Abstract
Purpose
Compared to low-grade disease, high-grade prostate cancers exhibit a higher rate of disease progression. As a result, there has been a trend to treat high-risk disease with methods other than surgery. The purpose of this study is to evaluate the long-term survival following radical prostatectomy (RRP) for non-metastatic Gleason 8–10 prostate adenocarcinoma (CaP).
Methods
All patients 75 years or less with Gleason 8–10 CaP that underwent RRP were identified from the SEER 18 database. Patients with metastatic disease, those who underwent other modalities of treatment, or with more than one primary cancer, were excluded. Data were analyzed for demographics, stage at presentation, treatment modality, and overall survival and cancer-specific survival.
Results
A total of 30,379 men met inclusion criteria. Mean age was 62.5 years and 82.5 % of patients were white. A total of 52.8 % of patients had T2 disease, and 73.1 % had node-negative disease, 80.2 % of patients underwent pelvic lymph node dissection, and 12.9 % underwent adjuvant radiation therapy. Overall survival for the entire cohort was 92.8, 78.6, 59.5, 38.6, and 20.0 % for 5, 10, 15, 20, and 25 years, respectively. Cancer-specific survival was 96.4, 89.5, 82.0, 72.9, and 68.8 % for 5, 10, 15, 20, and 25 years, respectively.
Conclusions
Although historically underutilized in patients with poorly differentiated disease, radical prostatectomy provides excellent long-term survival and should be offered to healthy patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the most common cancer in men. In 2012, approximately 241,740 patients in the USA were diagnosed with prostate cancer and approximately 28,170 patients were died from the disease [1].
Compared to low-grade disease, high-grade prostate cancers exhibit a higher rate of disease progression [2]. Up to 85 % of men with high-grade disease will have distant metastases by 5 years [3], and if left untreated, up to 87 % of these men will die of the disease within 15 years of diagnosis [4]. Multiple studies have shown pathologic grade to be an independent risk factor for disease prognosis [5, 6]. Patients with poorly differentiated prostate cancer usually have a high tumor volume and are more likely to have extraprostatic disease.
There is reluctance among some urologists to offer radical prostatectomy because of the higher incidence of lymph node metastasis, local and systemic recurrence, and poor survival [7, 8]. As a result, there has been a trend to treat high-risk disease with methods other than surgery. An investigation of prostate cancer treatment patterns from the Cancer of the Prostate Strategic Urological Research Endeavor disease registry showed that when compared to surgical treatment, patients with Gleason 8–10 cancers were 1.9 times more likely to have external beam radiation therapy and 3.9 times more likely to receive primary androgen deprivation [9].
The current study evaluates the long-term survival following radical prostatectomy (RP) for poorly differentiated prostatic adenocarcinoma.
Methods
All patients with a diagnosis of poorly differentiated adenocarcinoma of the prostate between 1973 and 2009 were identified from the SEER 18 registry dataset (1973–2009) of the US National Cancer Institute. SEER is a population-based cancer registry and currently collects and publishes cancer incidence and survival data from population-based cancer registries covering approximately 28 percent of the US population. The SEER Program registries routinely collect data on patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and overall survival (OS) and cancer-specific survival (CSS).
Database search, patient population, and definitions
The SEER 18 database was searched in March 2013 for all men from the SEER 18 registry with a diagnosis of prostate cancer (ICD-09 code 16.9), adenocarcinoma (ICD-09-03 code 8140/3), and poorly differentiated (grade III) disease. Limitations were set to include patients between 20 and 75 years in whom surgery was performed and to exclude patients with metastatic disease, those that had radiation prior to surgery, patients that had intra-operative radiation, or those patients in which the sequence of radiation with surgery was unknown. A total of 91,135 patients were generated with this search. There were 76,308 patients with the surgery procedure codes 50, 60, or 70 that correspond to RP, RP with PLND, and prostatectomy. After excluding the Gleason 7 prostate cancers, 30,379 patients with Gleason 8, 9, or 10 CaP were identified and included in the study.
The lymph node fields were used to confirm the RRP patients that underwent PLND. The clinical T and the N stages were determined by different EOD fields. Gleason 8–10 cancers are classified as poorly differentiated cancer in the SEER database. In the year 2003, Gleason 7 was also classified as poorly differentiated cancers. The CS site-specific staging fields were used to identify the patients with Gleason 8–10 prostate cancer and exclude the Gleason 7 cancers from the analysis. Pathologic staging is only available for patients diagnosed after 1995 and was not included, and therefore, the clinical stage (cT) was used for this analysis. The ISPOR Scientific Task Force Report guidelines for conducting retrospective database studies were followed.
