Abstract
Purpose
Positive surgical margins (PSMs) after radical prostatectomy (RP) are a known factor associated with biochemical recurrence (BCR) and raise the issue of adjuvant treatment by radiotherapy versus salvage treatment at recurrence. To help this choice, our study aimed to analyze BCR-free survival and factors associated with BCR in patients with PSM and undetectable postoperative prostate-specific antigen (PSA).
Methods
Between 2005 and 2008, 630 patients had RP for localized prostate cancer in our center. We included patients with PSM, uninvaded nods, undetectable postoperative PSA and no adjuvant treatment. The 5-year BCR-free survival was calculated using Kaplan–Meier method. Logistic regression models were used to determine the factors associated with BCR in univariate and multivariate analyses (Cox model).
Results
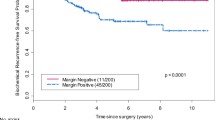
The PSM rate was 32.7 % (n = 206 patients), and 110 patients corresponded to the inclusion criteria. The median follow-up was 72 months. The BCR rate was 30 % with a 5-year BCR-free survival of 83.9 %. The factors significantly associated with BCR were preoperative PSA, predominance and percentage of Gleason 4, tumor volume, PSM length and predominance of Gleason 4 at the margin. In the multivariate analysis, the remaining two significant factors were PSM length [OR 4.35, 95 % CI (1.011–1.421), p = 0.037] and tumor volume [OR 4.29, 95 % CI (1.011–1.483), p = 0.038].
Conclusion
Over a 5-year follow-up, only one-third of patients experienced BCR. It might be reasonable to postpone adjuvant radiotherapy for patients with PSM and undetectable PSA after RP. Tumor volume and PSM length were associated with BCR and should be taken into account in the postoperative treatment management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Positive surgical margins (PSMs) are a frequent situation that is encountered after radical prostatectomy (RP) for localized prostate cancer, with series reporting a rate of 10–40 % [1]. Yet, the impact of PSM on the clinical outcome and on the risk of biochemical recurrence (BCR) is still unclear. Several studies concluded that a positive surgical margin is an independent factor of local recurrence with a two- to fourfold increased risk [2, 3]. However, according to other studies, 40–50 % of patients with PSM did not show recurrence with a BCR rate varying widely from 11 to 64 % [4]. Therefore, dealing with PSM after RP is a debatable situation where the issue of an adjuvant treatment is raised. According to the last urologic guidelines [5–7], patients at high risk of local recurrence after RP (e.g., PSM, seminal vesicle invasion or extra-prostatic extension) can be treated either by immediate adjuvant radiotherapy or by salvage radiotherapy before prostate-specific antigen (PSA) level exceeds 0.5 ng/ml. While the choice is still an option, urologists and radiotherapists frequently disagree about the indication of adjuvant radiation therapy [8]. In order to find new helpful arguments, the purpose of our study was to determine the BCR-free survival and the recurrence risk factors for patients who underwent RP with PSM and undetectable postoperative PSA and who did not receive adjuvant radiotherapy.
Materials and methods
Patient selection
We reviewed our pathologic database including 630 patients who underwent RP for clinically localized prostate cancer in our institution between 2005 and 2008. Our study included patients who had PSM and undetectable postoperative PSA, who did not receive adjuvant radiotherapy nor androgen-deprivation therapy. Exclusion criteria were patients with positive lymphadenectomy (N+), detectable postoperative PSA, any neo-adjuvant or adjuvant therapy and incomplete follow-up. The study was approved by the local ethics committee, and all patients gave their informed consent before the study.
