Abstract
Purpose
To retrospectively evaluate the accuracy of dual-energy CT (DECT) in the detection of the chemical composition of urinary calculi in correlation with infrared spectroscopic stone analysis.
Methods
We reviewed the CT scans of 255 patients who underwent DECT due to a clinical suspicion of urolithiasis. Out of this group, we included 64 patients with clinically symptomatic urolithiasis requiring stone removal. After surgical removal of the stone by ureterorenoscopy, chemical composition was analyzed with infrared spectroscopy. We correlated DECT stone characterization results with chemical stone composition based on dual-energy indices (DEI). A total of 213 renal and ureteral stones could be removed and chemically analyzed.
Results
A total of 213 calculi were evaluated. Thirty eight out of sixty four (59 %) patients had >1 stone. DECT was used to differentiate stones by using DEI. Stones harboring calcium (CA) were color-coded in blue, while stones containing uric acid (UA) were colored red. Median DEI in UA-containing stones were 0.001. Non-UA-containing stones had a DEI between 0.073 for pure CA stones and 0.077 containing CA and other substances (p = 0.001; p = 0.03, respectively). Sensitivity of DECT was 98.4 % for differentiation of UA from non-UA-containing calculi. Specificity was 98.1 %. Mean effective radiation dose of DECT was 4.18 mSv (0.44–14.27 mSv), thus comparable to conventional CT scans of the abdomen. Conventional measurement of Hounsfield units did not correlate with stone composition.
Conclusion
DECT with image post-processing reliably discriminates UA-containing calculi from all other stones, but the study offered limitations. Discrimination within the non-UA stones cannot be reliably achieved but is clinically insignificant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urolithiasis, the formation of urinary tract calculi, is one of the most common causes of acute and recurrent flank pain. The majority of affected patients are young men with their first episode of urolithiasis occurring between 20 and 30 years of age [1]. The lifetime risk of stone formation has increased over the last 10 years and amounts to ca. 12 % in men and ca. 6 % in women with a high risk of recurrence [2].
Patients with calculi usually present with acute flank pain, vomiting and sometimes fever, but may also be asymptomatic. Standard evaluation includes a detailed medical history and physical examination. If urolithiasis is clinically suspected, the diagnosis should be confirmed by imaging [3]. About 50 % of all patients undergo radiologic imaging. Non-enhanced low-dose computed tomography (CT) is recommended as the most accurate technique in the detection of urinary calculi and has become the imaging test of choice [4]. CT has excellent sensitivity and specificity for detecting the presence of stones, is reliable in determining their size and location and supports clinical treatment decisions. For successful treatment of urolithiasis, the chemical composition of the stone is of paramount importance. Eighty percent of all calculi contain calcium and its derivates; 10–15 % of all calculi are uric acid stones [5]. Stones consisting of uric acid can be managed medically without an invasive therapeutic procedure. It is also well established that certain stones, like cystine and calcium oxalate monohydrate stones, are mostly resistant to extracorporeal shockwave lithotripsy (ESWL) [6]. Ideally, chemical stone composition should be determined before treatment, but conventional non-enhanced CT cannot reproducibly predict stone composition. This is a major limitation of conventional CT scans for imaging stones [7].
Dual-energy CT (DECT) is a promising new technology that has the potential to improve our current ability to discriminate stone composition. On a conventional “single-energy” CT, the Hounsfield unit (HU) value of the stone is related to its attenuation coefficient, which also depends on the energy of the interacting photons, not only on its composition and density, i.e., at lower tube kilovoltages the attenuation of the stone will be greater than at high kVp settings. On a DECT scanner, two X-ray tubes can be operated with different energy levels to discriminate different material compositions [8]. We aimed to evaluate the feasibility of differentiating uric acid stones from non-uric acid-containing stones by using dual-energy CT. Preliminary studies have proven the feasibility of a simultaneous localization and characterization of calculi with in vitro experiments [6, 9, 10]. The purpose of this study is to evaluate the accuracy of DECT in vivo to detect the chemical composition of urinary calculi and correlate the imaging findings with infrared spectroscopic stone analysis.
