Abstract
Purpose
To evaluate the association of gender with survival following radical cystectomy (RC) for patients with pT4 bladder cancer.
Materials and methods
We reviewed our institutional registry of 2,088 patients treated with RC between 1980 and 2005 to identify 128 with pT4 tumors, including 91 males and 37 females. Survival was estimated using the Kaplan–Meier method and compared with log-rank test. Cox hazard regression models were used to analyze the association of clinicopathologic demographics, including gender, with outcome.
Results
A total of 7 women and 30 men with pT4 tumor received perioperative chemotherapy. Median postoperative follow-up was 10.5 years, during which time 27 patients experienced local recurrence (LR) and 120 died, including 90 who died from bladder cancer. Women with pT4 tumor trended to have higher 5-year LR-free survival (72 vs. 59 %; p = 0.83), cancer-specific survival (31 vs. 17 %; p = 0.50), and overall survival (19 vs. 11 %; p = 0.33), although these differences did not reach statistical significance. On multivariate analysis, moreover, gender was not significantly associated with LR (HR 0.96; p = 0.93), cancer-specific mortality (HR 1.05; p = 0.87), or all-cause mortality (ACM) (HR 1.14; p = 0.58). Instead, poor ECOG performance status and pN+ disease were associated with an increased risk of ACM, while removal of a greater number of lymph nodes was associated with decreased ACM.
Conclusion
We did not find gender-specific disparities in survival following RC for pT4 bladder cancer. Prognosis was instead driven by patient performance status and lymph node status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer is the fourth most common malignancy in males and ninth most common cancer in females in the USA with 72,570 new cases and 15,210 deaths estimated for 2013 [1]. Consistently, tumor stage has been found to stratify patients’ risk of disease recurrence and mortality after surgery. Indeed, pT4 tumors have been associated with approximately 27 % 5-year survival [2]. Nevertheless, substantial heterogeneity in the natural history of these patients has been demonstrated. In particular, gender disparities have been reported, with female patients having been found to experience worse outcome than males with such locally advanced bladder tumors [3, 4]. The etiology for this noted difference in outcome by gender remains to be established but has been postulated to result from delayed presentation, healthcare disparities, and different pelvic anatomy [5, 6].
Since pT4 classification represents the stage for which gender difference arises in the designation (tumor invading any of the following: prostatic stroma, seminal vesicles, uterus, vagina, pelvic wall, and abdominal wall) [7], studying this subset of locally advanced bladder cancer patients may provide insight into anatomical variables, which may influence outcomes. In addition, the natural history following radical cystectomy (RC) and prognostic variables predicting outcome of pT4 bladder cancer remains poorly understood, due to relatively limited number of patients with this stage of disease who undergo surgical extirpation and lack of centralized pathology review.
Here, then, we evaluated gender-specific outcomes for patients with pT4 bladder cancer from our dataset and determined clinicopathologic features associated with survival in these patients.
Materials and methods
After Institutional Review Board approval was obtained, we reviewed our institutional Cystectomy Registry to identify 2,088 patients who underwent with RC and bilateral pelvic lymphadenectomy for bladder cancer at Mayo Clinic between 1980 and 2005. Clinicopathologic features recorded included age, gender, ECOG performance status, pathologic tumor stage, lymph node status, histologic subtype, surgical margin status, and receipt of perioperative (neoadjuvant/adjuvant) chemotherapy. A single genitourinary pathologist (J.C.C.) re-reviewed all pathologic specimens without the knowledge of patient outcome. Tumor stage was assigned according to the 2010 AJCC classification [7].
Radical cystectomy was performed by multiple surgeons using standard techniques. All patients underwent an open approach to RC. Postoperative assessments were generally conducted according to institutional protocol every 3 months in the first 2 years after surgery, biannually for the next 3 years, and annually thereafter. We defined local recurrence as tumor recurrence in the soft tissue of the initial surgical bed or lymph node metastasis within the dissection template. Vital status for patients in the Cystectomy Registry is updated each year. If a patient has died in the previous year, a death certificate is ordered to determine the cause of death. If the death certificate is unclear regarding cause, the medical history is reviewed by an urologist to determine the cause of death. If a death certificate cannot be obtained, the cause of death is verified with the patient’s family or local physician. For patients followed elsewhere, the Cystectomy Registry at our institution monitors outcomes annually by correspondence. The lost-to-follow-up rate for the Cystectomy Registry is 2.9 %.
Continuous features were summarized with medians and interquartile ranges (IQR); categorical features were summarized with frequency counts and percentages.