Database
RP was performed by experienced surgeons, using open surgery (retropubic approach) or laparoscopy. All prostate specimens were inked (green ink on the left side of the specimen, black on the right side), entirely enclosed and sectioned at 5-mm intervals and examined following the standard Stanford protocol as described in the International Society of Urological Pathology (ISUP) Consensus Conference (last update [9]). After 2005, all prostate specimens were cross-checked by two uropathologists. If PSMs were found, further examinations were assessed with sections at 30-μm intervals. Surgical margins were considered as positive when tumor cells reached the inked surface of the prostate specimen, with re-evaluation in doubtful cases. The characteristics of the surgical margins including the length, the site (intra- or extra-prostatic) and the Gleason score at the margin were described. Patients were followed up with clinical examination and PSA test at 3-month intervals during the first year, every 6 months the second year, and annually thereafter. We retrospectively collected clinical data (age, preoperative and postoperative PSA, PSA level during follow-up) and histological data on prostate specimen (prostate weight, tumor volume, Gleason score, pathologic T stage, seminal vesicle invasion, extra-prostatic extension, perineural invasion). The main tumor volume was calculated by the formula: \({\text{volume}} \; = \left( {\varPi /6} \right) \times {\text{width}} \times \text{ }{\text{length}} \times {\text{height}}\) in mm3.
Study outcomes
The primary outcome of the study was the 5-year BCR-free survival. BCR was defined as a PSA level >0.2 ng/ml confirmed by a second consecutive blood test. The secondary outcome was the analysis of the factors that were associated with BCR.
Statistical analyses
The 5-year BCR-free survival was estimated using the Kaplan–Meier method. Logistic regression models were used to determine the factors associated with BCR in univariate analyses. Significant variables on the univariate analysis were included in the multivariate analysis using Cox model. Statistical tests were performed as two-sided with a p value <0.05 considered as significant. Statistics were calculated using the SPSS software (version 11.0, SPSS Inc., Chicago, IL, USA).
Results
Among the 630 patients who underwent RP between 2005 and 2008, 206 had a PSM (32.7 %), with 110 patients corresponding to the inclusion criteria. The clinical and histological characteristics of the 110 patients are described in Table 1. Seventy-four (67.3 %) and thirty-six (32.7 %) patients had a prostate specimen, respectively, staged pT2 and pT ≥ 3. The tumors were registered as Gleason 7 in 76 cases (69 %). The mean follow-up was 72 months. The BCR rate was 30 % (n = 33) with a 5-year BCR-free survival of 83.9 ± 0.04 % (Fig. 1). One patient (0.9 %) died from metastatic progression after BCR, and one (0.9 %) patient died from a metastatic kidney cancer. No other metastatic progression was noted. The mean surgical margins length was 3.0 ± 3.1 mm (median 2.0 mm, range 0.1–15.0 mm). In the univariate model, factors significantly associated with BCR were the preoperative PSA, the percentage of Gleason 4 on the prostate specimen, the Gleason 4 predominance on the prostate specimen, the tumor volume, the margin length and the predominance of Gleason 4 at the margin (Table 2). In our selected PSM population, neither age, pathological Gleason score, the presence of extra-prostatic perineural invasion, nor extra-prostatic extension were significant factors associated with BCR. In the multivariate model, the factors that remained significant for BCR risk were the tumor volume [OR 4.29, 95 % CI (1.011–1.483), p = 0.038] and the length of the PSM [OR 4.35, 95 % CI (1.011–1.421), p = 0.037].
Discussion
Radical prostatectomy is one of the standard treatments for men with localized prostate cancer. The two major aims of surgical treatment are the oncological outcome, through complete removal of the tumor, and the functional outcome, through conservation of urinary and erectile functions. PSM can occur as a result of this balance and is a difficult situation to deal with. While many studies have tried to determine the oncological impact of PSM, the choice between adjuvant radiotherapy and salvage radiotherapy remains debatable [5].
In our study, we had a PSM rate of 33 %. This result was comparable to previous published series where PSM rate varied from 10 to 40 % [1]. PSM definition has been clarified as any tumor cell reaching the inked surface of the prostate specimen [10]. Many artifacts can influence the diagnosis and lead to false positives, such as diathermy-induced lesions, intra-prostatic surgical incision or tissue processing [11]. PSM diagnosis could also depend on the pathologist experience. The PSM rate reported in the present study could be explained by our pathologic procedure: All prostate specimens were analyzed by senior uropathologists, with millimeter sections and a double-check inducing accurate diagnosis of true PSM. PSM rate has also been related to pathologic stage (24–63 % of PSM for pT3), Gleason score, tumor volume, preoperative PSA and obesity or to surgical expertise [12].