Materials and methods
Patient examinations
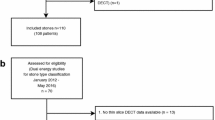
In this retrospective study, the data from a total of 255 patients treated in our department were analyzed. Between February 2007 and November 2010, 255 patients with symptomatic urolithiasis were included. The age range was 18–91 years (mean age: 53 years), including 77 female and 178 male patients. All patients underwent unenhanced DECT for assessment of suspected or known urinary stone disease. In n = 64 patients (15 women and 49 men), the chemical composition of the calculi was analyzed with infrared spectroscopy after surgical removal of the stone by ureterorenoscopy. All patients gave written informed consent prior to their DECT examination. In 38 patients, DECT examinations were performed on a first-generation dual-source scanner (Somatom Definition, Siemens Medical Solutions, Forchheim, Germany) from 2007 until 2009 using tube potentials of 80 and 140 kVp; 26 patients were scanned on a second-generation scanner (Somatom Definition Flash, Siemens Medical Solutions, Forchheim, Germany) at 100 and 140 kVp (Figs. 1, 2).
Image acquisition and post-processing
All patients were examined using a dual-source CT system in an unenhanced dual-energy CT mode in a single spiral acquisition (Somatom Definition and Somatom Definition Flash). The system consists of two X-ray tubes positioned at a 90 degree angle [11]. Due to two different types of scanners being used for the examinations, the scan parameters of the second-generation scanner (Siemens Somatom Flash) are shown in parentheses. There is a 64-slice detector (128-slice) corresponding to each of the tubes: The “A” detector operates similar to a standard single-source detector and the “B” detector which also features the same design and a field of view of 27 cm (33 cm). The tube potentials of 80 and 140 kV (100 and 140 kV) comprise standard values for dual-energy CT. The tube current–time products were set to the values of 200 and 96 mAs. Collimation on each detector was 14 × 1.2 mm (32 × 0.6 mm), and the pitch was 0.55 (0.6) to limit image noise which particulary benefits larger patients resulting in an improved characterization of calculi [12, 13]. The detailed scan parameters are listed in Table 1. The scan range covered the abdomen from the diaphragm to the ischial tuberosities and was obtained in an inspiratory breathhold (Figs. 3, 4).
Images were reconstructed in both axial and coronal planes with 2-mm slice thickness using a dual-energy-specific soft tissue kernel (D30f). To achieve low image noise and a spectral appearance similar to that of 120 kV images, the weighted-average images––70 % (50 %) density information from the 80 kV (100 kV) and 30 % (50 %) from the 140 kV image—were calculated additionally. This allows a reliable detection of small low-density urinary calculi.
All examinations were retrospectively evaluated in consensus by two experienced radiologists blinded to the results of the infrared spectroscopy of the stone. Every scan was read on a multimodality workstation equipped with dual-energy post-processing software (Siemens MMWP VA 21). For detection, localization and size measurement of calculi, the weighted-average images provided by the image reconstruction system were evaluated. The size of the stones was measured digitally on axial images. Location of calculi was stated as being in one of the following: in the right or left renal parenchyma, the pelvicalyceal system, in the proximal, mid- or distal ureter or the bladder. Stone densities were measured in Hounsfield units (HU) on images from the “A” tube, from the “B” tube, and from the weighted-average images. Objective assessment was done by manually putting a 1–5 mm2 circular region of interest (ROI), depending on the size of the calculus, to include at least half of the area of the calculus placed inside its highest density area on axial images. For placement of ROIs, images were displayed at bone window settings to minimize the partial volume effects between the stone and surrounding soft tissue. The software automatically copies ROIs drawn on one of the series to the respective other series for including exactly the same voxels and then calculates the mean attenuation value. The DEI were calculated for each calculus. The dual-energy software uses the information of both tubes to generate a color-coded image. The algorithm includes the ratio of mean attenuation using both tubes at different kV and assumes a mixture of water, calcium, and UA for every image voxel. A voxel with a similar behavior to calcium is color-coded in blue and one that is similar to uric acid in red. Voxels that show a linear density at both tube potentials remain gray. From the measured density values, the DEI were calculated according to the following formula: (HU80 (100) − HU140)/(HU80 (100) + HU140 + 2000).
