Abstract
Objectives
To compare oncological outcomes of a consecutive retropubic radical prostatectomy (RRP) and robot-assisted radical prostatectomy (RARP) series performed by a single surgeon who had performed >750 prior RRPs and was starting to perform RARPs.
Materials and methods
Prospectively collected longitudinal data of 277 RRP and 730 RARP cases over a 5-year period were retrospectively analyzed. The RARP series were divided into 3 subgroups (1st, <250 cases; 2nd, 250–500; and 3rd, >500) according to the surgical period. The positive surgical margin (PSM) and biochemical recurrence-free survival (BCRFS) rates were compared at each pathological stage.
Results
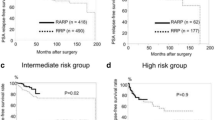
The pT2 PSM rates showed no significant difference between the RRP (7.8 %) and RARP series (1st, 9.5 %; 2nd, 14.1 %; and 3rd, 9.8 %) throughout the study period (P = 0.689, 0.079, and 0.688, respectively). Although the pT3 PSM rates of the 1st (50.6 %) and 2nd RARP series (50.0 %) were higher than that of the RRP series (36.0 %; P = 0.044 and P = 0.069, respectively), the 3rd RARP series had a comparable pT3 PSM rate (32.4 %, P = 0.641). The 3-year BCRFS rates of the RRP and RARP series were similar at each pathological stage (pT2, 92.1 vs. 96.8 %, P = 0.517; pT3, 60.0 vs. 67.3 %, P = 0.265, respectively).
Conclusions
The pT2 PSM and short-term BCRFS rates were similar between RRP and RARP, and RARP showed comparable pT3 PSM rate with RRP after >500 cases of surgical experience. Our data suggest that an experienced robotic surgeon at a high-volume center may achieve comparable oncological outcomes with open prostatectomy even in locally advanced disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Retropubic radical prostatectomy (RRP), which is the most common treatment option for clinically localized prostate cancer, provides excellent long-term cancer control [1]. Regarding functional outcomes, such as continence and potency recovery, robot-assisted radical prostatectomy (RARP) is reported to be generally superior to RRP in several studies including our previous report [2, 3], although conflicting results have been reported [4]. The main goal of RARP remains cancer control, though excellent functional outcomes are also of key importance. Several single-arm RARP studies [5, 6] and comparative studies between RRP and RARP reported that the short-term oncological outcomes are similar between the two techniques [3, 7]. However, well-controlled prospective studies comparing the outcomes based on concurrent RRP and RARP series performed by a single surgeon are rare.
In our present study, we compared oncological outcomes between the two operative methods and sought to identify a learning curve of RARP that is comparable to RRP in terms of the positive surgical margin (PSM) and short-term biochemical recurrence-free survival (BCRFS) rates.
Materials and methods
Study population
After institutional review board approval, prospectively collected longitudinal data of 1,128 concurrent RRP and RARP series at a tertiary referral center between July 2007 and June 2012 were retrospectively analyzed. A total of 121 patients who received neoadjuvant therapy (n = 89), adjuvant therapy (n = 12), or both (n = 20) were excluded, leaving 1,007 patients (277 RRP and 730 RARP) who formed the cohort for the current study. The first few RARP cases were included in the analysis.
All surgeries were performed by a single senior surgeon (H.A.) who had performed >750 prior RRPs and was starting to perform RARP. The choice of surgical method was determined on a joint decision between the patient and the surgeon. Key surgical procedures at prostatectomy were performed in the same way except for the transperitoneal antegrade approach for RARP and extraperitoneal retrograde approach for RRP. As described in our previous study [2], nerve-sparing procedures were performed for all preoperatively potent patients on sides where cancer extension was not expected, and no electrocautery was used. Pelvic lymph node dissection was performed in intermediate and high-risk patients according to D’Amico’s criteria [8], and involved 77.6 % (215/277) of RRP patients and 59.9 % (437/730) of RARP patients.
Supersensitive prostate-specific antigen (PSA) levels were measured at 3, 6, and 12 months after surgery, then every 6 months up to 3 years, and then annually thereafter. The median follow-up was 32.0 months (interquartile range 15.6–45.9). Biochemical recurrence (BCR) following prostatectomies was defined as a serum PSA level of 0.2 ng/mL or greater, with a second confirmatory level of PSA of 0.2 ng/mL.
Pathological analysis
Both the prostate apex and base were examined by the cone method. All pathology slides were reviewed by a single uropathologist (Y.C.), and the PSM was defined as the tumor extending to the inked surface of the specimen [9]. PSM was stratified into four groups by tumor location: apex, posterolateral, base, and multifocal [10]. Pathological staging was performed with the 2002 TNM classification.