Approximately 10–20 % of the patients who underwent RP for localized prostate cancer will experience BCR. This rate can increase to 40–60 % when adverse pathologic factors are associated (such as PSM, seminal vesicle invasion or extra-prostatic extension) [13]. PSMs have been related to BCR with a two- to fourfold increased risk [2, 3]. In our study, the BCR rate was 30 % with a 5-year BCR-free survival of 83.9 %. PSM length and tumor volume were two significant pathologic features associated with BCR. The association between increasing margin length and BCR risk has been previously reported [14–16] but contested by other studies [17, 18]. The International Society of Urological Pathology (ISUP) recommended reporting the margin length in millimeters despite any consensus on a significant prognosis-associated threshold [10]. In other series, BCR was also related to the Gleason grade at the margin [19, 20]. In our univariate analysis, Gleason 4 at the margin was associated with BCR, but this result was not significant in the multivariate model. At this stage, no consensus exists to define significant margin-associated variables and their influence on biochemical recurrence [1].
Corcoran et al. [21] showed a significant increased risk of BCR exclusively in intermediate-risk cancer. Other studies supported this result with the hypothesis that either a high or a low risk cancer would or would not expose to BCR regardless of the presence of a PSM, because of its inherent aggressiveness or indolence [22, 23]. Furthermore, the majority of patients (60–70 %) with adverse pathologic features such as PSM and/or extra-prostatic extension without seminal vesicle invasion would be cured by RP alone [13, 24]. In our multivariate analysis, extra-prostatic extension was not significantly associated with BCR for patients with PSM. Thus, the mere presence of a PSM does not necessarily mean BCR and should not lead to systematic adjuvant therapy. Knowing that PSMs have been associated with an increased risk of BCR, it is now the associated factors which could predict this recurrence that must be identified. The best approach in order to propose an adjuvant therapy for patients with PSM should clearly be based on a global evaluation of associated clinical and histological adverse factors.
How to select patients who would benefit from adjuvant radiotherapy and spare those who would not relapse? Three large randomized trials compared adjuvant radiotherapy versus observation for pathologically advanced prostate cancer. The Southwest Oncology Group (SWOG) 8794 trial reported greater metastasis-free survival and overall survival in the postoperative radiotherapy arm [25]. The European Organization for Research and Treatment of Cancer (EORTC) 22,911 trial reported a significantly improved 10-year cumulative biochemical progression-free survival for the postoperative irradiation group compared with the wait-and-see group (60.6 vs. 41 %, respectively, p < 0.001), but no significant difference was found for clinical progression or overall survival, likewise for the German Cancer Society (ARO 96-02) trial [26, 27]. In the EORTC and SWOG trials, the postoperative PSA was not necessarily undetectable prior to study. Furthermore, in the three trials, many patients from the wait-and-see groups who experienced BCR did not receive salvage radiation therapy or received it after the PSA level had reached a level at which salvage treatment is known to be less effective. Thus, for patients with adverse pathologic factors, early salvage radiotherapy has not yet been compared to immediate adjuvant therapy. The current Radiotherapy Adjuvant versus Early Salvage following Radical Prostatectomy (RAVES), Radiotherapy and Combined Androgen Deprivation after Local Surgery (RADICALS) and Groupe d’Etude des Tumeurs Uro-Génitales-17 (GETUG-17) randomized trials, including pT3R1 patients for either adjuvant or salvage radiotherapy, will study this issue [28–30].