Statistical analysis
The primary and secondary end points of the study were CSS and OS. Data were presented as mean ± SD or median (IQR—interquartile range). Kaplan–Meier analysis, life table analysis, competing risk analysis, and the log-rank tests were used. Multivariate Cox regression analysis was conducted, and the model was adjusted for age at diagnosis, race, node status, year of diagnosis, T stage, and N stage. Hazard ratios with 95 % confidence intervals are shown where appropriate. The JMP®10 software was used for statistical analysis.
Results
Baseline characteristics
A total of 30,379 men met inclusion criteria. The mean age was 62.5 years, and 82.5 % of patients were white. 52.8 % of patients had T2 disease, and 73.1 % had node-negative disease. A total of 80.2 % of patients underwent pelvic lymph node dissection, and 12.9 % patients underwent adjuvant radiation therapy. The baseline characteristics are shown in Table 1.
Survival
Overall and cancer-specific survival
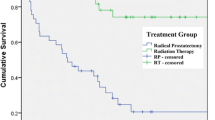
The overall survival for the entire cohort was 92.8, 78.6, 59.5, 38.6, and 20.0 % for 5, 10, 15, 20, and 25 years, respectively. Cancer-specific survival was 96.4, 89.5, 82.0, 72.9, and 68.8 % for 5, 10, 15, 20, and 25 years, respectively. Both the overall survival and the cancer-specific survival are shown in Table 2 as well as in Kaplan–Meier survival plots in Fig. 1.
The overall survival and cancer-specific survival by baseline characteristics are represented in Table 2 as well as in Cox regression multivariate analysis tables given in Table 3. Age did not significantly affect survival, and there was no difference in survival seen in race between whites and blacks; however, patients in the other races had a significantly higher survival (p < 0.001).
cT stage and N stage
Overall survival for T staging was 85.5 % at 10 years and 41.4 % at 20 years for cT1, 80.2/43.1 % for T2, 73.5/33.1 % for cT3 (NOS), 80.4 %/NA for T3a, 62.5/26.5 % for cT3b, and 64.3/28.6 % for T4 (p < 0.0001). Cancer-specific survival for cT1 was 93.9 % for 10 years and 84.1 % for 20 years, 91.2/77.9 % for cT2, 90.0/72.0 % for T3 (NOS), 92.3 %/NA for cT3a, 74.3/51.5 % for cT3b, and 77.7/61.6 % for cT4 (p < 0.0001). The overall survival by N stage was found to be 81.1 % at 10 years and 42.2 % at 20 years for N0, and 59.4/25.0 % for N1 staging (p < 0.0001). For 10-/20-year overall survival, the cancer-specific survival was 91.5/76.8 % for N0 stage and 71.3/47.9 % for N1 stage at p < 0.0001.
Overall survival by number of nodes was found to be at 81.1 % at 10 years and 42.2 % at 20 years for 0 node, 62.6/29.2 % for 1 node, 60.2/20.9 % for 2 nodes, 51.1 %/NA for 3 nodes, and 47.6 %/NA for ≥ 4 nodes (p < 0.0001). Cancer-specific survival for number of nodes was found to be 91.5 % at 10 years and 76.8 % at 20 years for 0 node, 75.2/54.0 % for 1 node, 72.0/41.8 %for 2 nodes, 60.5 %/NA for 3 nodes, and 57.9 %/NA for ≥4 nodes (p < 0.0001).
A significant decrease in survival was found in patients with a stage cT3 or worse. Also, patients who were positive for nodes were found to have a significant decrease in survival than those who were negative for nodes. Finally, patients with increased number of nodes were found to have a worse prognosis compared to patients with less number of positive nodes—specifically those with 3 or more positive nodes as compared to 2 or less positive nodes. These are all represented in Table 2 as well as in Figs. 2, 3, and 4 in Kaplan–Meier plots.
Adjuvant/secondary therapies
Overall survival by pelvic lymph node dissection was not found to be significant (79.1/37.6 % 10- and 20-year OS, respectively, without PLND, compared to 78.3/38.7 % with PLND). However, cancer-specific survival was found to be 91.1 % at 10 years and 73.6 % at 20 years in those without PLND, compared to 89.1 % at 10 years and 72.9 at 20 years (p < 0.01). Overall survival of adjuvant radiotherapy was found to be 79.8 % at 10 years and 38.9 % at 20 years in those without treatment, and 69.4 % at 10 years and 34.2 % at 20 years in those who had received treatment (p < 0.0001). Cancer-specific survival was 90.7 % at 10 years and 74.6 % at 20 years without radiotherapy, and 80.3 % at 10 years and 61.6 % at 20 years in those who had received radiotherapy (p < 0.0001). Therefore, patients who underwent PLND and radiotherapy were found to have hazard ratios that were worse as compared to those without the treatment. This is represented in Table 3 as well as in Fig. 5.
