Abstract
Purpose
This large dose-ranging study explored the benefits of different combinations of mirabegron and solifenacin on health-related quality of life (HRQoL), based on patient-reported outcomes (PROs), and patients (‘responders’) achieving clinically meaningful improvements in efficacy and HRQoL.
Methods
SYMPHONY (NCT01340027) was a Phase II, placebo- and monotherapy-controlled, dose-ranging, 12-week trial. Adult patients with overactive bladder (OAB) for ≥3 months were randomized to 1 of 12 groups: 6 combination (solifenacin 2.5/5/10 mg + mirabegron 25/50 mg), 5 monotherapy (solifenacin 2.5/5/10 mg, or mirabegron 25/50 mg), or placebo. Change from baseline to end of treatment was assessed, versus placebo and solifenacin 5 mg in: PROs (OAB-q [Symptom Bother/total HRQoL] and Patient Perception of Bladder Condition score), and responders achieving minimally important differences (MIDs) in PROs and predetermined clinically meaningful improvements in efficacy (e.g. <8 micturitions/24 h). Changes in PROs and responders were analysed using an ANCOVA model and logistic regression, respectively.
Results
The Full Analysis Set included 1278 patients. Combination therapy of solifenacin 5/10 mg + mirabegron 25/50 mg significantly improved PROs versus solifenacin 5 mg and placebo, and significantly more responders achieved MIDs in PROs and efficacy. Micturition frequency normalization was approximately twofold greater with 10 + 25 mg (OR 2.06 [95 % CI 1.11, 3.84; p = 0.023]) and 5 + 50 mg (OR 1.91 [95 % CI 1.14, 3.21; p = 0.015]) versus solifenacin 5 mg.
Conclusion
Combining mirabegron 25/50 mg and solifenacin 5/10 mg improves objective and subjective efficacy outcomes compared with placebo or solifenacin 5 mg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The symptoms of overactive bladder (OAB; urinary urgency, often accompanied by increased daytime frequency and nocturia, with or without urgency incontinence [1, 2]) have a detrimental effect on a patient’s daily activities and quality of life (QoL) [3, 4], which encompasses socio-demographic, clinical, and psychological factors. This underlines the importance of evaluating the patient’s perception of effectiveness of a treatment [5], since meaningful improvements in their QoL contribute to long-term persistence with treatment [6].
In OAB trials, the conventional approach to measure efficacy involves patient-recorded bladder diaries that are used to calculate mean change from baseline in OAB symptoms over a given period. Subjective patient-reported outcomes (PROs), in which health-related QoL (HRQoL) and the patient’s perception of symptom bother are assessed using validated health questionnaires, complement these objective efficacy assessments. The Overactive Bladder Questionnaire (OAB-q) and Patient Perception of Bladder Condition (PPBC) are robust, validated tools that are routinely used in OAB trials, and are highly responsive to improvements in efficacy assessed using bladder diaries [7–10]. While it is desirable to show a statistically significant treatment effect both objectively and subjectively, it is of greater importance to demonstrate that the magnitude of the effect is clinically meaningful to patients. This can be achieved using responder analyses, whereby a continuous efficacy variable (e.g. micturition frequency) or PRO (e.g. OAB-q) is categorized into a binary variable (‘responder’ or ‘non-responder’) according to a predefined cut-off point. Efficacy responders are identified according to symptom resolution or ‘normalization’ (e.g. <8 micturitions/24 h) or accepted definitions of clinically important improvements e.g. a 50 % reduction in daily urinary incontinence episodes [11, 12]. A daily urinary frequency of ≥8 is associated with a significant negative impact on HRQoL and symptom bother compared with participants experiencing a daily urinary frequency <8 [12]; consequently, micturition frequency normalization is defined as a daily frequency <8. For PRO responders, a 1- or 2-point improvement in PPBC, and a 10-point improvement in OAB-q, was identified as being a minimally important difference [9, 13–15] (MID; defined as ‘the smallest difference in score in the domain of interest that patients perceive as beneficial and which would mandate, in the absence of troublesome side effects and excessive costs, a change in patient management’) [16].