Statistical analysis
To evaluate the learning curve of RARP, consecutive RARP series were divided into 3 subgroups (1st, <250 cases; 2nd, 250–500 cases; and 3rd, >500 cases) according to surgical period. Overall and stage-specific PSM and 3-year BCRFS rates were compared between the RRP and RARP series. In addition, a cumulative summation graph of the PSM for the RRP and RARP subgroups was drawn to visualize the learning curve of RARP [11].
BCFRS was analyzed with the Kaplan–Meier method with a log-rank test. Predictive factors for PSM and BCRFS were identified by multivariate logistic regression analysis and a Cox proportional hazards regression model, respectively. All tests were two sided, with P < 0.05 considered significant. All statistical analyses were performed with SAS software, version 9.2 (SAS institute, Cary, NC).
Results
Patient characteristics and pathological outcomes are summarized in Table 1. The RARP group was significantly associated with a younger age, lower body mass index, and lower clinical stage than the RRP group. Similarly, the RARP group was associated with a lower pathological stage and pathological Gleason score than the RRP group. Nerve-sparing surgeries were more frequently performed in the RARP group than the RRP group (76.1 vs. 52.7 %, P < 0.001).
The overall, pT2, and pT3 PSM rates of RRP and RARP were 20.9 and 23.3 %, 7.8 and 11.2 %, and 36.0 and 44.7 %, respectively (P = 0.426, 0.233, and 0.122, respectively). Table 2A shows the PSM rates stratified by RARP surgical period. The pT2 PSM rates showed no significant difference between the RRP (7.8 %) and RARP series (1st, 9.5 %; 2nd, 14.1 %; and 3rd, 9.8 %) throughout the study period (P = 0.689, 0.079, and 0.688, respectively). While the pT3 PSM rates of the 1st (50.6 %) and 2nd RARP series (50.0 %) were higher than that of the RRP series (36.0 %; P = 0.044 and P = 0.069, respectively), the 3rd RARP series had comparable pT3 PSM rates (32.4 %, P = 0.641). Figure 1 shows the variation in the PSM rates for the consecutive surgical series (Fig. 1a) and the pT3 tumors (Fig. 1b) of each procedure. Overall, PSM rates after 500 RARP cases significantly decreased compared to those of less than 500 cases (17.4 vs. 26.0 %, P = 0.011). Similarly, pT3 PSM rates also significantly decreased after 500 cases RARP (50.3 vs. 32.4 %, P = 0.010). The PSM locations are summarized in Table 2B. The most common site of PSM in the RRP group was the apex (37.9 %) and followed by the posterolateral side (27.6 %) and the base (3.4 %), and the apex was also the most common site of PSM in the RARP group (34.1 %) and followed by the posterolateral side (28.2 %) and the base (7.1 %).
Three-year BCRFSs of the RRP and RARP were similar in each pathological stage (pT2 92.1 vs. 96.8 %, P = 0.517; pT3 60.0 vs. 67.3 %, P = 0.265, respectively). In the multivariate analysis including various parameters, PSA, pathologic stage, pathologic Gleason score, and operative period of RARP were significant predictors for PSM, while pathologic stage, pathologic Gleason score, and PSM were significant predictors for BCRFS (Table 3A). In pT3 tumors, operative period of RARP was also significant predictors for PSM (Table 3B).
Discussion
In the present study, we assessed PSM and short-term BCRFS of consecutive RARP series performed by a single surgeon who was an expert of RRP and started to perform RARPs. Stage-specific analysis showed comparable pT2 PSM and 3-year BCRFS rates between the two operative methods. However, pT3 PSM rate on early phase of the learning curve of the RARP series was significantly higher than that of the RRP series, and with the accumulation of RARP cases, the operative methods had little impact on the pT3 PSM rates.
The PSM rates of RRP and RARP comparative studies are well summarized in the study of Tewari et al. [12]. In their meta-analysis, the weighted means of the overall PSM rates were 24.2 % for RRP and 16.2 % for RARP; the pT2 rates were 16.6 % for RRP and 10.7 % for RARP; and the pT3 rates were 42.6 % for RRP and 37.2 % for RARP. The overall PSM rate of our RARP series (23.3 %) was slightly higher than that of the meta-analysis (16.2 %). This finding may be due to differences in the study population, i.e., our study population included more high-risk patients.