Discussion
There are several advantages to performing RP in patients with high-grade prostate cancer. It provides accurate tumor staging and grading by pathologic examination of the surgical specimen and can help predict which patients are more likely to benefit from adjuvant therapy. In addition, a significant proportion of these patients are down-staged on RP. Two recent reports suggest that more than one-third of the patients with a GS of 8–10 on biopsy will be down-staged to GS of ≤7 in the RRP specimen [10, 11], and another recent study shows that almost half of the diagnosed GS 8–10 cancers will have a lower Gleason score in the RP specimen [12].
In comparison with other treatments, studies show that RP can often provide a better outcome. Based on outcomes of one randomized controlled clinical trial, when watchful waiting and RP are compared, RP was associated with a lower risk of cancer recurrence, cancer-related death, and improved survival [13]. Tewari et al. [14] found that in Gleason 8–10 prostate cancer, the risk of cancer-specific death following RP was 68 % lower than for conservative treatment and 49 % lower than for RT (p < 0.001 and 0.053, respectively) and concluded that RP had the greatest impact on decreasing cancer-specific mortality in men with poorly differentiated prostate cancer. Finally, Shao et al. [15] found in a multivariate analysis that compared to radiation therapy in intermediate-/high-risk patients (Gleason 8–10), RP had a significantly higher prostate cancer-specific survival rate (76.3 % as compared to 63.3 %).
In this study, the survival after RP in high-grade prostate cancer was a major point of interest. The 10- and 20-year overall survival was 78.6 and 38.6 %, respectively, while the 10- and 20-year cancer-specific survival was 89.5 and 72.9 %, respectively. Previous studies also report improved survival following prostatectomy for Gleason 8–10 prostate cancer. Mian et al. [16] studied a group of 188 patients with GS 8–10 who underwent RRP. The 5- and 7-year OS rates were 71 and 55 %, respectively. Lughezzani et al. [17] found the 5- and 10-year CSS in a study of 578 patients with GS 8–10 was 87.3 and 69.5 %, respectively. Tewari et al. [14] studied men with Gleason 8–10 prostate cancer undergoing RP and found that a median OS was 9.7 years, while median CSS was more than 14 years. Gerber et al. [18] studied men with high-grade prostate cancer undergoing RP and found that 10-year CSS was 77 % (range 65–86 %).
There are several pathologic criteria that help determine prognosis. The most important factors are tumor grade, surgical margin status, extracapsular disease, seminal vesicle invasion, and pelvic lymph node involvement [16, 19, 20]. Mian et al. [16] noted through multivariate analysis that pathologic tumor stage was the most significant predictor of disease recurrence after RRP for Gleason 8–10 cancers, and Brimo et al. [21] found that the tumor grade at the positive margin and total length of positive margin are major independent predictors of prognosis after RP.
In particular, seminal vesicle invasion and lymph node involvement are independent prognostic factors of substantial importance both in this study and in previous studies. In this study, the 10- and 20-year OS for patients with seminal vesicle involvement (T3b) was 62.5 and 26.5 %, respectively, while the 10- and 20-year CSS was 74.3 and 51.5 %, respectively. Several other studies have also demonstrated seminal vesicle invasion to be an independent risk factor, as well as being associated with an increased risk of recurrence of the disease and increased mortality [4, 22, 23].
