Abstract
Purpose
To review the etiology and pathogenesis of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS).
Methods
A literature review for the years 1985–2012 was performed using the MEDLINE database of the United States National Library of Medicine.
Results
The evidence for ongoing infection in men with CP/CPPS is lacking. However, men with CP/CPPS are twice as likely to have had a sexually transmitted disease (STD), and bacteria from men with CP/CPPS may be phenotypically different from those that cause cystitis or acute prostatitis. Evidence continues to support an alteration in both the afferent and efferent autonomic nervous systems. Functional brain imaging suggests changes in the gray matter as well as the importance of the anterior insula and anterior cingulated gyrus in pain processing. Neural function can be modulated by immune and endocrine factors. Alterations in cytokine function and autoimmunity appear to play a role in the immune dysfunction. Alterations in the hypothalamic–pituitary–adrenal axis can mediate the endocrine effects, similar to many other chronic pain conditions. Genetics may play a role in who may develop chronic pain after an initial insult. Finally, any biological changes must then be processed through the psychosocial environment, including the tendency to catastrophize, and degree of spousal support, to produce a given individual patient’s pain experience.
Conclusions
Infection with atypical bacteria or sequelae of an STD may lead to CP/CPPS in some men. Such a biological insult in the context of alterations in psychoimmunoneurendocrine factors produces the chronic pain experience.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a condition of chronic pelvic pain in men [1]. The diagnosis is made on the basis of symptoms of pain with or without voiding symptoms in the absence of other identifiable causes [2]. The etiology of symptoms in a given patient can be from many causes. Whether there are identifiable, repeatable patterns of causes of symptoms, or just individual patients with distinct individual phenotypes is unknown. However, in the last several decades, we have identified some of the factors that play a role in men with CP/CPPS.
Infection
The symptoms of CP/CPPS are similar to those of a true prostatic infection. Therefore, infection has been commonly assumed by patients and clinicians alike to be the cause of the symptoms, both historically and to this day. In the CPCRN study, using the 4-glass urine test for localization, men with CP/CPPS and asymptomatic controls showed almost identical numbers of bacteria isolated from urine, prostatic fluid, and post-prostate massage urine [3]. Eight percent of men had uropathogenic bacteria and roughly 70 % had some form of bacteria in each group. This indicates that asymptomatic men appear to routinely have bacteria in the prostate, but may not by themselves produce disease or symptoms. Search for other infectious agents such as virus [4] or for other bacteria identifiable by polymerase chain reaction (PCR) [5] has not proven an infectious cause; there was however an association with improvement on antibiotics in men with bacteria detected by PCR compared to those with no detectable bacteria [6].
Since the discovery of H. pylori as the causative organism in stomach ulcers, a search for a previously unknown source has been carried out in many other unexplained conditions including CP/CPPS. A report indicated that blood samples examined for H. pylori antibodies were positive in 76 % of men with CP/CPPS compared to 62 % in controls (p < 0.05). Although this is significantly greater, a large number of the patients without symptoms were seropositive [7]. Nanobacteria have also been suggested as a possible infectious source in CPPS, as they commonly occur in prostatic stones, and anti-nanobacterial therapy has proven effective in small uncontrolled studies [8–11].
In a study of 30,000 male health professionals, men who reported a history of sexually transmitted disease were found to have 1.8-fold higher odds of prostatitis [12]. However, studies that have looked for the presence of sexually transmitted organisms such as Chlamydia trachomatis, Trichomonas vaginalis, Ureaplasma urealyticum, or Mycoplasma hominis have failed to show persistent infection [13]. Although the presence of an active infection was not evident in men participating in the NIH cohort study, patients with CP/CPPS were found to have a significantly greater history of urethritis compared to age-matched controls [14]. In susceptible men, urethritis could serve as a source of inflammation that could cause chronic pelvic pain well after the resolution of infection.
It is possible that more common bacteria can cause CP/CPPS, but the ones that do are likely different from those that may be present and cause no disease process. Uropathogenic bacteria including uropathogenic E. coli (UPEC) express virulence factors that produce the ability to infect [15]. Several reports have indicated that the bacteria isolated from men with CP/CPPS are phenotypically different than those from men without pain, including the ability to inhibit complement [16–20]. The bacteria from men with CP/CPPS have also been shown in a rat model to produce several different effects from E. coli that produce cystitis: (1) the strain CP1 from a man with CP/CPPS adhered to, invaded and proliferated within prostate epithelial cells in culture; (2) in a live animal model, CP1 induced and sustained chronic pelvic pain that persisted after bacterial clearance from the genitourinary tract; (3) the pelvic pain was produced in the NOD strain of mice but not C57BL/6J mice, indicating genetic despite similar invasion and proliferation in each species; this indicates a genetic susceptibility to pain but not the infection itself [21]. The NOD mice that developed pain are genetically prone to develop autoimmune diseases of specific organs [22]. The bacterium CP1 in this study was phenotypically different from cystitis strains in that it was group B1 and not B2, and lacked virulence factors found on bacteria associated with acute prostatitis. This study may be the equivalent of the Rosetta stone for CP/CPPS as it recreates many of the clinical phenotypes seen in our patients.
