Abstract
Purpose
The aim of this study was to investigate the effect of renal cortex transient receptor potential melastatin 7 (TRPM7) suppression on renal ischemia reperfusion injury induced by transplantation in mice.
Methods
M7shRNA was used to decrease the expression of TRPM7 in NRK-52e cells. The mice were subjected to renal intra-parenchymal injection with lentivirus containing M7shRNA to produce hypo-expression of TRPM7 in renal cortex. Cell hypoxia mode and syngeneic renal transplantation in vivo were established. Then the effects of M7shRNA were measured by fluorescent probe for reactive oxygen species (ROS), intracellular calcium and magnesium; Western blot was applied for p38-MAPKs and Bax expression in cell studies. In vivo studies, mice were killed 24 h, 48 h, 72 h, 7 days and 21 days, respectively, after transplantation and the kidneys were dissected. Serum creatinine was measured, and the H&E, Masson’s trichrome staining, TUNEL, Kim-1 and α-smooth muscle actin were used to evaluate pathological change.
Results
In cell model of hypoxia, the level of ROS in NRK-52e-M7shRNA was significantly lower than that in both NRK-52e and NRK-52e control cells, while the activation of p38-MAPKs was limited. In renal transplanted mice, renal function of M7shRNA group was conspicuously better than PBS- and vector-control-treated group. The histological examination showed that renal tubule injury and interstitial fibrosis were lower in M7shRNA-treated group compared with PBS and vector-control group.
Conclusions
Suppression of renal cortex TRPM7 could alleviate kidney injury induced by transplantation in mice. The mechanism may involve reducing the early stage of ischemia reperfusion injury by inhibition of intracellular Ca2+, Mg2+ and ROS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal failure is a major clinical problem without effective therapy and contributes greatly to patient morbidity and mortality [1, 2]. Transplantation is considered to be one of the most important replacement therapies. However, renal ischemia–reperfusion injury (RIRI) is unavoidable in renal transplantation, which firstly leads to acute tubular necrosis (ATN) and then causes interstitial fibrosis [3, 4]. Fibrosis would finally result in graft dysfunction [3, 5]. Therefore, how to alleviate the renal injury in early ischemia–reperfusion (IR) is of great important significance in renal transplantation.
The transient receptor potential melastatin 7 (TRPM7) is a bifunctional protein characterized by ion channel (e.g., Ca2+ and Mg2+) and kinase activity [6, 7]. Like most of calcium channel, TRPM7 activity might be promoted by ischemia and consequently lead to overload of free and total intracellular calcium, which results in reactive oxygen species (ROS) production and cell death. However, the action of TRPM7 is still effective even if other known calcium influx pathways are pharmacologically blocked [8]. Studies showed that TRPM7 up-regulation in cells increases levels of ROS and nitric oxide (NO) and stimulates p38 mitogen-activated protein kinase (p38 MAPK) and Jun N-terminal kinase (JNK) [9]. Another investigation showed role of Mg2+ in regulation of TRPM7 on intracellular ROS levels during cell stress is similar to that of calcium [10]. The MAPKs (p38, ERK-1/2 and JNK) are a family of protein kinases playing an important role in apoptosis and survival signaling, and the ROS and calcium are the most important impact factors in the ischemia reperfusion. ROS up-regulation can damage cellular components such as proteins, lipids and DNA [11]. Intracellular Ca2+ overload is also involved in cell death during IR. Our previous study showed reperfusion time after ischemia could affect the expression of TRPM7. The expression of TRPM7 was up-regulated in the early stage of RIRI, and the majority of varied TRPM7 protein in RIRI was located in renal tubules [12]. From the animal experiment, we knew that kidney was dysfunctional in the early reflow injury and RIRI is always occurred in renal tubules [13]. Therefore, TRPM7 may play an important role in RIRI.
TRPM7 has been reported to be involved in neurons cell death during ischemia reperfusion via increasing intracellular Ca2+ overload and oxidative stress [8, 14]. Suppression of hippocampal TRPM7 can provide protection against brain ischemia [15]. And depletion of TRPM7 in hippocampal has no negative effect on animal survival [15]. In this study, we aim to find whether suppression of TRPM7 located under renal cortex could protect mice kidney in renal transplantation or not.