We acknowledge several limits in the present study. First, the data were retrospectively collected. The PSM rate is suspected of being dependent on the surgeon and pathologist expertise, with the risk of center bias. Our relatively small cohort could be explained by our stringent selection criteria, which only included patients with undetectable postoperative PSA and no adjuvant nor neo-adjuvant therapy. In the present analysis, we studied the onset of BCR. This criteria was widely studied in the literature despite its low clinical significance for the patient, unlike prostate cancer-specific mortality or metastasis-free survival. However, the main question with PSM is whether adjuvant radiotherapy is preferable to salvage radiotherapy at time of recurrence. Thus, if BCR has a low clinical significance, it definitely has a therapeutic impact for the patient, justifying the pertinence of its analysis in the controversy regarding adjuvant therapy after radical prostatectomy.
Conclusions
In our study, two-thirds of the patients with PSM after RP, with undetectable postoperative PSA and no adjuvant therapy, did not experience BCR. Close attention must be paid on PSM length and tumor volume, pathologic features that might be related to BCR. As a PSM does not always mean BCR, prospective studies are needed to clearly identify, through clinical and histological data, which patients are likely to benefit from adjuvant radiotherapy with the objective of sparing the many others from overtreatment.
References
Fontenot PA, Mansour AM (2013) Reporting positive surgical margins after radical prostatectomy: time for standardization. BJU Int 111(8):E290–E299. doi:10.1111/j.1464-410X.2012.11640.x
Staerman F, Soulie M, Tostain J, de Fromont M, Davin JL, Coulange C (2006) Management of positive margins after total prostatectomy for localized prostate cancer. Progres en urologie: journal de l’Association francaise d’urologie et de la Societe francaise d’urologie 16(3):286–291
Cormier L, Bastide C, Beuzeboc P, Fromont G, Hennequin C, Mongiat-Artus P, Peyromaure M, Ploussard G, Renard-Penna R, Richaud P, Rozet F, Soulie M, Salomon L (2014) Prostate cancer surgical margin: CCAFU review. Progres en urologie : journal de l’Association francaise d’urologie et de la Societe francaise d’urologie 24(6):334–345. doi:10.1016/j.purol.2013.11.006
Simon MA, Kim S, Soloway MS (2006) Prostate specific antigen recurrence rates are low after radical retropubic prostatectomy and positive margins. J Urol 175(1):140–144. doi:10.1016/s0022-5347(05)00050-9 ; discussion 144–145
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N (2014) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65(1):124–137. doi:10.1016/j.eururo.2013.09.046
Thompson IM, Valicenti RK, Albertsen P, Davis BJ, Goldenberg SL, Hahn C, Klein E, Michalski J, Roach M, Sartor O, Wolf JS Jr, Faraday MM (2013) Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol 190(2):441–449. doi:10.1016/j.juro.2013.05.032
Salomon L, Bastide C, Beuzeboc P, Cormier L, Fromont G, Hennequin C, Mongiat-Artus P, Peyromaure M, Ploussard G, Renard-Penna R, Rozet F, Azria D, Coloby P, Molinie V, Ravery V, Rebillard X, Richaud P, Villers A, Soulie M (2013) CCAFU Recommendations 2013: prostate cancer. Progres en urologie : journal de l’Association francaise d’urologie et de la Societe francaise d’urologie 23(Suppl 2):S69–S101. doi:10.1016/s1166-7087(13)70048-4
Lavallee LT, Fergusson D, Mallick R, Grenon R, Morgan SC, Momoli F, Witiuk K, Morash C, Cagiannos I, Breau RH (2013) Radiotherapy after radical prostatectomy: treatment recommendations differ between urologists and radiation oncologists. PLoS One 8(11):e79773. doi:10.1371/journal.pone.0079773