When stratified by pathological stage, our pT2 PSM rate for RARP was comparable to that of the meta-analysis (11.2 vs. 10.7 %, respectively) and was even similar in our initial 250 RARP series (9.5 vs. 10.7 % for the meta-analysis). These results indicate that the learning curve to achieve a comparable pT2 PSM rate is not too steep for an experienced, high-volume open surgeon. In another study by Doumerc et al. [13], the learning curve started to plateau for the overall PSM rate after 150 cases, and for the pT2 PSM rate, it started to flatten after 140 cases.
In current study, the pT3 PSM rate of RARP was 44.7 %, which was higher than the RRP rate of 36.0 %. In other studies, pT3 PSM rates of RARP have been reported as ranging from 37 to 57 % [2, 10, 14, 15]. For example, Zorn et al. [14] reported that the PSM rate in pT3 disease was 50 %, even after 200 RARP surgeries. The higher PSM rate in the RARP group compared to RRP in our series may be attributable to several factors. The learning curve of RARP could be one cause. As shown in the cumulative summation graph of PSM (Fig. 1), the PSM rate for RRP was consistent during the entire study period, whereas the overall PSM rate for RARP improved after approximately 500 cases (17.4 vs. 26.0 % for the initial 500 cases, P = 0.011). In addition, our multivariate analysis for predictive factors of PSM also indicated that >500 cases of RARP were needed to achieve a comparable PSM rate to RRP (OR = 1.155, P = 0.579). This finding indicates that differences in pT3 PSM rates between RRP and RARP decrease with accumulating experience with RARP.
It is not clear how many cases are needed to overcome the learning curve of RARP, specifically in locally advanced tumors. Our results suggest that more than 500 cases are required to achieve comparable pT3 PSM rates between RARP and RRP. In one study published by the Vanderbilt University group [16], it was reported that even for an experienced open surgeon, a minimum of 150 RARP cases was needed to achieve comparable outcomes to RRP, although it was not specific for pT3 tumors. However, some other studies have reported that much more cases are needed to overcome the learning curve [17–19]. For example, in a multi-institutes’ study from 3 high-volume centers (UPenn, Karolinska, Cornell) specifically focusing on pT3 tumors [19], the learning curve of pT3 tumors started to plateau after 1,000–1,500 cases. From our results and prior studies, we believe that the learning curve for RALP, specifically in pT3 tumors, is not as short as that reported previously [20, 21], even for an experienced open surgeon.
With regards to BCR, our present stage-specific analysis showed similar 3-year BCRFS rates between RRP and RARP. In the multivariate analysis for BCRFS, the surgical experience with RARP was not significant, which was similar to finding in other studies [7, 22, 23]. However, given that the PSM was a significant predictor for BCR in our multivariate analysis (HR = 1.786, P = 0.005) and that our BCRFS results were based on a short follow-up period, a further long-term follow-up is needed to confirm whether PSM following RARP translates into BCR, specifically in pT3 tumors of the early RARP series.
The treatment of high-risk prostate cancer is still challenging, but good long-term cancer-specific survival data have been reported after radical surgery for these patients [24–27]. In addition, there are growing evidences that RARP is a feasible option for men with high-risk prostate cancer, as reported by several studies demonstrating equivalent oncological outcomes of RARP and RRP [28, 29]. Our present finding that an experienced robotic surgeon could achieve PSM and short-term BCRFS rates comparable with those of RRP, even in pT3 tumors, may be a additional evidence that supports a role for RARP as one of the treatment options for high-risk prostate cancer.
We acknowledge that our results are non-equal group comparison due to lack of randomization, and further, long-term follow-up is needed to confirm comparable BCRFS between groups. In addition, given that lower rate of nerve sparing in the RRP group may predispose that group to having a lower PSM rate, this bias should be considered. Despite possible limitations, our results were based on a single surgeon’s concurrent RRP and RARP series and a pathological review under the same protocol by a single uropathologist.
Conclusion
In our stage-specific analysis, the pT2 PSM and short-term BCRFS rates of RARP were similar between RRP and RARP, whereas RARPs were associated with higher pT3 PSM rate than RRP. Meanwhile, pT3 PSM rate of RARP showed a trend for similar outcomes to that of RRP after >500 cases of RARP surgical experience. Although RRP is still gold standard for locally advanced prostatic cancer, our data suggest that an experienced robotic surgeon of a high-volume center may achieve comparable oncological outcomes with open prostatectomy, even in locally advanced disease.