Materials and methods
Reagents
Antibody for TRPM7 was kindly provided by Professor Loren Runnels (UMDNJ-Robert Wood Johnson Medical School; NJ, USA) and beta-actin was from Santa Cruz Biotechnology (Santa Cruz; CA, USA). Other antibody for Western blot was from CST (Beverly; MA, USA). Kidney injury molecule-1(Kim-1) and α-smooth muscle actin (α-SMA) were both bought from BOSTER (Wuhan; Hubei, China). Fluo-4-AM (Calcium Indicator), Mag-fluo-4 (Magnesium Indicator) and CM-H2DCFDA (ROS Indicator) were obtained from Life Technologies (Carlsbad; CA, USA). Transfection using opti-MEM and Lipofectamine 2000 was bought from Life Technologies (Carlsbad; CA, USA). TUNEL kit was obtain from Roche (Indianapolis; IN, USA). QDs conjugated streptavidin (QDs-SA) probes were purchased form Wuhan JiaYuan Quantum Dots co., Ltd (Wuhan. China).
In vitro experiments
Cell culture and transfection
The NRK-52e cell line was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % fetal bovine serum incubated at 37 C in a 5 % CO2 incubator. Lentivirus plasmid encoding vector-control 5′-AAT TCT CCG AAC GTG TCA CGT-3′ or vector-shRNA-TRPM7 target rat TRPM7, 5′-GCA CCC CTC AGT TGC GAA AGA-3′ was kindly provided by Professor Loren Runnels (UMDNJ-Robert Wood Johnson Medical School, NJ, USA) and transfecting NRK-52e cell using opti-MEM and Lipofectamine 2000 [16].
Cell hypoxia injury
NRK-52e, NRK-52e-control and NRK-52e-M7shRNA cells were washed twice with Hank’s without glucose, and then treated with 5 mM of Na2S2O4. The cultures were incubated at 37◦C for 1 h. Cells were washed twice with Hank’s and cultured in DMEM containing 10 % fetal bovine serum incubated at 37 C in a 5 % CO2 incubator for 24 h. After that, ROS levels of cells were analyzed using the flow cytometer.
Measurement of ROS levels, intracellular Ca2+ and Mg2+
NRK-52e cells after hypoxia injury were trypsinized, washed with PBS and resuspended in phenol red-free culture medium. The measurement of Ca2+ and Mg2+ used the same protocol as previous study [17].
Western blot analysis for MAPKs cell signal pathway and Bax
NRK-52e, NRK-52e-control and NRK-52e-shRNAM7 cells lysed underwent a hypoxia injury with RIPA buffer. Samples of the lysates were resolved by SDS-PAGE and analyzed by Western blot analysis using standard procedures.
In vivo experiments
Mice
Male BALB/c mice with 6–8 weeks of age were purchased from the laboratory ABLS-3 of Wuhan University (Wuhan, China) and housed under standard condition and received humane care. All animal experiments were conducted in accordance with the UK Animals (Scientific Procedures) Act 1986 and associated guidelines, and the EEC Directive of 1986 (86/609/EEC).The study was approved by the Ethical Committee, Wuhan University School of Medicine.
Group protocols
There were four groups including: sham group (n = 4), PBS-treated group (n = 30), vector-control-treated group (n = 30), M7shRNA-treated group (n = 30). Mice were killed at 24 h, 48 h, 72 h, 7 days and 21 days, respectively, and the kidneys were dissected. Every group had six mice in different time points except for sham group. We measured H&E and Kim-1, TUNEL staining, α-SMA, Masson’s trichrome of tissues from the mice killed at 48 h, 72 h, 7 days and 21 days, respectively. The serum creatinine (Scr) was measured between 24 h and 7 days.
Intrarenal lentivirus delivery
In vivo virus transduction to express M7shRNA in mice was performed as investigation above [16] with slight modification. On the anesthetized mice, after temporary occlusion of left renal pedicle, a 31G needle was inserted at the middle of the left kidney parallel to the long axis and was carefully pushed toward the upper pole and then the lower pole. As the needle was slowly removed, 100 μl lentivirus (vector-control or Vector-M7shRNA, ~1 × 105 IU/μl) or PBS was injected. Mice were subjected to renal IR 72 h after injection. The determination of transgene expression was measured by flow cytometer and quantum dots (QDs)-based immunofluorescence technology [18].