With regard to lymph node involvement, in this study, 7.3 % of patients had positive lymph nodes. This correlates with the findings of Mian et al. [16] who found that for men with Gleason 8–10 prostate cancers, 6 % had positive nodes. However, other studies note a higher incidence. Lau et al. [24] found the rate of nodal involvement to be 27 %, and Schiavina et al. [25] found 26 % of lymph node metastases in high-risk prostate cancer. Along with this, our study found the 10- and 20-year OS for patients with positive lymph nodes to be 59.4 and 25.0 %, respectively, while the 10- and 20-year CSS was 71.3 and 47.9 %, respectively. Using Cox regression multivariate analysis to define CSS, we found that with one node or two nodes, the hazard ratio was 2.44 and 2.58, respectively. With 3 or ≥4 nodes, the hazard ratio was 3.295 and 4.892, respectively; these numbers were significantly higher. We can therefore conclude that there is a worse prognosis with increased positive nodes, specifically with three or more positive nodes as compared to two or less. Other studies have demonstrated worsening prognosis with increasing number of positive nodes. Cheng et al. [26] found that the 5- and 10-year CSS for patients with lymph node metastasis was 94 and 83 %, compared to those without lymph node metastasis being 99 and 97 %. This study also found that the hazard ratio for cancer-specific death was 1.5 for one positive node, 6.1 for two positive nodes, and 4.3 for three or more positive nodes. Briganti et al. [27] found that patients with more than two positive nodes had a significantly worse CSS outcome at 15-year follow-up (62 %) than did those patients with 2 or less positive nodes (84 %). Finally, Daneshmand et al. [28] found that patients with 1 or 2 positive lymph nodes had a recurrence-free survival of 70 and 73 %, respectively, as compared to 49 % in those with 3 or more positive nodes.
In our study, there was no change in the overall survival of the patients treated with RP and pelvic lymph node dissection as compared to those who did not receive PLND. However, we did note that there was improved CSS survival. Therefore, it can be hypothesized that PLND does not affect the overall survival, but does improve staging which may lead to better adjuvant treatment options and possibly indirect improved survival. The extent of lymph node dissection could not be ascertained in our study, and this may also lead to have a lower positive nodal rate and hence demonstrated no overall survival benefit. Patients that required adjuvant radiotherapy had a significantly poorer survival when compared to patients that did not require adjuvant radiation. This likely reflects the fact that this group of patients had worse pathology and not that adjuvant radiation confers a negative survival benefit.
This study has the distinct advantage of assessing long-term survival following RP in a very large series of patients with Gleason 8–10 prostate cancer. This is the first study to demonstrate excellent 20-year cancer-specific survival of men with Gleason 8–10 prostate cancer. This study does have certain limitations which are inherent to large population database studies that may have confounded the results. Firstly, because this is a cancer database representing many institutions, there was no standardization with regard to how the pathologic specimens were interpreted. Secondly, data regarding PSA measurement and surgical margin status are not recorded in the SEER database and could not be analyzed. In addition, there are no available data regarding dosing of adjuvant radiation, and therefore, no dose-dependent benefits or complications could be ascertained. Finally, there are no data available regarding patient comorbidity, adjuvant anti-hormonal therapy, or metastasis-free survival.
Conclusion
Excellent long-term survival can be achieved with prostatectomy for Gleason 8–10 prostate cancer. Pelvic lymph node dissection for these patients does not seem to increase survival significantly, but increasing the number of positive nodes was associated with worse survival. Patients that required adjuvant radiotherapy had a significantly poorer survival when compared to patients that did not require adjuvant radiation.
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
Epstein JI, Carmichael MJ, Pizov G et al (1993) Influence of capsular penetration on progression following radical prostatectomy: a study of 196 cases with long-term follow-up. J Urol 150(1):135–141
Sgrignoli AR, Walsh PC, Steinberg GD et al (1994) Prognostic factors in men with stage D1 prostate cancer: identification of patients less likely to have prolonged survival after radical prostatectomy. J Urol 152:1077–1081
Albertsen PC, Hanley JA, Gleason DF et al (1998) Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA 280:975
Hull GW, Rabbani F, Abbas F et al (2002) Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol 167:528
Sweat SD, Bergstralh EJ, Slezak J, Blute ML, Zincke H (2002) Competing risk analysis after radical prostatectomy for clinically nonmetastatic prostate adenocarcinoma according to clinical Gleason score and patient age. J Urol 168:525–529
Paulson DF, Piserchia PV, Gardner W (1980) Predictors of lymphatic spread in prostatic adenocarcinoma: uro-oncology research group study. J Urol 123:697
