystoscopy was performed to confirm an anatomical stricture and all patients showed a segment of narrow urethra preventing the passage of a 17-Fr cystoscope.
Osman reviewed 16 retrospective studies. He reported among the 221 patients included that the majority of the strictures were idiopathic (49 %), while trauma or previous instrumentation was documented in 7 % and 39 %, respectively [4]. In our series, similar results have been observed with an incidence of idiopathic stricture of 42 %; iatrogenic strictures were evident in 11 patients (42 %); and previous trauma was documented in 15 % of the patients. Osman also reported that 96.9 % of patients had previously undergone urethral dilatation, and 3.1 % had a previous failed urethroplasty [4]. We have reported results consistent with this literature in terms of previous treatments: 73 % of our patients presented with a recurrent stricture following previous failed dilatations/urethrotomies, and in 1 patient (4 %) a urethroplasty was performed previously in a different center.
The success rate of endoscopic treatments is higher for newly diagnosed urethral stricture compared to redo procedures. After a mean follow-up of 47 months, patients treated with a repeat urethral dilatation are stricture free in only 27.2 % of cases, whereas the chances in success are reported to be closer to 60 % for primary cases [4]. Romman et al. [13] reported the outcomes of 49 patients who underwent progressive urethral dilatation up to 41 Fr and found an overall success rate of 49 % after 46 months; the failure of the procedure was demonstrated to be higher in the group of patients already treated with a urethral dilatation compared to patients in which it was performed for the first time (39 vs 17 %) after a mean follow-up of 8 ± 12 months.
Blavias reported a success rate for urethral dilatations in only 6 %, and 16 of the 17 patients included in his series required further urethral dilatations or a urethroplasty [7]. Smith demonstrated a success rate of 57 % after 21 months in 7 patients treated with urethral dilatation but all the patients included continued to perform ISD daily after surgery. Our results are consistent with the literature, and a poor outcome has been observed particularly in patients with a history of recurrent strictures: None of them were successfully treated with this technique, and a redo dilatation was necessary in 50 % despite performing regular self-dilatation.
Female urethroplasty using graft or flap has provided satisfactory results, but the series available are small, and the mean follow-up is lower than that for urethral dilatation. Montorsi showed a success rate of 88 % after a mean follow-up of 12 months in 17 patients treated with a vaginal flap for a distal urethral stricture [14]. Similar outcomes are reported by Onol and Blaivas with the same technique after a follow-up of 36 and 53 months, respectively [6, 7]. Only 2 patients had a vaginal flap urethroplasty in our center, and in both cases complete cure of the stricture was observed; after a mean follow-up of 31.5 months, none of the patients required ISD or further procedures. The majority of the patients reported in the present study have been treated with a ventral onlay BMG urethroplasty. In this group, we have observed the most convincing results in terms of cure of the stricture, cure of pain and improvement of UTI in 93, 67 and 88 %, respectively, at a mean follow-up of 12.1 months. Postoperatively we have documented 3 cases of mild SUI which resolved with conservative treatment. The low risk of SUI following the ventral incision of the urethra is explained by the horseshoe-shaped female urethral sphincter, which is composed mainly of fibrous tissue at the 6 O’clock position minimizing the risk of incontinence following Urethrotomy in this position. Onol reports a success rate of 100 % in 6 patients treated with a BMG and Martius fat pad at 17 months postoperatively [6]. The same success rate was reported by Blaivas and Castillo in a total of 5 patients treated with a BMG at 25 and 18 months of follow-up, respectively [7, 15].
Conclusion
Urethral stricture is present in 4.9 % of all women presenting with LUTS to our centre, and this is far commoner than previously reported. There is not a homogenous definition or diagnostic criteria for female urethral stricture, and its treatment represents a challenging reconstructive urological problem and includes urethral dilatation, urethrotomy and various forms of urethroplasty. Female patients with a urethral stricture were treated with urethral dilatation or urethrotomy in 31 % and urethroplasty in 69 % with temporary improvement in flow, no improvement in urethral pain and no cure of urethral stricture in those women having urethral dilatation or urethrotomy and improvement in flow in 100 %, cure of pain in 67 % and cure of stricture in 93 %.
References
Carr LK, Webster GD (1996) Bladder outlet obstruction in women. Clin North Am 23:385–391
Groutz A, Blaivas JG, Chaikin DC (2000) Bladder outlet obstruction in women: definition and characteristics. Neurourol Urodyn 19:213–220
Kuo HC (2005) Videourodynamic characteristics and lower urinary tract symptoms of female bladder outlet obstruction. Urology 66:1005–1009
Osman NI, Mangera A, Chapel CR (2013) A systematic review of surgical techniques used in the treatment of female urethral stricture. Eur Urol 64:965–973
Keegan KA, Nanigian DK, Stone AR (2008) Female urethral stricture disease. Curr Urol Rep 9:419–423
Onol FF, Antar B, Kose O, Erdem MR, Onol SY (2011) Techniques and results of urethroplasty for female urethral strictures: our experience with 17 patients. Urology 77:1318–132
Blavias JG, Santos JA, Tsui JF, Deibert CM, Rutman MP, Purohit RS, Weiss JP (2012) Management of urethral stricture in women. J Urol 188:1778–1782
Solomon E, Yasmin H, Jenks J, Pakzad M, Hamid R, Ockrim J et al (2014) MP76-02 The development and validation of a new nomogram for diagnosis bladder outlet obstruction in women. J Urol 191(4):e882
Edwards L, Malvern J (1974) The urethral pressure profile: theoretical considerations and clinical application. Br J Urol 46(3):325–335
WWW.HSCIC.GOV.UK/HES. Accessed Mar 2016
Brannan D (1951) Stricture of the female urethra. J Urol 66:242–253
Smith AL, Ferlise VJ, Rovner ES (2006) Female urethral strictures: successful management with long-term clean intermittent catheterization after urethral dilatation. BJU Int 98:96–99
Romman AN, Alhalabi F, Zimmern PE (2012) Distal intramural urethral pathology in women. J Urol 188:1218–1223
Montorsi F, Salonia A, Centemero A et al (2002) Vestibular flap urethroplasty for strictures of the female urethra. Impact on symptoms and flow pattern. Urol Int 69:12–16
Castillo OA, Sepulveda F, Feria-Flores MA (2011) Urethroplasty with dorsal oral mucosa graft in female urethral stenosis. Actas Urol Esp 35:246–249
Authors’ Contribution
Spilotros contributed to protocol/project development, data collection and manuscript writing/editing; Malde helped with data management; Solomon contributed to data analysis; Grewal and Mukhtar contributed to data collection; Pakzad, Hamid and Ockrim helped with final review of the manuscript. Greenwell helped with protocol/project development, manuscript writing/editing and final review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical statements
In lieu of a formal ethics committee, the principles of the Helsinki Declaration were followed.
Rights and permissions
About this article
Cite this article
Spilotros, M., Malde, S., Solomon, E. et al. Female urethral stricture: a contemporary series. World J Urol 35, 991–995 (2017). https://doi.org/10.1007/s00345-016-1947-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-016-1947-7