Syngeneic renal transplantation
We used a well-characterized syngeneic renal transplantation model described in detail [19]. Briefly, at 9 weeks of age, the mice with transfected vector-control/shRNAM7 lentivirus were randomized to undergo left nephrectomy. The transfected kidney kept with ureter, and a 10-mm-diameter bladder patch was flushed with chilled Collins organ preservative buffer and then stored at 4 ◦C. Meanwhile, recipient mice were received a left-side nephrectomy. An end-to-side anastomosis between the donor’s (lentivirus transfected) renal vessels and the recipient’s (normal BABL/c) was performed, and then, the 10-mm-diameter donor’s bladder patch was also sutured on the recipient’s bladder. After that, a right-side native nephrectomy was performed. The abdomen was rinsed with sterile normalized saline. The fascial and skin layers were closed with 6-0 silk, and the animal was allowed to recover in a clean, isolated cage.
Quantum dots-based immunofluorescence technology (QDs-IFC)
Three serial sections (4 μm thickness) were obtained for each specimen, which were used for QDs-IFC. The specimen treatment by QDs-IFC was similar to conventional IHC, with the following major procedures as previously described [18].
Renal function and histology detection
The measurement of Scr was performed from post-transplantation day 1 to 14. Level of Scr was measured in the Department of Clinical Laboratory at the Zhongnan Hospital of Wuhan University.
Mice were killed at 24 h, 48 h, 72 h, 7 days and 21 days, respectively, and the kidneys were dissected. The tissue slices were fixed in 4 % formalin and then processed for histological examination using standard techniques. Formalin-fixed tissue was embedded in paraffin, and 4-μm sections were stained with H&E (mice kidney at 48 h) and Masson’s trichrome (mice kidney at 21 days). The tubulointerstitial injury was evaluated by calculating the percentage of the affected area fraction as described previously [20], 6 fields per section at 200× magnification. Jablonski scale [21] (renal injury scores of 0–4) for assessment of tubules necrotic injury was conducted by an experienced renal pathologist. Immunohistochemistry for detecting KIM-1 (mice kidney at 48 h) and α-SMA (mice kidney at 7 days) was performed as described [22]. We used TUNEL staining (green fluorescence) to detect apoptosis 72 h after transplantation by situ cell death detection kit.
Statistical analysis
The SPSS version 17.0 for Windows (SPSS, Chicago, IL, USA) was used for the statistical analysis. All of these data have been presented as the mean ± SEM. Statistical analysis was performed using unpaired t test, and one-way ANOVA (analysis of variance) was used in comparisons with more than two groups. P < 0.05 was taken as statistically significant.
Result
Suppression of TRPM7 in NRK-52e cells and kidney of mice
The Western blot was not suitable to indirectly detect TRPM7 levels in this experiment due to the limited expression of TRPM7. Then, we took the QDs-based immunofluorescence technology (QDs-IFC) and fluorescent probe to confirm the suppression of TRPM7. None of the mice died or showed signs of ARF after lentiviral injection to their kidneys.
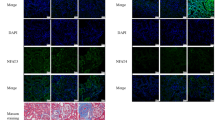
Figure 1a showed the effect of suppression of TRPM7 in NRK-52e cells. The red fluorescence showed the expression of TRPM7, and the green was beta-actin, and then the merge was yellow. The red fluorescence and the yellow of merge in NRK-52e-M7shRNA cells were much weaker than those in vector-control and NRK-52e cells. Figure 1b showed the TRPM7 expression in mice kidney. The untreated and vector-control-treated group mice showed a robust expression of red fluorescence, whereas the M7shRNA-treated group mice showed much less expression.