Sogani PC, Israel A, Lieberman PH et al (1985) Gleason grading of prostate cancer: a predictor of survival. Urology 25:223
Meng MV, Elkin EP, Latini DM, Duchane J, Carroll PR (2005) Treatment of patients with high risk localized prostate cancer: results from cancer of the prostate strategic urological research endeavor (CaPSURE). J Urol 173:1557–1561
Manoharan M, Bird VG, Kim SS, Civantos F, Soloway MS (2003) Outcome after radical prostatectomy with a pretreatment prostate biopsy Gleason score of ≥8. BJU Int 92:539–544
Boorjian SA, Karnes RJ, Crispen PL, Rangel LJ, Bergstralh EJ, Sebo TJ et al (2009) The impact of discordance between biopsy and pathological Gleason scores on survival after radical prostatectomy. J Urol 181:95–104
Donohue J, Bianco F, Kuroiwa K et al (2006) Poorly differentiated prostate cancer treated with radical prostatectomy: long term outcome and incidence of pathological downgrading. J Urol 176(3):991–995
Bill-Axelson A, Holmberg L, Ruutu M, Haggman M, Andersson SO, Bratell S et al (2011) Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 364:1708–1717
Tewari A, Divine G, Chang P et al (2007) Long-term survival in men with high grade prostate cancer: a comparison between conservative treatment, radiation therapy and radical prostatectomy—a propensity scoring approach. J Urol 177(3):911–915
Shao YH, Kim S, Moore D et al (2013) Cancer-specific survival after metastasis following primary radical prostatectomy compared with radiation therapy in prostate cancer patients: results of a population-based, propensity score–matched analysis. Eur Urol. Online 21 May 2013. ISSN 0302-2838. doi:10.1016/j.eururo.2013.05.023
Mian BM, Troncoso P, Okihara K et al (2002) Outcome of patients with Gleason score 8 or higher prostate cancer following radical prostatectomy alone. J Urol 167:1675–1680
Lughezzani G, Gallina A, Larcher A et al (2013) Radical prostatectomy represents an effective treatment in patients with specimen-confined high pathological Gleason score prostate cancer. BJU Int 111(5):723–730
Gerber GS, Thisted RA, Scardino PT et al (1996) Results of radical prostatectomy in men with clinically localized prostate cancer. JAMA 276:615
Grossfeld GD, Chang JJ, Broering JM et al (2000) Impact of positive surgical margins on prostate cancer recurrence and the use of secondary cancer treatment: data from the CaPSURE database. J Urol 163:1171–1177
Boorijan SA, Thompson RH, Siddiqui S et al (2007) Long-term outcome after radical prostatectomy for patients with lymph node positive prostate cancer in the prostate specific antigen era. J Urol 178(3):864–870
Brimo F, Partin AW, Epstein JI (2010) Tumor grade at margins of resection in radical prostatectomy is an independent predictor of prognosis. Urology 76(5):1206–1209
Pierorazio PM, Ross AE, Schaeffer EM et al (2011) A contemporary analysis of outcomes of adenocarcinoma of the prostate with seminal vesicle invasion (pT3b) after radical prostatectomy. J Urol 185(5):1691–1697
Hubanks M, Boorjian SA, Frank I et al (2013) The presence of extracapsular extension is associated with an increased risk of death from prostate cancer after radical prostatectomy for patients with seminal vesicle invasion and negative lymph nodes. Urol Oncol. Online 6 February 2013. ISSN 1078-1439. doi:10.1016/j.urolonc.2012.09.002
Lau WK, Bergstralh EJ, Blute ML, Slezak JM, Zincke H (2002) Radical prostatectomy for pathological Gleason 8 or greater prostate cancer: influence of concomitant pathological variables. J Urol 167:117
Schiavina R, Scattoni V, Castellucci P et al (2008) 11C-choline positron emission tomography/computerized tomography for preoperative lymph-node staging in intermediate-risk and high-risk prostate cancer: comparison with clinical staging nomograms. Eur Urol 54(2):392–401
Cheng L, Zincke H, Blude M et al (2001) Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer 91(1):66–73
Briganti A, Karnes J, Filippo Da Pozzo L et al (2009) Two positive nodes represent a significant cut-off value for cancer specific survival in patients with node positive prostate cancer. A new proposal based on a two-institution experience on 703 consecutive N+ patients treated with radical prostatectomy, extended pelvic lymph node dissection and adjuvant therapy. Eur Urol 55(2):261–270
Daneshmand S, Quek M, Stein J et al (2004) Prognosis of patients with lymph node positive prostate cancer following radical prostatectomy: long term results. J Urol 172(6):2252–2255
Acknowledgments
All authors of this research paper have directly participated in the planning, execution, or analysis of this study. All authors of this paper have read and approved the final version submitted. There are no directly related manuscripts or abstracts, published or unpublished, by any author(s) of this paper. The contents of this manuscript have not been copyrighted or published previously; are not now under consideration for publication elsewhere; and will not be copyrighted, submitted, or published elsewhere, while acceptance by the Journal is under consideration.
Conflict of interest
There is no actual or potential conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pokala, N., Trulson, J.J. & Islam, M. Long-term outcome following radical prostatectomy for Gleason 8–10 prostatic adenocarcinoma. World J Urol 32, 1385–1392 (2014). https://doi.org/10.1007/s00345-014-1253-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-014-1253-1