Combining efficacy and PRO variables, into a composite responder analysis, overcomes potential heterogeneity in response between these variables, and identifies patients simultaneously achieving clinically important improvements, both symptomatically and subjectively.
Antimuscarinics (e.g. solifenacin) and the β3-adrenoceptor agonist, mirabegron, the two classes of oral pharmacotherapies used to treat OAB, exhibit similar efficacy, but unlike antimuscarinics, mirabegron is not associated with anticholinergic side effects [17]. Patients are usually initiated on an antimuscarinic, with dose escalation or change of antimuscarinic if symptom improvement is inadequate. However, dose escalation increases anticholinergic burden, the risk of bothersome side effects and treatment discontinuation [18, 19]. Using a combination of mirabegron and antimuscarinic may improve efficacy without compromising tolerability, thus promoting treatment persistence.
SYMPHONY (NCT01340027) was a Phase II, randomized, double-blind, factorial design, dose-ranging, placebo- and monotherapy-controlled study that investigated the efficacy and safety of combinations of solifenacin (2.5/5/10 mg) plus mirabegron (25/50 mg). Combination therapy significantly improved mean volume voided, micturition frequency, and urgency versus solifenacin 5 mg monotherapy, without increasing bothersome adverse events [20]. The objective of this prespecified secondary analysis was to evaluate PROs (OAB-q and PPBC) utilized in the study and identify patients achieving clinically relevant improvements in objective measures of symptom severity (incontinence episodes and micturition frequency) and PROs. Combinations (2.5/5/10 + 25/50 mg) and their respective monotherapies were compared with placebo and solifenacin 5 mg (the recommended starting dose in clinical practice) [21].
Patients and methods
Study design
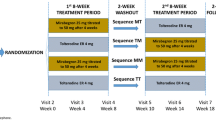
Male and female patients aged ≥18 years with OAB for ≥3 months were screened for eligibility (Supplementary Table 1). Following a 2-week, single-blind placebo run-in period to washout prohibited medications and existing OAB medications (prior use of solifenacin or mirabegron was not excluded), patients with ≥8 micturitions/24 h and ≥1 urgency episode/24 h (with or without incontinence), based on a 3-day electronic patient bladder diary, were randomized (Supplementary File 1) in a 2:1 ratio to one of 12 treatment groups of primary and secondary interest for 12 weeks (Supplementary Fig. 1). The patient diary was completed daily for 3 consecutive days prior to study visits at baseline, and during weeks 2, 4, 8, and 12, to record micturition frequency, volume voided, urgency severity, and incontinence episodes.
Patient-reported outcomes
PROs were assessed using an electronic diary to complete the OAB-q at baseline and weeks 4, 8, and 12, and the PPBC questionnaire at baseline and week 12. The OAB-q consists of an 8-item Symptom Bother scale (0–100; lower scores indicate better QoL) and 25-item HRQoL scale (0–100; higher scores indicate better QoL) [22]. The PPBC consists of a 6-point Likert scale ranging from 1 (‘no problems at all’) to 6 (‘many severe problems’) [9].
Efficacy responder analyses
Responder analyses were conducted at weeks 2, 4, 8, and 12. There were three predefined efficacy responses, one based on micturition frequency (patients with ≥8 micturitions/24 h at baseline and ≤8 micturitions/24 h post-baseline, and a reduction from baseline) and two based on incontinence (patients who were incontinent at baseline and became continent during treatment, and patients with a ≥50 % reduction in incontinence episodes/24 h during treatment).
Patient-reported outcome responder analyses
Individual PRO responder analyses were based on MIDs from baseline in the PPBC (≥1-point or ≥2-point improvement), and the OAB-q (≥10-point improvement in Symptom Bother or total HRQoL).
Exploratory responder analyses: double/triple responders
Five composite responder analyses were explored post hoc based on the proportion of patients who achieved micturition frequency normalization plus MIDs in either one PRO (OAB-q [Symptom Bother or HRQoL] or PPBC): a ‘double responder’, or two PROs (PPBC and OAB-q [Symptom Bother or HRQoL]): a ‘triple responder’.