References
Zincke H, Bergstralh EJ, Blute ML, Myers RP, Barrett DM, Lieber MM, Martin SK, Oesterling JE (1994) Radical prostatectomy for clinically localized prostate cancer: long-term results of 1,143 patients from a single institution. J Clin Oncol 12(11):2254–2263
Kim SC, Song C, Kim W, Kang T, Park J, Jeong IG, Lee S, Cho YM, Ahn H (2011) Factors determining functional outcomes after radical prostatectomy: robot-assisted versus retropubic. Eur Urol 60(3):413–419. doi:10.1016/j.eururo.2011.05.011
Rocco B, Matei DV, Melegari S, Ospina JC, Mazzoleni F, Errico G, Mastropasqua M, Santoro L, Detti S, de Cobelli O (2009) Robotic versus open prostatectomy in a laparoscopically naive centre: a matched-pair analysis. BJU Int 104(7):991–995. doi:10.1111/j.1464-410X.2009.08532.x
Barry MJ, Gallagher PM, Skinner JS, Fowler FJ Jr (2012) Adverse effects of robotic-assisted laparoscopic versus open retropubic radical prostatectomy among a nationwide random sample of medicare-age men. J Clin Oncol 30(5):513–518. doi:10.1200/JCO.2011.36.8621
Menon M, Bhandari M, Gupta N, Lane Z, Peabody JO, Rogers CG, Sammon J, Siddiqui SA, Diaz M (2010) Biochemical recurrence following robot-assisted radical prostatectomy: analysis of 1,384 patients with a median 5-year follow-up. Eur Urol 58(6):838–846. doi:10.1016/j.eururo.2010.09.010
Liss MA, Lusch A, Morales B, Beheshti N, Skarecky D, Narula N, Osann K, Ahlering TE (2012) Robot-assisted radical prostatectomy: 5-year oncological and biochemical outcomes. J Urol 188(6):2205–2210. doi:10.1016/j.juro.2012.08.009
Barocas DA, Salem S, Kordan Y, Herrell SD, Chang SS, Clark PE, Davis R, Baumgartner R, Phillips S, Cookson MS, Smith JA Jr (2010) Robotic assisted laparoscopic prostatectomy versus radical retropubic prostatectomy for clinically localized prostate cancer: comparison of short-term biochemical recurrence-free survival. J Urol 183(3):990–996. doi:10.1016/j.juro.2009.11.017
D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280(11):969–974
Rosen MA, Goldstone L, Lapin S, Wheeler T, Scardino PT (1992) Frequency and location of extracapsular extension and positive surgical margins in radical prostatectomy specimens. J Urol 148(2 Pt 1):331–337
Patel VR, Coelho RF, Rocco B, Orvieto M, Sivaraman A, Palmer KJ, Kameh D, Santoro L, Coughlin GD, Liss M, Jeong W, Malcolm J, Stern JM, Sharma S, Zorn KC, Shikanov S, Shalhav AL, Zagaja GP, Ahlering TE, Rha KH, Albala DM, Fabrizio MD, Lee DI, Chauhan S (2011) Positive surgical margins after robotic assisted radical prostatectomy: a multi-institutional study. J Urol 186(2):511–516. doi:10.1016/j.juro.2011.03.112
Williams AK, Chalasani V, Martinez CH, Osbourne E, Stitt L, Izawa JI, Pautler SE (2011) Cumulative summation graphs are a useful tool for monitoring positive surgical margin rates in robot-assisted radical prostatectomy. BJU Int 107(10):1648–1652. doi:10.1111/j.1464-410X.2010.09634.x
Tewari A, Sooriakumaran P, Bloch DA, Seshadri-Kreaden U, Hebert AE, Wiklund P (2012) Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol 62(1):1–15. doi:10.1016/j.eururo.2012.02.029
Doumerc N, Yuen C, Savdie R, Rahman MB, Rasiah KK, Pe Benito R, Delprado W, Matthews J, Haynes AM, Stricker PD (2010) Should experienced open prostatic surgeons convert to robotic surgery? The real learning curve for one surgeon over 3 years. BJU Int 106(3):378–384. doi:10.1111/j.1464-410X.2009.09158.x
Zorn KC, Gofrit ON, Orvieto MA, Mikhail AA, Zagaja GP, Shalhav AL (2007) Robotic-assisted laparoscopic prostatectomy: functional and pathologic outcomes with interfascial nerve preservation. European urology 51 (3):755-762; discussion 763. doi:10.1016/j.eururo.2006.10.019
Ficarra V, Novara G, Secco S, D’Elia C, Boscolo-Berto R, Gardiman M, Cavalleri S, Artibani W (2009) Predictors of positive surgical margins after laparoscopic robot assisted radical prostatectomy. J Urol 182(6):2682–2688. doi:10.1016/j.juro.2009.08.037
Herrell SD, Smith JA Jr (2005) Robotic-assisted laparoscopic prostatectomy: what is the learning curve? Urology 66(5 Suppl):105–107. doi:10.1016/j.urology.2005.06.084