Suppression of TRPM7 in NRK-52e cells and mice renal cortex. The red fluorescence showed the expression of TRPM7, the green was beta-actin, and the merge was yellow (Magnification ×400). a The effect of TRPM7 suppression in NRK-52e cells. b The effect of TRPM7 of suppression in mice renal cortex. Representative images of six independent experiments (Magnification ×200). n = 6 per group
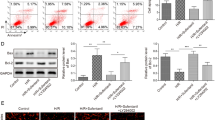
TRPM7 is an ion channel for maintaining intracellular calcium and magnesium. So the levels of intracellular Ca2+ and Mg2+ could indirectly represent the function of TRPM7. We labeled calcium and magnesium with Fluo-4 AM and Mag-fluo-4, respectively, and then detected with flow cytometer. Figure 2a, b showed the function of the TRPM7 channel was significant deceased in NRK-52e-M7shRNA cell compared with NRK-52e and vector-control cells.
TRPM7-knockdown NRK-52e cells have decreased intracellular Ca2+ and Mg2+ levels and the depletion of TRPM7 reduced activation of p38 MAPKs, Bax and lowered ROS production in response to cell hypoxia. Measurement of free Ca2+ (a) and Mg2+ (b) concentration in NRK-52e-M7shRNA and NRK-52e control cells by flow cytometer using the Fluo-4-AM (Calcium Indicator), Mag-fluo-4 (Magnesium Indicator). c Measurement of ROS production in cells labeled with CM-H2DCFDA (ROS Indicator) by flow cytometer. The left side of the histogram showed ROS levels of cells in normal culture and right-side histogram was for the hypoxia-treated cells. d Western blots analyses of levels of activated MAPKs and the total amount of each protein, Bax and beta-actin in NRK-52e cells, Vector-C cells and M7shRNA cells at 24 h after hypoxia. Results are mean ± SD for four independent experiments; *P < 0.05
Depletion of TRPM7-reduced activity of MAPKs and lowers ROS and Bax levels in IR
TRPM7 knockdown cells (NRK-52e-M7shRNA) had a significant lower concentration of ROS compared with NRK-52e and vector-control cells (Fig. 2c). These data indicated that TRPM7 had an effect on ROS levels both in normal or hypoxia culture. Western blots showed that the p38 MAPK signal was inhibited in NRK-52e-M7shRNA and deletion of TRPM7 could reduce apoptotic signaling in early stage of hypoxia (Fig. 2d).
M7shRNA-treated mice showed ameliorative renal tubular necrosis and apoptosis after syngeneic renal transplantation
To confirm the treatment effects mediated by TRPM7 suppression, we examined histopathological changes at 48 h after transplantation. The result of the H&E (200×) and immunohistochemical staining (200×) for Kim-1 showed us that renal tubule injury was lower in M7shRNA-treated group compared with PBS and vector-control group (Fig. 3a–h). The number of positive TUNEL-renal tubule cells per field of view (FOV, 100×) was larger than that in PBS and vector-control-treated group than M7shRNA-treated group (Fig. 3a–e).
Decreased Levels of Kim-1 and alleviated renal injury in M7shRNA-treated mice with syngeneic transplantation. The renal tubule injury was evaluated by H&E for 48 h after transplantation. (a sham group; b PBS-treated group; c Vector-C group; d M7shRNA-treated group). Quantitative analysis of ATN (e) of transplantation mice (×200). Sham group n = 4, others n = 6, results are mean ± SD; *P < 0.05. Compared with PBS-treated (f) and Vector-C-treated (g) group mice, M7shRNA group (h) mice had less Kim-1 expression at 48 h after transplantation. n = 6 in each group. The renal tubule apoptosis was measured by TUNEL for 72 h after transplantation (a sham group; b PBS-treated group; c Vector-C group; d M7shRNA-treated group). The positive apoptotic nuclei were marked by green fluorescence (×100). M7shRNA-treated group showed less positive apoptosis per FOV compared with PBS and Vector-C-treated group (e). Sham group n = 4, others n = 6, results are mean ± SD; *P < 0.05
Ameliorative fibrosis was found in M7shRNA-treated group after mice renal transplantation
M7shRNA-treated group mice showed decreased expression of α-SMA (200×) which was associated with interstitial damage and fibrosis at 7 days after transplantation (Fig. 4a–d). And the presence of collagen deposition, measured with Masson’s trichrome staining (200×), was also reduced in the M7shRNA group at 21 days after transplantation (Fig. 4a-e).