Statistical analyses
This study was planned with 140 evaluable patients in each primary interest group (solifenacin 5 mg; solifenacin 2.5/5 mg + mirabegron 25/50 mg), and 70 evaluable patients in each secondary interest group (placebo; solifenacin 2.5 mg; solifenacin 10 mg; mirabegron 25 mg; mirabegron 50 mg; solifenacin 10 mg + mirabegron 25/50 mg). Sample size was calculated according to the primary efficacy analysis [20], based on treatment differences versus placebo in previous studies [23–26]. Assuming drop-out rates of 20 % during screening and 10 % after the 2:1 randomization to primary or secondary interest groups, approximately 1658 patients had to be enrolled to have 1190 evaluable patients. Randomization was stratified by sex, age (<65 years and ≥65 years), and geographic region (Western Europe and Eastern Europe).
Changes from baseline in OAB-q and PPBC were analysed using an ANCOVA model that included mirabegron dose (0, 25, and 50 mg), solifenacin dose (0, 2.5, 5, and 10 mg) and their corresponding dose combination interaction term to reflect the factorial design. The model further included the main factors of sex, age group, and geographic region, and baseline measurement as a covariate. The ANCOVA model presented least squares mean estimates and 2-sided 95 % confidence intervals (CIs) for mean changes from baseline within each treatment combination group.
The proportion of efficacy and PRO responders in monotherapy and combination groups was compared with placebo and solifenacin 5 mg and summarized using point estimates and two-sided 95 % CIs, based on normal approximation. Nominal p values for the odds ratios between relevant combination groups were calculated based on logistic regression analyses adjusting for treatment combination group, sex, age group, geographic region, and baseline measurement. Patients missing an efficacy measurement for a responder analysis at a scheduled week or at end of treatment (EoT) were excluded from the analysis for that visit.
PROs (PPBC and OAB-q), their respective responder analyses, and the micturition frequency responder analysis were assessed in the Full Analysis Set (FAS); randomized patients who received ≥1 dose of study drug and had volume voided measurements in a 3-day micturition diary at baseline and ≥1 post-baseline diaries. Responder analyses based on incontinence were assessed in the Full Analysis Set-Incontinence (FAS-I); FAS patients with ≥1 incontinence episode at baseline.
Results
Patient demographics and baseline characteristics
Overall 1306 patients were randomized and received double-blind medication (Supplementary Fig. 2); 1278 and 281 patients were included in the FAS and FAS-I, respectively. A total of 67 (5.1 %) patients, distributed equally across the treatment groups, discontinued the study; primary reasons for discontinuation included withdrawal by patient (n = 27 [2.1 %]), adverse events (n = 18 [1.4 %]), and protocol violation (n = 14 [1.1 %]). Demographic and baseline characteristics were generally consistent across groups. Approximately 20 % of patients were incontinent at baseline; patients receiving placebo recorded the lowest number of incontinence and micturition episodes (Table 1). Baseline PROs indicated moderate-to-severe levels of Symptom Bother, HRQoL, and PPBC (Table 2).
Patient-reported outcomes
In the FAS, at EoT, the 5 + 25 mg and 5 + 50 mg combinations of primary interest significantly improved PPBC versus placebo and solifenacin 5 mg (p < 0.05) (Table 2). Significant improvements in the Symptom Bother score were evident for three combinations of primary interest (2.5 + 50 mg, 5 + 25 mg, and 5 + 50 mg) versus placebo and solifenacin 5 mg (p < 0.05). Significant improvements in total HRQoL were observed with the 5 + 50 mg combination of primary interest versus placebo, and for three combinations of primary interest (2.5 + 50 mg, 5 + 25 mg, and 5 + 50 mg) versus solifenacin 5 mg (p < 0.05).