Samaratunga H, Montironi R, True L, Epstein JI, Griffiths DF, Humphrey PA, van der Kwast T, Wheeler TM, Srigley JR, Delahunt B, Egevad L (2011) International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. In: Working group 1: specimen handling. Mod Pathol 24(1):6–15. doi:10.1038/modpathol.2010.178
Tan PH, Cheng L, Srigley JR, Griffiths D, Humphrey PA, van der Kwast TH, Montironi R, Wheeler TM, Delahunt B, Egevad L, Epstein JI (2011) International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. In: Working group 5: surgical margins. Mod Pathol 24(1):48–57. doi:10.1038/modpathol.2010.155
Evans AJ, Henry PC, Van der Kwast TH, Tkachuk DC, Watson K, Lockwood GA, Fleshner NE, Cheung C, Belanger EC, Amin MB, Boccon-Gibod L, Bostwick DG, Egevad L, Epstein JI, Grignon DJ, Jones EC, Montironi R, Moussa M, Sweet JM, Trpkov K, Wheeler TM, Srigley JR (2008) Interobserver variability between expert urologic pathologists for extraprostatic extension and surgical margin status in radical prostatectomy specimens. Am J Surg Pathol 32(10):1503–1512. doi:10.1097/PAS.0b013e31817fb3a0
Fleshner NE, Evans A, Chadwick K, Lawrentschuk N, Zlotta A (2010) Clinical significance of the positive surgical margin based upon location, grade, and stage. Urol Oncol 28(2):197–204. doi:10.1016/j.urolonc.2009.08.015
Stephenson AJ, Bolla M, Briganti A, Cozzarini C, Moul JW, Roach M 3rd, van Poppel H, Zietman A (2012) Postoperative radiation therapy for pathologically advanced prostate cancer after radical prostatectomy. Eur Urol 61(3):443–451. doi:10.1016/j.eururo.2011.10.010
Shikanov S, Marchetti P, Desai V, Razmaria A, Antic T, Al-Ahmadie H, Zagaja G, Eggener S, Brendler C, Shalhav A (2013) Short (≤1 mm) positive surgical margin and risk of biochemical recurrence after radical prostatectomy. BJU Int 111(4):559–563. doi:10.1111/j.1464-410X.2012.11340.x
Cao D, Humphrey PA, Gao F, Tao Y, Kibel AS (2011) Ability of linear length of positive margin in radical prostatectomy specimens to predict biochemical recurrence. Urology 77(6):1409–1414. doi:10.1016/j.urology.2010.10.059
Ochiai A, Sotelo T, Troncoso P, Bhadkamkar V, Babaian RJ (2008) Natural history of biochemical progression after radical prostatectomy based on length of a positive margin. Urology 71(2):308–312. doi:10.1016/j.urology.2007.08.042
Leite KR, Hartmann C, Reis ST, Viana N, Dall’Oglio MF, Sant’Anna AC, Nesrallah A, Nesrallah L, Antunes AA, Camara-Lopes LH, Srougi M (2014) Biochemical recurrence rates are similar for pT2-positive surgical margins and pT3a. International braz j urol: official journal of the Brazilian Society of Urology 40(2):146–153. doi:10.1590/s1677-5538.ibju.2014.02.03
Marks RA, Koch MO, Lopez-Beltran A, Montironi R, Juliar BE, Cheng L (2007) The relationship between the extent of surgical margin positivity and prostate specific antigen recurrence in radical prostatectomy specimens. Hum Pathol 38(8):1207–1211. doi:10.1016/j.humpath.2007.01.006
Savdie R, Horvath LG, Benito RP, Rasiah KK, Haynes AM, Chatfield M, Stricker PD, Turner JJ, Delprado W, Henshall SM, Sutherland RL, Kench JG (2012) High Gleason grade carcinoma at a positive surgical margin predicts biochemical failure after radical prostatectomy and may guide adjuvant radiotherapy. BJU Int 109(12):1794–1800. doi:10.1111/j.1464-410X.2011.10572.x
Cao D, Kibel AS, Gao F, Tao Y, Humphrey PA (2010) The Gleason score of tumor at the margin in radical prostatectomy is predictive of biochemical recurrence. Am J Surg Pathol 34(7):994–1001. doi:10.1097/PAS.0b013e3181e103bf