Alleviated fibrosis was found in M7shRNA-treated mice group after renal transplantation. (1) Immunohistochemical for α-SMA at 7 days after transplantation (a sham group; b PBS-treated group; c Vector-C group; d M7shRNA-treated group). The deposition of collagen was determined by Masson trichrome staining on 21 days after operation (a sham group; b PBS-treated group; c Vector-C group; d M7shRNA-treated group) (×200). Representative images of six independent experiments. The renal function measured by Scr (e) showed M7shRNA was better than PBS-treated and Vector-C-treated group mice. Sham group n = 4, others n = 6, results are mean ± SD; *P < 0.05
Levels of Scr were down in M7shRNA-treated mice with syngeneic renal transplantation
In cell levels, we found suppression of TRPM7 could protect the NRK-52e, which is a rat renal tubule cells. Then we employed the technology of renal intra-parenchymal injection to product mice with lower TRPM7 levels in renal cortex. We established the model of syngeneic renal transplantation as previously described [19], and Scr (mg/dl) was detected at day 1, day 2, day 7 and day 14, respectively. Scr obtained in M7shRNA-treated group (1.69 ± 0.34; 3.11 ± 0.25; 1.59 ± 0.39; 1.16 ± 0.23; n = 6, P < 0.05) was significantly better than PBS-treated group (3.06 ± 0.41; 3.96 ± 0.23; 2.44 ± 0.26; 1.70 ± 0.16; n = 6, P < 0.05) and Vector-C group (2.81 ± 0.44; 3.91 ± 0.37; 2.66 ± 0.39; 1.8 ± 0.21; n = 6, P < 0.05) (Fig. 4e).
Discussion
Renal transplantation is a common operation in urology. Glomerular disease, obstructive nephropathy, chronic interstitial nephritis, renal vascular diseases and hereditary nephropathy all contribute to renal failure. The transplant is an important therapy for renal failure; however, the RIRI could not be avoided during transplantation. Even if we try our best to decrease the time cost in surgery such as nephrectomy or transplantation, we could not control the time cost in donor’s transportation. Unfortunately, RIRI is one of the most important causes for chronic renal allograft dysfunction [3]. So alleviating the RIRI is critical to prolong time of donor kidney, and it is also helpful for saving social resource. These studies showed that suppression of renal TRPM7 can alleviate the kidney injury induced by the renal ischemia reperfusion and protect kidney in transplantation.
There were four reasons to choose suppression of TRPM7 as new strategy to protect kidney in RIRI: (1) The ROS level was down-regulated. The same phenomenon was also reported in HEK-293 cells and Swiss 3T3 fibroblasts cells by Loren et al. [9, 10]. The core of the RIRI was happened in mitochondria and ROS played one of the most important roles in the mitochondrion injury [23, 24]. After we decreased the ROS levels in M7shRNA cells, it would alleviate injury in hypoxia. When the M7shRNA-expressed lentivirus was transfected into renal tubule where always being suffered from IR, the renal function could be protected. And in our previous investigation, we found the main change of expression of TRPM7 was mostly occurred in renal tubule in earlier RIRI [12]. (2) The intracellular Ca2+ and Mg2+ was decreased. Though there are many channels could maintain magnesium homeostasis, such as MagT1 of which expression would be up-regulated in HEK-293 cells when the concentration of extracellular Mg2+ in the growth media is reduced [25], knockdown of TRPM7 would still decrease cellular free magnesium and so with calcium [10]. TRPM7 is a still available even if other known calcium influx pathways are pharmacologically blocked [8]. TRPM7 plays an essential role in calcium overload which was also an important impact factor in RIRI. Obviously, the intracellular Ca2+ decreased by suppression TRPM7 was an important factor for protecting kidney. In recent studies, magnesium played a same role as calcium in the TRPM7′s control on ROS levels during cell stress [10]. Decreased intracellular magnesium by suppression TRPM7 channel could also protect renal function. (3) The activity of MAPKs cell signal pathway was inhibited. The MAPKs (p38, ERK-1/2 and JNK) are a family of protein kinases playing an important role in apoptosis and survival signaling. The lower of the expression of p38 and JNK would be better for liver transplantation [26] and heart underwent prolonged hypothermic ischemia [27]. In the cell model of hypoxia, the activity of p38 and JNK was significantly inhibited, which was beginning of apoptosis; however, in the mice model of ischemia reperfusion, we could not get same result with cells. We suppose the main reason was the lentivirus injected into the renal cortex and corticomedullary junction, and then transfection of M7shRNA was only took place in part of the renal cortex which the IR happened firstly, but not the whole kidney. (4) From previous investigation, mast cell survived belonged to TRPM7, knockdown the TRPM7 would lead to cell death of the mast cell line [28]. The role of mast cells in IR of heart [29], lung [30] and skeletal muscle [31] was also reported, and decreased number or function or enzyme release will protect the organ from the IR.