Efficacy responder analyses
In the FAS-I, two combinations of primary interest (5 + 25 mg and 5 + 50 mg) were associated with statistically significant improvements versus solifenacin 5 mg (p < 0.05) for responders achieving zero incontinence episodes and for responders achieving ≥50 % reduction in incontinence episodes/24 h (Fig. 1). The odds of achieving zero incontinence episodes with 5 + 25 mg (OR 6.12 [95 % CI 1.47, 25.60; p = 0.013]) and 5 + 50 mg (OR 5.49 [95 % CI 1.17, 25.77, p = 0.031) were over fivefold greater than with solifenacin 5 mg, while the odds of achieving ≥50 % reduction in incontinence were approximately tenfold greater than solifenacin 5 mg. Improvements in the incontinence responder rates did not translate into statistically significant differences versus placebo presumably due to the high placebo response, low baseline frequency of incontinence, and the small number of ‘wet’ patients.
In the FAS, the proportion of patients achieving micturition frequency normalization at EoT was 65.4 and 61.6 % with the 10 + 25 mg and 5 + 50 mg combinations, respectively, which was statistically significantly greater than placebo or solifenacin 5 mg (p < 0.05). The odds of achieving micturition frequency normalization were approximately twofold greater with the 10 + 25 mg (OR 2.06 [95 % CI 1.11, 3.84; p = 0.023]) and 5 + 50 mg (OR 1.91 [95 % CI 1.14, 3.21, p = 0.015]) groups than with solifenacin 5 mg.
Patient-reported outcome responder analyses
In the FAS, more than 80 % of patients achieved ≥1-point improvement in PPBC with the 5 + 50 mg combination at EoT which was statistically significant versus placebo and solifenacin 5 mg (p < 0.05) (Fig. 2). The proportion achieving a major (≥2 point) improvement in PPBC was significantly higher with the 5 + 50 mg combination versus placebo (p = 0.038), and the 5 + 25 mg and 5 + 50 mg combinations versus solifenacin 5 mg (p = 0.020 and p = 0.012, respectively). The odds of achieving an improvement in PPBC (≥1 or ≥2 points) were approximately twofold higher with the 5 + 25 mg and 5 + 50 mg combination groups of primary interest versus solifenacin 5 mg and placebo. Responder rates for the ≥10-point improvement in Symptom Bother were significantly higher with 5 + 25 mg (OR 2.14 [95 % CI 1.02, 4.48, p = 0.043]) and 5 + 50 mg (OR 2.61 [95 % CI 1.22, 5.58, p = 0.013]) versus placebo. Responder rates for the ≥10-point improvement in total HRQoL were significantly higher with 5 + 50 mg versus placebo (OR 2.45 [95 % CI 1.22, 4.94, p = 0.012) and solifenacin 5 mg (OR 2.21 [95 % CI 1.19, 4.09, p = 0.012]). These differences translated into approximately twofold higher odds for responders for Symptom Bother and HRQoL versus solifenacin 5 mg and placebo.
Exploratory composite responder analyses
For the double and triple responder analyses, the 5 + 50 mg and 10 + 25 mg combinations were associated with significantly higher responder rates versus solifenacin 5 mg and placebo (p < 0.05) in four of the five composite responder analyses. The odds of achieving micturition frequency normalization plus one or two of the PRO criteria (PPBC/Symptom Bother/HRQoL) were more than twofold higher with the 5 + 50 mg or 10 + 25 mg groups compared with solifenacin 5 mg and placebo in three of the five composite responder analyses (Supplementary Tables 2 and 3).
Discussion
In OAB trials, demonstrating statistically significant improvements in mean values of OAB symptoms is desirable but what is most pertinent is whether patients achieve a meaningful response with their treatment in terms of improving daily activities and psychological well-being, since these factors are more likely to influence patient behaviour and persistence with treatment. The International Continence Society recommends the evaluation of PROs in OAB trials [27]. Responder analyses represent an additional tool for translating changes in subjective or objective measures into a clinically meaningful binary outcome i.e. responder or non-responder.