Corcoran NM, Hovens CM, Metcalfe C, Hong MK, Pedersen J, Casey RG, Peters J, Harewood L, Goldenberg SL, Costello AJ, Gleave ME (2012) Positive surgical margins are a risk factor for significant biochemical recurrence only in intermediate-risk disease. BJU Int 110(6):821–827. doi:10.1111/j.1464-410X.2011.10868.x
Choo MS, Cho SY, Ko K, Jeong CW, Lee SB, Ku JH, Hong SK, Byun SS, Kwak C, Kim HH, Lee SE, Jeong H (2014) Impact of positive surgical margins and their locations after radical prostatectomy: comparison of biochemical recurrence according to risk stratification and surgical modality. World J Urol 32(6):1401–1409. doi:10.1007/s00345-013-1230-0
Ploussard G, Drouin SJ, Rode J, Allory Y, Vordos D, Hoznek A, Abbou CC, de la Taille A, Salomon L (2014) Location, extent, and multifocality of positive surgical margins for biochemical recurrence prediction after radical prostatectomy. World J Urol 32(6):1393–1400. doi:10.1007/s00345-014-1243-3
Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT (2002) Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol 167(2 Pt 1):528–534
Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, Messing E, Forman J, Chin J, Swanson G, Canby-Hagino E, Crawford ED (2009) Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 181(3):956–962. doi:10.1016/j.juro.2008.11.032
Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, Verbaeys A, Bosset JF, van Velthoven R, Colombel M, van de Beek C, Verhagen P, van den Bergh A, Sternberg C, Gasser T, van Tienhoven G, Scalliet P, Haustermans K, Collette L (2012) Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 380(9858):2018–2027. doi:10.1016/s0140-6736(12)61253-7
Wiegel T, Bartkowiak D, Bottke D, Bronner C, Steiner U, Siegmann A, Golz R, Storkel S, Willich N, Semjonow A, Stockle M, Rube C, Rebmann U, Kalble T, Feldmann HJ, Wirth M, Hofmann R, Engenhart-Cabillic R, Hinke A, Hinkelbein W, Miller K (2014) Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol 66(2):243–250. doi:10.1016/j.eururo.2014.03.011
Pearse M, Fraser-Browne C, Davis ID, Duchesne GM, Fisher R, Frydenberg M, Haworth A, Jose C, Joseph DJ, Lim TS, Matthews J, Millar J, Sidhom M, Spry NA, Tang CI, Turner S, Williams SG, Wiltshire K, Woo HH, Kneebone A (2014) A Phase III trial to investigate the timing of radiotherapy for prostate cancer with high-risk features: background and rationale of the Radiotherapy-Adjuvant Versus Early Salvage (RAVES) trial. BJU Int 113(Suppl 2):7–12. doi:10.1111/bju.12623
Parker C, Clarke N, Logue J, Payne H, Catton C, Kynaston H, Murphy C, Morgan R, Morash C, Parulekar W, Parmar M, Savage C, Stansfeld J, Sydes M (2007) Radiotherapy and androgen deprivation in combination after local surgery (RADICALS). Clin Oncol (Royal College of Radiologists (Great Britain)) 19(3):167–171. doi:10.1016/j.clon.2007.01.001
Guerif S, Latorzeff I, Lagrange JL, Hennequin C, Supiot S, Garcia A, Francois P, Soulie M, Richaud P, Salomon L (2014) Postoperative radiotherapy of prostate cancer. Cancer radiotherapie: journal de la Societe francaise de radiotherapie oncologique 18(5–6):517–523. doi:10.1016/j.canrad.2014.07.149
Conflict of interest
None.
Ethical statement
The study was approved by the local ethics committee, and all patients gave their informed consent before the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pettenati, C., Neuzillet, Y., Radulescu, C. et al. Positive surgical margins after radical prostatectomy: What should we care about?. World J Urol 33, 1973–1978 (2015). https://doi.org/10.1007/s00345-015-1580-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-015-1580-x