Stone analysis
Following retrieval of all stones by endourologic techniques, they were sent to the laboratory for chemical analysis. Seven patients with calculi, for which extracorporeal treatment was possible, underwent extracorporeal shockwave lithotripsy (ESWL). Fifty two patients were treated by ureterorenoscopy to extract calculi using either semirigid or flexible endoscopes. Five patients with very large stones were treated by percutaneous nephrolithotomy (PCNL). The stones were analyzed by infrared spectroscopy (Nicolet 380 FT-IR, Thermo Electron Corporation) at our laboratory. Stones were classified by the predominant component. Calculi with a chemical composition of more than 80 % of a single component on infrared spectroscopy were considered as pure. Results of the chemical analysis were documented and correlated with the results of dual-energy CT and image post-processing.
Statistical analysis
The ratios were expressed as means ± standard deviations. p values <0.05 were considered statistically significant. All statistical analysis was performed using IBM SPSS Statistics (version 23, IBM). The comparison was made using the averages of DE indices for the single-energy and attenuation for the weighted-average images. A Student’s t-test for independent samples was performed to check for significant differences between the average values for the weighted-average images and the DE indices.
Results
In all 64 patients, a total of 213 stones could be localized and extracted from the urinary tract. In 12 patients, the calculi were located in the renal parenchyma and in 33 patients in the renal pelvicalyceal system. The remaining stones were found in the ureter: in the distal ureter (n = 11), in the middle ureter (n = 7) and in the proximal ureter (n = 18). The size of stones ranged from 4.6 to 30.6 mm with a median of 10.8 ± 7.1 mm.
Pure calculi with a single chemical component >80 % were classified into uric acid (n = 9), calcium oxalate monohydrate (n = 26), calcium oxalate dihydrate (n = 4), calcium hydroxyapatite (n = 7), brushite (n = 1) and cystine (n = 1). Mixed stones were classified into the following groups: mixed uric acid-containing (n = 2) and mixed non-uric acid-containing stones (n = 14).
Using the weighted-average images from the DECT scan simulating a regular 120 kV scan, stone densities for mixed calcium-containing stones were 1287 HU (976 HU). The density values of uric acid stones ranged from 352 to 730 HU (435 and 472 HU) at 120 kV, from 364 to 775 HU (438–472 HU) at 80/100 kV, and at 140 (Sn 140) kV from 346 to 601 HU (437–471 HU). Mean density values, standard deviations and the associated mean DE ratios for different stone groups are given in Tables 2 and 3.
The results of DECT scans and postoperative infrared spectroscopic analysis had an agreement of 98.4 %. Only one pure uric acid stone of a diameter of 5.4 mm was classified as a calcium-containing stone with blue color by DECT examination, and stone analysis showed a chemical composition of uric acid (90 %) and calcium hydroxyapatite (10 %). Detection of uric acid calculi and differentiation from non-UA-containing calculi with a sensitivity of 98.4 % (8/53) and a specificity of 98.1 % (52/53) were possible with a positive predictive value of 88.9 % and a negative predictive value of 91.1 %. For all other stones, namely COM, COD, brushite, cystine, calap, and mixed calculi, sensitivity and specificity were both 100 %.
The DE ratio of uric acid was 0.018 (0.001). The DE ratio from pure uric acid calculi was significantly different from stones of other chemical composition with a p value of 0.001, respectively, and 0.03 for the Flash scanner. Comparing mixed stones, COM, COD, calap and brushite, there were no statistically significant differences at p values ranging from 0.08 to 0.95 (0.15–0.91). Examinations performed on any of the two both dual-source scanners could not discriminate different groups of mixed calcium-containing stones. There was no significant difference found between these groups with a high overlap of the HU and DE ratios.