Improvements in PROs related to HRQoL and the proportion of patients achieving clinically relevant improvements in efficacy and HRQoL was significantly greater with combinations of mirabegron (25 or 50 mg) and solifenacin (5 or 10 mg) versus placebo and solifenacin 5 mg. These results are consistent with the primary efficacy analysis which demonstrated significant improvements in mean volume voided per micturition in all combinations with solifenacin 5 or 10 mg versus solifenacin 5 mg, in micturition frequency with three combination groups (5 + 50 mg, 10 + 25 mg, and 10 + 50 mg) versus solifenacin 5 mg and placebo, and in urgency episodes with all combinations (except 2.5 + 25 mg) versus solifenacin 5 mg [20].
Patients receiving higher dose combinations were at least twice as likely to achieve resolution of incontinence (‘dry’ rate), a significant reduction (≥50 %) in daily incontinence, or normalization of micturition frequency, versus solifenacin 5 mg and placebo. In view of the absence of between-group differences on mean change from baseline in incontinence episodes (probably as a consequence of the very small population with urgency incontinence and low baseline frequency of incontinence [~1 episode/24 h]) as previously reported [20], the significant differences in responder rates for incontinence are surprising. The odds of achieving clinically important improvements in PROs associated with the PPBC, HRQoL, and Symptom Bother were approximately twofold or higher with combinations including solifenacin 5 mg versus both placebo and solifenacin 5 mg, suggesting the responder rates on incontinence and/or micturition frequency are clinically relevant. This was reflected in the composite responder analyses, in particular, the 5 + 50 mg and 10 + 25 mg groups, where the odds of achieving micturition frequency normalization plus MIDs in one (‘double responder’) or two (‘triple responder’) PRO variables (PPBC/HRQoL/Symptom Bother) were approximately twofold higher versus solifenacin 5 mg and placebo. The comparator groups (solifenacin 5 mg and placebo) did not differentiate as expected for the efficacy and PRO responder analyses, partly as a result of the high placebo response, which would have contributed to the inconsistent treatment differences observed in this study. It is also worth considering the lower baseline severity of micturition frequency in the placebo group as another potential factor responsible for the high placebo response.
The apparent benefit with higher dose combinations, particularly 5 + 50 mg, is supported by the Phase IIIb BESIDE study, which investigated incontinent OAB patients refractory to initial solifenacin 5 mg who were subsequently treated with a combination of mirabegron 50 mg + solifenacin 5 mg, or solifenacin monotherapy (5 or 10 mg) [28]. In BESIDE, the odds of becoming ‘dry’, achieving a 50 % reduction in incontinence, micturition frequency normalization, and MIDs for Symptom Bother and HRQoL were, respectively, 47, 51, 29, 75 and 50 % higher with combination versus solifenacin 5 mg [28]. Responder rates and PROs from a post hoc sub-analysis of placebo-controlled Phase III studies of mirabegron 50 mg monotherapy also suggest a benefit with combination: the odds of achieving micturition frequency normalization, and MIDs in Symptom Bother and HRQoL, were respectively, 57, 43, and 46 % higher with mirabegron 50 mg versus placebo [29]. Combination groups in SYMPHONY (5 + 50 mg, 10 + 25 mg) were associated with odds that were approximately twofold or higher versus placebo (i.e. >100 %) for the same responder variables.
In SYMPHONY, BESIDE, and pooled Phase III mirabegron 50 mg studies, the magnitude of improvement in PROs correlated with clinically meaningful improvements in efficacy variables and treatment satisfaction [28, 29]. Moreover, a correlation analyses of pooled Phase III data for mirabegron 50 mg showed significant correlation between objective measures (micturition frequency and incontinence) and Symptom Bother score, total HRQoL, and PPBC [29]. Studies investigating antimuscarinics have also confirmed that subjective reporting of symptoms is highly predictive of treatment satisfaction [30] and objective findings based on bladder diaries [31].
Although this study was only powered to detect differences across treatment groups in mean volume voided per micturition, the study also demonstrated statistically significant efficacy on micturition frequency. Strengths of the study included a factorial design which allowed numerous combinations of mirabegron and solifenacin doses to be explored. Furthermore, responder analyses are a clinically important accompaniment to primary efficacy and safety data as they evaluate treatment from the patient’s perspective and allow simple meaningful outcomes, such as ‘the odds of achieving continence are twice as likely with combination’, to be communicated to the patient.