Comparison of clinical and pathological features between females and males were made using Wilcoxon test for continuous variables, and χ 2 or Fisher’s exact test for categorical variables. Overall survival (OS), cancer-specific survival (CSS), local recurrence-free survival (LRFS), and distant metastases-free survival (DMFS) were estimated using the Kaplan–Meier method and compared with the log-rank test. Patients were censored at last follow-up or death if the end point of interest had not been attained. Cox proportional hazards models were used to assess the association of clinicopathologic features with the outcome.
Statistical analysis was done using SAS®, version 9.2. (SAS Institute, Cary, NC). All tests were two-sided, with p ≤ 0.05 considered to indicate statistical significance.
Results
We identified a total of 128 (6.1 %) patients with pT4 disease, including 91 males and 37 females. Median age at surgery was 72 years (IQR 64, 74) for females and 70 years (64, 77) for males (p = 0.83). Table 1 lists the clinicopathologic features of this cohort. Interestingly, as can be seen, males were more likely to have lymph node invasion than females (49.4 vs. 25 %; p = 0.01). Demographics were otherwise not significantly different between the genders. In addition, of the patients with pT4a disease, we noted invasion of the prostate in 50 of the men, invasion of the vaginal wall in 20 females, involvement of the small or large bowel in 14 patients, and pathologic evidence of uterine invasion in 9 women. Of note, four males underwent orthotopic neobladder reconstruction, while the remaining male and female patients received an ileal conduit type of urinary diversion.
Median postoperative follow-up for pT4 patients alive at last follow-up was 10.5 years (IQR 9.7, 12.1) for the whole cohort; [female 11.5 years (IQR 10.4, 12.8); males 9.4 years (IQR 6.3, 10.5)]. During this time, 27 patients experienced local recurrence, 43 developed distant metastases, and 120 died, with 90 dying from bladder cancer.
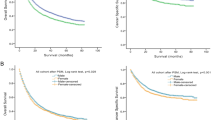
We then investigated the LRFS, DMFS, CSS, and OS using the Kaplan–Meier method, stratified by gender. Figures 1, 2, 3, and 4 depict the outcomes with the numbers of patient at risk. We found no statistically significant difference in 5-year outcomes according to gender. That is, the 5-year LRFS for females versus males was 71.9 versus 58.2 % (p = 0.83; Fig. 1), while the 5-year DMFS was 52.9 versus 41.8 %, respectively (p = 0.40; Fig. 2). Likewise, 5-year CSS (31.1 vs. 17.2 %; p = 0.50; Fig. 3) and OS (18.9 vs. 11.0 %; p = 0.33; Fig. 4) were also not significantly different between women and men. In addition, no significant difference in the 5-year CSS was noted among patients undergoing orthotopic neobladder reconstruction (25 %) compared with patients undergoing conduit diversion (22.5 %) (p = 0.40). Similarly, no significant difference in 5-year OS was noted between these as well (25 vs. 14.4 %; p = 0.21). Moreover, we found no statistically significant difference in the 3-year CSS for patients with invasion of the bowel (13 %) versus patients with invasion of the uterus (17 %) (p = 0.69). Similarly, no significant difference was noted in the 3-year OS for patients with bowel invasion (7 %) versus patients with uterine invasion (11 %) (p = 0.94). We also evaluated comparative CSS and OS for pT4a patients with tumor involvement of the vaginal wall versus prostate. Of note, we again found no statistically significant differences in outcomes, as the 3-year CSS for patients with vaginal wall invasion was 49 versus 24 % for patients with prostate involvement (p = 0.32). Likewise, the 3-year OS for patients with vaginal versus prostate invasion was 40 versus 20 %, respectively (p = 0.31).
Next, we used Cox proportional hazard models to evaluate the association of patient age, gender, performance status, nodal status, soft tissue margin status, total number of lymph nodes removed, perioperative chemotherapy, and PT4 stage with all-cause and cancer-specific mortality (Table 2).
We found that the presence of positive lymph nodes (HR 1.62; p = 0.02) and worse ECOG performance status (HR 1.70; p < 0.001) were associated with a significantly increased risk of all-cause mortality, while an increase in total numbers of lymph nodes removed tended to decrease the risk of death (p = 0.05). Poor ECOG performance status was also associated with a significantly increased risk of death from bladder cancer (HR 1.72; p < 0.001), while the presence of positive lymph nodes tended to increase the risk of cancer death as well (HR 1.57; p = 0.07).