Zorn KC, Wille MA, Thong AE, Katz MH, Shikanov SA, Razmaria A, Gofrit ON, Zagaja GP, Shalhav AL (2009) Continued improvement of perioperative, pathological and continence outcomes during 700 robot-assisted radical prostatectomies. Can J Urol 16(4):4742–4749; discussion 4749
Samadi DB, Muntner P, Nabizada-Pace F, Brajtbord JS, Carlucci J, Lavery HJ (2010) Improvements in robot-assisted prostatectomy: the effect of surgeon experience and technical changes on oncologic and functional outcomes. J Endourol 24(7):1105–1110. doi:10.1089/end.2010.0136
Sooriakumaran P, John M, Wiklund P, Lee D, Nilsson A, Tewari AK (2011) Learning curve for robotic assisted laparoscopic prostatectomy: a multi-institutional study of 3794 patients. Minerva Urol Nefrol 63(3):191–198
Di Pierro GB, Baumeister P, Stucki P, Beatrice J, Danuser H, Mattei A (2011) A prospective trial comparing consecutive series of open retropubic and robot-assisted laparoscopic radical prostatectomy in a centre with a limited caseload. Eur Urol 59(1):1–6. doi:10.1016/j.eururo.2010.10.026
Philippou P, Waine E, Rowe E (2012) Robot-assisted laparoscopic prostatectomy versus open: comparison of the learning curve of a single surgeon. J Endourol 26(8):1002–1008. doi:10.1089/end.2011.0569
Masterson TA, Cheng L, Boris RS, Koch MO (2012) Open versus robotic-assisted radical prostatectomy: A single surgeon and pathologist comparison of pathologic and oncologic outcomes. Urol Oncol. doi:10.1016/j.urolonc.2011.12.002
Magheli A, Gonzalgo ML, Su LM, Guzzo TJ, Netto G, Humphreys EB, Han M, Partin AW, Pavlovich CP (2011) Impact of surgical technique (open vs. laparoscopic vs. robotic-assisted) on pathological and biochemical outcomes following radical prostatectomy: an analysis using propensity score matching. BJU Int 107(12):1956–1962. doi:10.1111/j.1464-410X.2010.09795.x
Mitchell CR, Boorjian SA, Umbreit EC, Rangel LJ, Carlson RE, Karnes RJ (2012) 20-Year survival after radical prostatectomy as initial treatment for cT3 prostate cancer. BJU Int 110(11):1709–1713. doi:10.1111/j.1464-410X.2012.11372.x
Xylinas E, Drouin SJ, Comperat E, Vaessen C, Renard-Penna R, Misrai V, Bitker MO, Chartier-Kastler E, Richard F, Cussenot O, Roupret M (2009) Oncological control after radical prostatectomy in men with clinical T3 prostate cancer: a single-centre experience. BJU Int 103 (9):1173-1178; discussion 1178. doi:10.1111/j.1464-410X.2008.08208.x
Joniau S, Hsu CY, Gontero P, Spahn M, Van Poppel H (2012) Radical prostatectomy in very high-risk localized prostate cancer: long-term outcomes and outcome predictors. Scand J Urol Nephrol 46(3):164–171. doi:10.3109/00365599.2011.637956
Engel J, Bastian PJ, Baur H, Beer V, Chaussy C, Gschwend JE, Oberneder R, Rothenberger KH, Stief CG, Holzel D (2010) Survival benefit of radical prostatectomy in lymph node-positive patients with prostate cancer. Eur Urol 57(5):754–761. doi:10.1016/j.eururo.2009.12.034
Pierorazio PM, Mullins JK, Eifler JB, Voth K, Hyams ES, Han M, Pavlovich CP, Bivalacqua TJ, Partin AW, Allaf ME, Schaeffer EM (2013) Contemporaneous comparison of open versus minimally-invasive radical prostatectomy for high-risk prostate cancer. BJU Int. doi:10.1111/j.1464-410X.2012.11757.x
Punnen S, Meng MV, Cooperberg MR, Greene KL, Cowan JE, Carroll PR (2013) How does robot-assisted radical prostatectomy (RARP) compare with open surgery in men with high-risk prostate cancer? BJU Int. doi:10.1111/j.1464-410X.2012.11493.x
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jinsung Park and Dae-Seon Yoo have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Park, J., Yoo, DS., Song, C. et al. Comparison of oncological outcomes between retropubic radical prostatectomy and robot-assisted radical prostatectomy: an analysis stratified by surgical experience. World J Urol 32, 193–199 (2014). https://doi.org/10.1007/s00345-013-1168-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-013-1168-2