The mean CTDIvol for DECT scans of the abdomen was 11.9 ± 4.9 mGy/cm, with the minimum CTDIvol being 4.49 and the maximum 31.17. This resulted in a mean effective patient dose of 4.18 mSv ± 2.9 mSv (minimum, 0.44 mSv; maximum, 14.27 mSv). Considering the two scanners used for this study separately, there is an effective patient dose with a minimum 0.95 and maximum 14.27 mSv for the Definition scanner and a minimum of 0.44 and maximum 10.46 mSv for the Flash scanner with an equal mean effective dose of 4.2 mSv.
Discussion
Information regarding stone composition is of utmost value for decision of treatment strategies and also predicting success of lithotripsy to optimize management of urolithiasis. As yet, there was no reliable method of determining stone composition prior to retrieval and analysis. The knowledge of stone composition prior to retrieval would improve patient management. This may include treatment of uric acid stones with medical chemolitholysis, which has reported success rates as high as 70–80 % and could avoid patients from potentially unnecessary and invasive therapies [14]. ESWL could also be spared for patients with cystine or calcium oxalate monohydrate stones. This would potentially reduce costs and results in more cost-effective management of patients with urolithiasis [15].
Stone detection and discrimination in a single acquisition using DECT are possible. DECT is an accurate, noninvasive examination in patients with known or suspected urinary calculi. A number of preliminary studies have shown the feasibility to differentiate calculi in vitro [5, 8].
This in vivo study with 64 patients, including 213 examined urinary stones, was performed to demonstrate whether dual-energy CT is a reliable imaging examination to detect and discriminate uric acid from stones of other chemical composition. Most of our findings were stones consisting COM, concordant with the regional epidemiology [16]. DE ratio of 0.001 detected uric acid calculi with a sensitivity of 88.9 % (8/9) and a specificity of 98.1 % (52/53) with a positive predictive value of 88.9 % and a negative of 91.1 %. For all other stones, namely COM, COD, brushite, cystine, calap, and mixed calculi, both the sensitivity and the specificity were 100 %.
It is obvious that single-energy CT scans are an inadequate examination for stone characterization as there is a substantial overlap in 120 kV density between uric acid, mixed calculi, struvite, and cystine [17]. Using the information of dual-energy CT scans with two separate X-ray tubes and a simultaneous acquisition, it has recently become possible. It can be ideally used to reliably differentiate between different chemical compositions of urinary calculi. In contrast to other previous studies, our study was performed in vivo with a larger patient population. Using dual-energy CT, we could differentiate between the clinically important categories of uric acid and non-uric acid stones. This is not possible in scans with one X-ray spectrum due to the overlap of density values [18].
In the initial part of our study, we performed a dual-energy CT of the kidneys, ureter, and bladder as part of the workup for suspected urolithiasis. In the later part of the study, the protocol was modified to minimize radiation exposure by performing a low-dose scan of the entire abdomen. If calculi were identified by the radiologist while the patient was on the scanner table, a short dual-energy acquisition was performed in the stone-containing area only. This could reduce the effective dose significantly to 2–4 mSv. Qu et al. [19] showed a minimal effect in characterization of stones using DECT with a 75 % radiation dose reduction protocol related to a 16-mGy routine dose. This could be also an effective possibility to reduce radiation exposure.
Our study has a few limitations. Firstly, some rare stone types are underrepresented; there were just one brushite and one cystine-containing stone in our patient population, which could be detected and analyzed afterward. For these types of stones, no statistically meaningful data could be obtained. To determine whether a further differentiation between the subgroups of non-uric acid-containing stones is possible, further prospective studies with a larger sample size are required. Likewise, the retrospective study harbored potential bias, the number of included patients is small, and the use of different techniques of CT scan should be mentioned.
In conclusion, DECT can be employed to reliably predict the composition of urinary calculi as a noninvasive in vivo examination. This is very useful for the clinical management of patients with urolithiasis and may also increase cost-effectiveness of the stone treatment.