Discussion
Here, in a single institutional study of 128 patients with pT4 bladder cancer and pathologic specimen re-reviewed and median follow-up for 10.5 years, we did not identify gender-specific disparities in outcomes following RC. Instead, prognosis in these patients was guided by performance status and lymph node involvement.
Of note, the AJCC staging system does not differentiate between male and female gender in the pT4 stage. However, the importance of gender in bladder cancer continues to be debated, with several population-based studies have demonstrated poor outcomes in females [8–10].
Although the biologic mechanism for this has not been established [11–13], one of the factors that may be responsible for the noted difference in outcome by gender is a difference in stage at presentation, with females presenting with higher stage than males [3]. Indeed, Mungan et al. [14] utilized the Netherlands Cancer Registry to evaluate gender-specific stage differences. They studied 21,795 patients with bladder cancer and found that females were more likely to present with advanced bladder cancer than males [14].
These investigators then evaluated gender differences in CSS after adjusting for stage [5], utilizing the surveillance, epidemiology, and end results (SEER) database in the USA and the Comprehensive Cancer Centers database from the Netherlands. Interestingly, analysis of the SEER database revealed a significant difference in 5-year CSS (F vs. M, 15.2 vs. 27.1 %); however, the Netherlands database did not demonstrate a statistically significant difference in 5-year CSS by gender (F vs. M, 9.6 vs. 11.7 %).
With regard to pT4 tumors specifically, May et al. [15] studied the CSS and predictive variables for 245 patients followed for 33 months with pT4a disease and noted worse CSS among females (15 vs. 35 %; p = 0.003). Furthermore, on multivariate analysis, female gender was found to be an independent predictor of worse CSS (HR 1.83; p = 0.008) [15]. Similarly, Tilki et al. [4] evaluated 583 patients with pT4 tumors from 12 institutions followed for 55 months. Likewise, on multivariate analysis, female gender was again associated with worse CSS (HR 1.67; p = 0.001) [4]. Nevertheless, both of these series were multicenter studies, lacking a centralized pathology review and containing shorter follow-up than here.
On the other hand, Dalbagani et al. [16], in a study of 300 patients who underwent RC, of which 38 patients had pT4 disease, noted no significant difference in survival according to gender on multivariate analysis (HR 1.15; p = 0.53). Similarly, Thrasher et al. [17] evaluated 531 patients with bladder cancer, of which 61 had pT4 disease and did not find gender as a significant factor for CSS on multivariate analysis as well.
Our study extends prior series on pT4 bladder tumors treated with RC in that: (1) All specimens here underwent pathologic re-review by a single genitourinary pathologist, and (2) our dataset contained longer follow-up period (10.8 years) with <3 % of patients lost to follow-up. We did not find an association of gender with outcome, suggesting that in the setting of such locally advanced disease, prognosis is primarily guided by established pathologic predictors such as nodal stage and performance status [18–21] rather than gender. Therefore, our data validate the current AJCC system [7] for pT4 stage of bladder cancer by classifying both male and female gender in pT4a subgroup.
We recognize that our study is limited by its retrospective, non-randomized design. We also acknowledge that the patients evaluated here represent a highly selected cohort, treated at a single, high-volume academic center. Lastly, it remains possible that with a larger sample size, significant differences in survival according to gender may be determined.
Conclusion
We did not find gender-specific disparities in survival following radical cystectomy for pT4 bladder cancer. Prognosis was instead driven by performance status and lymph node status. These data support the current staging classification of pT4 disease, which contains gender-specific designation in the same group.