Conclusion
Combination therapy of mirabegron 25/50 mg and solifenacin 5/10 mg was associated with statistically significant improvements in PROs indicative of HRQoL versus solifenacin 5 mg and placebo. This was accompanied by a significantly higher proportion of patients achieving clinically relevant improvements in micturition frequency and/or incontinence episodes and PROs, individually and simultaneously. Given the limitations of the study, further investigations into the potential benefit of combination therapy with mirabegron and solifenacin are recommended.
References
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U et al (2002) The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21(2):167–178
Drake MJ (2014) Do we need a new definition of the overactive bladder syndrome? ICI-RS 2013. Neurourol Urodyn 33(5):622–624
Abrams P, Kelleher CJ, Kerr LA, Rogers RG (2000) Overactive bladder significantly affects quality of life. Am J Manag Care 6(11 Suppl):S580–S590
Basra R, Kelleher C (2007) Disease burden of overactive bladder: quality-of-life data assessed using ICI-recommended instruments. Pharmacoeconomics 25(2):129–142
Palmtag H (2004) The patient’s perspective: redefining end points. Urology 64(6 Suppl 1):17–20
Balkrishnan R, Bhosle MJ, Camacho FT, Anderson RT (2006) Predictors of medication adherence and associated health care costs in an older population with overactive bladder syndrome: a longitudinal cohort study. J Urol 175(3 Pt 1):1067–1071
Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover J et al (2002) Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res 11(6):563–574
Coyne KS, Matza LS, Thompson CL (2005) The responsiveness of the Overactive Bladder Questionnaire (OAB-q). Qual Life Res 14(3):849–855
Coyne KS, Matza LS, Kopp Z, Abrams P (2006) The validation of the patient perception of bladder condition (PPBC): a single-item global measure for patients with overactive bladder. Eur Urol 49(6):1079–1086
Sussman D, Garely A (2002) Treatment of overactive bladder with once-daily extended-release tolterodine or oxybutynin: the antimuscarinic clinical effectiveness trial (ACET). Curr Med Res Opin 18(4):177–184
Payne CK, Kelleher C (2007) Redefining response in overactive bladder syndrome. BJU Int 99(1):101–106
Coyne KS, Payne C, Bhattacharyya SK, Revicki DA, Thompson C, Corey R et al (2004) The impact of urinary urgency and frequency on health-related quality of life in overactive bladder: results from a national community survey. Val Health 7(4):455–463
Coyne KS, Matza LS, Thompson C, Jumadilova Z, Bavendam T (2007) The responsiveness of the OAB-q among OAB patient subgroups. Neurourol Urodyn 26(2):196–203
Dyer KY, Xu Y, Brubaker L, Nygaard I, Markland A, Rahn D et al (2011) Minimum important difference for validated instruments in women with urge incontinence. Neurourol Urodyn 30(7):1319–1324
Coyne KS, Matza LS, Thompson CL, Kopp ZS, Khullar V (2006) Determining the importance of change in the overactive bladder questionnaire. J Urol 176(2):627–632
Jaeschke R, Singer J, Guyatt GH (1989) Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 10(4):407–415
Maman K, Aballea S, Nazir J, Desroziers K, Neine ME, Siddiqui E et al (2014) Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol 65(4):755–765
Benner JS, Nichol MB, Rovner ES, Jumadilova Z, Alvir J, Hussein M et al (2010) Patient-reported reasons for discontinuing overactive bladder medication. BJU Int 105(9):1276–1282
Sexton CC, Notte SM, Maroulis C, Dmochowski RR, Cardozo L, Subramanian D et al (2011) Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: a systematic review of the literature. Int J Clin Pract 65(5):567–585
Abrams P, Kelleher C, Staskin D, Rechberger T, Kay R, Martina R et al (2015) Combination treatment with mirabegron and solifenacin in patients with overactive bladder: efficacy and safety results from a randomised, double-blind, dose-ranging, phase 2 study (Symphony). Eur Urol 67(3):577–588
Astellas. VESICARE® (solifenacin succinate) product label. FDA.gov, Revised 2010 http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021518s008lbl.pdf