References
Litwin M, Saigal C (2007) Urologic diseases in America. NIH Publication No. 07–5512. Washington, DC: US Department of Health and Human Services. Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, US Government Publishing Office
Curhan GC (2007) Epidemiology of stone disease. Urol Clin North Am 34(3):287–293
Smith RC, Rosenfield AT, Choe KA, Essenmacher KR, Verga M, Glickman MG et al (1995) Acute flank pain: comparison of non-contrast-enhanced CT and intravenous urography. Radiology 194(3):789–794
Türk C, Knoll T, Petrik A, Sarica K, Straub M, Seitz C (2011) Guidelines on urolithiasis. European Association of Urology, pp 12–15
Saita A, Bonaccorsi A, Motta M (2007) Stone composition: where do we stand? Urol Int 79(Suppl 1):16–19
Deveci S, Coskun M, Tekin MI, Peskircioglu L, Tarhan NC, Ozkardes H (2004) Spiral computed tomography: role in determination of chemical compositions of pure and mixed urinary stones—an in vitro study. Urology 64(2):237–240
Nakada SY, Hoff DG, Attai S, Heisey D, Blankenbaker D, Pozniak M (2000) Determination of stone composition by noncontrast spiral computed tomography in the clinical setting. Urology 55(6):816–819
Johnson TR, Krauss B, Sedlmair M, Grasruck M, Bruder H, Morhard D et al (2007) Material differentiation by dual energy CT: initial experience. Eur Radiol 17(6):1510–1517
Ferrandino MN, Pierre SA, Simmons WN, Paulson EK, Albala DM, Preminger GM (2010) Dual-energy computed tomography with advanced postimage acquisition data processing: improved determination of urinary stone composition. J Endourol/Endourol Soc 24(3):347–354
Li XH, Zhao R, Liu B, Yu YQ (2013) Determination of urinary stone composition using dual-energy spectral CT: initial in vitro analysis. Clin Radiol 68(7):370–377
Yildirim D, Ozturk O, Tutar O, Nurili F, Bozkurt H, Kayadibi H et al (2014) A new method for computer-assisted detection, definition and differentiation of the urinary calculi. Ren Fail 36(8):1278–1282
Manglaviti G, Tresoldi S, Guerrer CS, Di Leo G, Montanari E, Sardanelli F et al (2011) In vivo evaluation of the chemical composition of urinary stones using dual-energy CT. Am J Roentgenol 197(1):76–83
Stolzmann P, Leschka S, Scheffel H, Rentsch K, Baumüller S, Desbiolles L et al (2010) Characterization of urinary stones with dual-energy CT: improved differentiation using a tin filter. Invest Radiol 45(1):1–6
Ngo TC, Assimos DG (2007) Uric acid nephrolithiasis: recent progress and future directions. Rev Urol 9(1):17–27
Eaton SH, Cashy J, Pearl JA, Stein DM, Perry K, Nadler RB (2013) Admission rates and costs associated with emergency presentation of urolithiasis: analysis of the Nationwide Emergency Department Sample 2006–2009. J Endourol/Endourol Soc 27(12):1535–1538
Ansari MS, Gupta NP, Hemal AK, Dogra PN, Seth A, Aron M et al (2005) Spectrum of stone composition: structural analysis of 1050 upper urinary tract calculi from northern India. Int J Urol 12(1):12–16
Motley G, Dalrymple N, Keesling C, Fischer J, Harmon W (2001) Hounsfield unit density in the determination of urinary stone composition. Urology 58(2):170–173
Mostafavi MR, Ernst RD, Saltzman B (1998) Accurate determination of chemical composition of urinary calculi by spiral computerized tomography. J Urol 159(3):673–675
Qu M, Yu L, Cardona DG, Liu Y, Duan X, Ai S et al (2015) Radiation dose reduction in dual-energy CT: does it affect the accuracy of urinary stone characterization? Am J Roentgenol 205(2):W172–W176
Author’s contribution
A. Spek wrote the manuscript and was involved in project development and data management; F. Strittmatter was involved in data collection and data management; A. Graser was involved in data collection and edited the manuscript; P. Kufer was involved in data collection and analysis; C. Stief was involved in project development; M. Staehler edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Spek, A., Strittmatter, F., Graser, A. et al. Dual energy can accurately differentiate uric acid-containing urinary calculi from calcium stones. World J Urol 34, 1297–1302 (2016). https://doi.org/10.1007/s00345-015-1756-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-015-1756-4