References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63(1):11–30. doi:10.3322/caac.21166
Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M, Raghavan D, Skinner DG (2001) Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 19(3):666–675
Fajkovic H, Halpern JA, Cha EK, Bahadori A, Chromecki TF, Karakiewicz PI, Breinl E, Merseburger AS, Shariat SF (2011) Impact of gender on bladder cancer incidence, staging, and prognosis. World J Urol 29(4):457–463. doi:10.1007/s00345-011-0709-9
Tilki D, Svatek RS, Karakiewicz PI, Isbarn H, Reich O, Kassouf W, Fradet Y, Novara G, Fritsche HM, Bastian PJ, Izawa JI, Stief CG, Ficarra V, Lerner SP, Schoenberg M, Dinney CP, Skinner E, Lotan Y, Sagalowsky AI, Shariat SF (2010) Characteristics and outcomes of patients with pT4 urothelial carcinoma at radical cystectomy: a retrospective international study of 583 patients. J Urol 183(1):87–93. doi:10.1016/j.juro.2009.08.145
Mungan NA, Aben KK, Schoenberg MP, Visser O, Coebergh JW, Witjes JA, Kiemeney LA (2000) Gender differences in stage-adjusted bladder cancer survival. Urology 55(6):876–880
Taub DA, Hollenbeck BK, Cooper KL, Dunn RL, Miller DC, Taylor JM, Wei JT (2006) Racial disparities in resource utilization for cystectomy. Urology 67(2):288–293. doi:10.1016/j.urology.2005.09.003
Edge SB, American Joint Committee on Cancer (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Scosyrev E, Noyes K, Feng C, Messing E (2009) Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 115(1):68–74. doi:10.1002/cncr.23986
Fleshner NE, Herr HW, Stewart AK, Murphy GP, Mettlin C, Menck HR (1996) The national cancer data base report on bladder carcinoma. The American college of surgeons commission on cancer and the American cancer society. Cancer 78(7):1505–1513
Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF (2011) Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev 20(8):1629–1637. doi:10.1158/1055-9965.epi-11-0246
Hoke GP, Stone BA, Klein L, Williams KN (1999) The influence of gender on incidence and outcome of patients with bladder cancer in Harlem. J Natl Med Assoc 91(3):144–148
Maralani S, Wood DP Jr, Grignon D, Banerjee M, Sakr W, Pontes JE (1997) Incidence of urethral involvement in female bladder cancer: an anatomic pathologic study. Urology 50(4):537–541. doi:10.1016/s0090-4295(97)00263
Pontari MA, Ruggieri MR (1999) Sex differences and role of nitric oxide in blood flow of canine urinary bladder. Am J Physiol 276(2 Pt 2):R407–R413
Mungan NA, Kiemeney LA, van Dijck JA, van der Poel HG, Witjes JA (2000) Gender differences in stage distribution of bladder cancer. Urology 55(3):368–371
May M, Bastian PJ, Brookman-May S, Fritsche HM, Tilki D, Otto W, Bolenz C, Gilfrich C, Trojan L, Herrmann E, Moritz R, Tiemann A, Muller SC, Ellinger J, Buchner A, Stief CG, Wieland WF, Hofner T, Hohenfellner M, Haferkamp A, Roigas J, Zacharias M, Nuhn P, Burger M (2011) Gender-specific differences in cancer-specific survival after radical cystectomy for patients with urothelial carcinoma of the urinary bladder in pathologic tumor stage T4a. Urol Oncol. doi:10.1016/j.urolonc.2011.09.011
Dalbagni G, Genega E, Hashibe M, Zhang ZF, Russo P, Herr H, Reuter V (2001) Cystectomy for bladder cancer: a contemporary series. J Urol 165(4):1111–1116
Thrasher JB, Frazier HA, Robertson JE, Dodge RK, Paulson DF (1994) Clinical variables which serve as predictors of cancer-specific survival among patients treated with radical cystectomy for transitional cell carcinoma of the bladder and prostate. Cancer 73(6):1708–1715
Boorjian SA, Kim SP, Tollefson MK, Carrasco A, Cheville JC, Thompson RH, Thapa P, Frank I (2013) Comparative performance of comorbidity indices for estimating perioperative and 5-year all cause mortality following radical cystectomy for bladder cancer. J Urol 190(1):55–60. doi:10.1016/j.juro.2013.01.010
Mayr R, May M, Martini T, Lodde M, Comploj E, Pycha A, Strobel J, Denzinger S, Otto W, Wieland W, Burger M, Fritsche HM (2012) Comorbidity and performance indices as predictors of cancer-independent mortality but not of cancer-specific mortality after radical cystectomy for urothelial carcinoma of the bladder. Eur Urol 62(4):662–670. doi:10.1016/j.eururo.2012.03.057
Mayr R, May M, Martini T, Lodde M, Pycha A, Comploj E, Wieland WF, Denzinger S, Otto W, Burger M, Fritsche HM (2012) Predictive capacity of four comorbidity indices estimating perioperative mortality after radical cystectomy for urothelial carcinoma of the bladder. BJU Int 110(6 Pt B):E222–E227. doi:10.1111/j.1464-410X.2012.10938
Megwalu II, Vlahiotis A, Radwan M, Piccirillo JF, Kibel AS (2008) Prognostic impact of comorbidity in patients with bladder cancer. Eur Urol 53(3):581–589. doi:10.1016/j.eururo.2007.10.069
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaushik, D., Frank, I., Eisenberg, M.S. et al. Gender-specific survival following radical cystectomy for pT4 bladder cancer. World J Urol 32, 1433–1439 (2014). https://doi.org/10.1007/s00345-013-1232-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-013-1232-y