Garely AD, Lucente V, Vapnek J, Smith N (2007) Solifenacin for overactive bladder with incontinence: symptom bother and health-related quality of life outcomes. Ann Pharmacother 41(3):391–398
Abrams P, Swift S (2005) Solifenacin is effective for the treatment of OAB dry patients: a pooled analysis. Eur Urol 48(3):483–487
Chapple CR, Dvorak V, Radziszewski P, Van Kerrebroeck P, Wyndaele JJ, Bosman B et al (2013) A Phase II dose-ranging study of mirabegron in patients with overactive bladder. Int Urogynecol J 24(9):1447–1458
Khullar V, Amarenco G, Angulo JC, Cambronero J, Hoye K, Milsom I et al (2013) Efficacy and tolerability of mirabegron, a beta(3)-adrenoceptor agonist, in patients with overactive bladder: results from a randomised European-Australian phase 3 trial. Eur Urol 63(2):283–295
Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S (2013) Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol 189(4):1388–1395
Mattiasson A, Djurhuus JC, Fonda D, Lose G, Nordling J, Stohrer M (1998) Standardization of outcome studies in patients with lower urinary tract dysfunction: a report on general principles from the Standardisation Committee of the International Continence Society. Neurourol Urodyn 17(3):249–253
MacDiarmid S, Al-Shukri S, Barkin J, Fianu-Jonasson A, Grise P, Herschorn S et al (2016) Mirabegron as add-on treatment to solifenacin in incontinent OAB patients with an inadequate response to solifenacin monotherapy: responder analyses and patient-reported outcomes from the BESIDE study. J Urol doi:10.1016/j.juro.2016.03.174. [Epub ahead of print]
Castro-Diaz D, Chapple CR, Hakimi Z, Blauwet MB, Delgado-Herrera L, Lau W et al (2015) The effect of mirabegron on patient-related outcomes in patients with overactive bladder: the results of post hoc correlation and responder analyses using pooled data from three randomized Phase III trials. Qual Life Res 24(7):1719–1727
Zinner N, Kobashi KC, Ebinger U, Viegas A, Egermark M, Quebe-Fehling E et al (2008) Darifenacin treatment for overactive bladder in patients who expressed dissatisfaction with prior extended-release antimuscarinic therapy. Int J Clin Pract 62(11):1664–1674
Van Kerrebroeck PE, Kelleher CJ, Coyne KS, Kopp Z, Brodsky M, Wang JT (2009) Correlations among improvements in urgency urinary incontinence, health-related quality of life, and perception of bladder-related problems in incontinent subjects with overactive bladder treated with tolterodine or placebo. Health Qual Life Outcomes 7:131
Acknowledgments
This study and editorial assistance were funded by Astellas Pharma Inc. The authors would like to thank Stuart Murray of Envision Scientific Solutions Ltd for editorial assistance.
Authors’ contribution
Protocol/project development: PA, CK, DS, IM, RK, AR, DN; Data collection or management: PA, CK, IM, AM, RvM, AP, DN; Data analysis: PA, CK, DS, RK, AM, IM, DN, AR, AP, RvM; Manuscript writing/editing: PA, CK, DS, RK, AM, IM, DN, AR, AP, RvM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DN, AR, RvM, and AP are employees of Astellas Pharma Inc. PA has received consultancy fees from Astellas, Ferring, Pfizer, and Ipsen. CK has received consultancy fees from Astellas and Allergan. RK has received consultancy fees from Astellas. DS has received research and consultancy fees from Astellas, consultancy fees from Allergan, and patent royalties from Endo. AM has received consultancy fees from Astellas and Meditrade. IM has no financial disclosures to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abrams, P., Kelleher, C., Staskin, D. et al. Combination treatment with mirabegron and solifenacin in patients with overactive bladder: exploratory responder analyses of efficacy and evaluation of patient-reported outcomes from a randomized, double-blind, factorial, dose-ranging, Phase II study (SYMPHONY). World J Urol 35, 827–838 (2017). https://doi.org/10.1007/s00345-016-1908-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-016-1908-1