There are still several limitations in our studies. The effect of lentivirus injection with Vector-M7shRNA in renal protection is significant, but the side effect to other organs is still unclear, especially, TRPM7 is an important divalent cation channel required for cell viability [6]. So we take the technology of renal intra-parenchymal injection to avoid the defection followed by suppression in whole body. Suppression of the TRPM7 in the whole kidney is the best design for this study. However, except knockout (KO) animal, it is very hard to find a way to suppress TRPM7 in the whole kidney. Fortunately, renal cortex is the most part of kidney baring the ischemia reperfusion injury. Then it is another feasible mode for this study. Above all, the KO mice with lower TRPM7 expression in the whole kidney should be necessary and the TRPM7 KO mice will be adopted in our next study. By the way, the detail mechanism and change of protein in the upstream/downstream also need to be measured. TRPM7 was took part not only in regulating ischemia injury but also in immune cell survival [28] cell adhesion [17] and cell motility [32]. Does TRPM7 also participate in transformation and migration of immune cells in transplant? So, the further investigation about it is very indispensable.
Conclusions
Suppression of renal cortex TRPM7 may alleviate kidney injury induced by transplantation in mice. The mechanism may involve reducing the early stage of ischemia reperfusion injury by inhibition of intracellular Ca2+, Mg2+ and ROS.
References
Jones DR, Lee HT (2007) Protecting the kidney during critical illness. Curr Opin Anaesthesiol 20:106–112
Tang IY, Murray PT (2004) Prevention of perioperative acute renal failure: what works? Best Pract Res Clin Anaesthesiol 18:91–111
Chapman JR, O’Connell PJ, Nankivell BJ (2005) Chronic renal allograft dysfunction. J Am Soc Nephrol 16:3015–3026
Schwarz A, Mengel M, Gwinner W, Radermacher J, Hiss M, Kreipe H et al (2005) Risk factors for chronic allograft nephropathy after renal transplantation: a protocol biopsy study. Kidney Int 67:341–348
Gulati P and Singh N (2013) Neuroprotective effect of tadalafil, a PDE-5 inhibitor, and its modulation by L-NAME in mouse model of ischemia-reperfusion injury. J Surg Res
Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ et al (2001) LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411:590–595
Runnels LW, Yue L, Clapham DE (2001) TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291:1043–1047
Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W et al (2003) A key role for TRPM7 channels in anoxic neuronal death. Cell 115:863–877
Su LT, Chen HC, Gonzalez-Pagan O, Overton JD, Xie J, Yue L et al (2010) TRPM7 activates m-calpain by stress-dependent stimulation of p38 MAPK and c-Jun N-terminal kinase. J Mol Biol 396:858–869
Chen HC, Su LT, Gonzalez-Pagan O, Overton JD, Runnels LW (2012) A key role for Mg2+ in TRPM7′s control of ROS levels during cell stress. Biochemical Journal 445:441–448
Gutteridge JM, Halliwell B (1992) Comments on review of free radicals in biology and medicine, second edition, by Barry Halliwell and John M. C. Gutteridge. Free Radic Biol Med 12:93–95
Meng Z, Wang X, Yang Z, Xiang F (2012) Expression of transient receptor potential melastatin 7 up-regulated in the early stage of renal ischemia-reperfusion. Transplant Proc 44:1206–1210
Lange C, Togel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR et al (2005) Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int 68:1613–1617
Wei WL, Sun HS, Olah ME, Sun X, Czerwinska E, Czerwinski W et al (2007) TRPM7 channels in hippocampal neurons detect levels of extracellular divalent cations. Proc Natl Acad Sci USA 104:16323–16328
Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H et al (2009) Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci 12:1300–1307
Kim M, Chen SW, Park SW, Kim M, D’Agati VD, Yang J et al (2009) Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int 75:809–823
Su LT, Agapito MA, Li M, Simonson WT, Huttenlocher A, Habas R et al (2006) TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem 281:11260–11270
Chen C, Peng J, Xia HS, Yang GF, Wu QS, Chen LD et al (2009) Quantum dots-based immunofluorescence technology for the quantitative determination of HER2 expression in breast cancer. Biomaterials 30:2912–2918
Siedlecki AM, Jin X, Thomas W, Hruska KA, Muslin AJ (2011) RGS4, a GTPase activator, improves renal function in ischemia-reperfusion injury. Kidney Int 80:263–271
Vieira JM Jr, Mantovani E, Rodrigues LT, Delle H, Noronha IL, Fujihara CK et al (2005) Simvastatin attenuates renal inflammation, tubular transdifferentiation and interstitial fibrosis in rats with unilateral ureteral obstruction. Nephrol Dial Transplant 20:1582–1591
Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J (1983) An experimental model for assessment of renal recovery from warm ischemia. Transplantation 35:198–204
Kakuta Y, Okumi M, Isaka Y, Tsutahara K, Abe T, Yazawa K et al (2011) Epigallocatechin-3-gallate protects kidneys from ischemia reperfusion injury by HO-1 upregulation and inhibition of macrophage infiltration. Transpl Int 24:514–522
Brinkkoetter PT, Song H, Losel R, Schnetzke U, Gottmann U, Feng Y et al (2008) Hypothermic injury: the mitochondrial calcium, ATP and ROS love-hate triangle out of balance. Cell Physiol Biochem 22:195–204
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287:C817–C833
Zhou H, Clapham DE (2009) Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc Natl Acad Sci USA 106:15750–15755
Uehara T, Peng XX, Bennett B, Satoh Y, Friedman G, Currin R (2004) c-Jun N-terminal kinase mediates hepatic injury after rat liver transplantation. Transplantation 78:324–332
Clanachan AS, Jaswal JS, Gandhi M, Bottorff DA, Coughlin J, Finegan BA et al (2003) Effects of inhibition of myocardial extracellular-responsive kinase and P38 mitogen-activated protein kinase on mechanical function of rat hearts after prolonged hypothermic ischemia. Transplantation 75:173–180
Wykes RC, Lee M, Duffy SM, Yang W, Seward EP, Bradding P (2007) Functional transient receptor potential melastatin 7 channels are critical for human mast cell survival. J Immunol 179:4045–4052
Mackins CJ, Kano S, Seyedi N, Schafer U, Reid AC, Machida T et al (2006) Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest 116:1063–1070
Vural KM, Liao H, Oz MC, Pinsky DJ (2000) Effects of mast cell membrane stabilizing agents in a rat lung ischemia-reperfusion model. Ann Thorac Surg 69:228–232
Abonia JP, Friend DS, Austen WG Jr, Moore FD Jr, Carroll MC, Chan R et al (2005) Mast cell protease 5 mediates ischemia-reperfusion injury of mouse skeletal muscle. J Immunol 174:7285–7291
Middelbeek J, Kuipers AJ, Henneman L, Visser D, Eidhof I, van Horssen R et al (2012) TRPM7 is required for breast tumor cell metastasis. Cancer Res 72:4250–4261
Acknowledgments
The authors would like to thank Professor Loren Runnels for his great assistance. The study was supported by grants from the National Natural Science Foundation of China (No.81172734 and No. 81202027) and Fundamental Research Funds for the Central Universities (No. 2012303020211).
Conflict of interests
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meng, Z., Cao, R., Wang, Y. et al. Suppression of renal TRPM7 may alleviate kidney injury in the renal transplantation. World J Urol 32, 1303–1311 (2014). https://doi.org/10.1007/s00345-013-1208-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-013-1208-y