Inflammation
The term “prostatitis” implies inflammation of the prostate gland. However, it is clear that not all men with CPPS have inflammation related to the prostate. Only about one-third of men with clinical CPPS have been found to have prostatic inflammation on biopsy [23]. In those with inflammation, the degree of that inflammation does not correlate with symptoms [24, 25]. Men with category IIIB CPPS do not have inflammation in seminal plasma, expressed prostatic secretions, or post-massage urine. Men with category IV prostatitis [1] do not have pain. They were previously called “asymptomatic,” but this may be only true from the standpoint of pain, as prostate inflammation seems to correlate with lower urinary tract symptoms (LUTS) [26].
Considerable effort has been made examining cytokines. Increased levels of IL-1 beta, IL-8, TNF-α, and ENA-78 have been found in EPS of patients with category IIIA prostatitis, but not in those with category IIIB prostatitis [27, 28]. Levels of the chemokines monocyte chemoattractant protein-1 and macrophage inflammatory protein-1-α were significantly elevated in patients with CP/CPPS compared to those without urological disease or BPH, regardless of WBC levels in EPS samples [29]. Furthermore, elevated MIP-1-α levels in CP/CPPS patients showed positive correlation not only with absolute NIH-CPSI scores, but also with pain components of the NIH-CPSI as well. The MCP-1 along with other pro-inflammatory cytokines TNF-α and IL-1β has recently been to be nonfunctional when incubated with expressed prostatic secretions from men with CPPS [30]. The mechanism involves heat labile extracellular proteases. Thus, it may not be the cytokines themselves in function or amount, but impairment of their normal function by an as-yet-unidentified protease in men with pelvic pain.
Autoimmune prostatitis in a rat model has been shown to produce pelvic pain, and pain localization to the prostate in this EAP model was suggested by attenuation of pain behaviors after intraprostatic lidocaine injection [31]. EAP mice also demonstrate increased total and activated mast cells in the prostate [32]. Mast cell-deficient mice showed attenuated pelvic pain behavior, but no difference in inflammation from control mice. Treatment of EAP mice with a mast cell stabilizer and histamine receptor antagonist resulted in a synergistic decrease in pelvic pain. In the same study, EPS from men with CPPS was found to have increased mast cell tryptase compared to controls. Other evidence for autoimmune prostatitis in men with CPPS includes increased T-cell response to seminal plasma compared to controls [33]. Men with CPPS demonstrate an increased lymphoproliferative response to prostate antigens compared to controls and those with chronic prostatitis [34]. A region of the PAP molecule, 173–192, results in greater activation of CD4 cells and release of interferon-γ in men with CPPS than controls [35]. Men with CP/CPPS have also been reported to have autoantibodies against human seminal vesicle secretory protein 2 (SVS2), which in mice deficient in the autoimmune regulator gene (AIRE) develop B- and T-cell immune responses to this protein [36]. These data indicate the likelihood of autoimmunity in some men with CPPS.
Nervous system
Since pain is mediated by the nervous system, it is reasonable to consider abnormalities of the nervous system as a cause of symptoms in patients with CP/CPPS. Men with CP/CPPS were 5 times more likely to self-report a history of nervous system disease compared to asymptomatic age-matched controls in the Chronic Prostatitis Collaborative Research Network (CPCRN) Study [14]. In the NIH cohort, the symptom that most contributed to the difference in neurologic disease was numbness and tingling in the limbs. Also significant was a history of vertebral disc disease/surgery. One of the markers that correlate with pain in men with CP/CPPS is nerve growth factor [37]. NGF is a neuropeptide that plays a role in nociception and regulates the sensitivity of adult neurons to capsaicin which excites C-fibers, in addition to mediating long-term depolarization via NMDA receptors [38]. NGF is reported to also decrease in men with CPPS who respond to treatment, but not in those who do not respond [39]. However, a recent trial of an antibody to NGF resulted in only a modest reduction in pain [40]. There was no selection of patients with elevation of NGF levels as a criterion for inclusion in the study.
Evidence has been growing over the last decade that men with CP/CPPS have alterations in the nervous system that may contribute to their symptoms. These efforts have been led by the group at the University of Washington. An early study of neurophysiologic testing examined differences in response to afferent fibers: A-β fibers which are large myelinated fibers mediating light touch and somatic pain, A-δ fibers which are smaller myelinated fibers that mediate cold stimuli and early sensations of pain, and C-fibers which are small unmyelinated fibers and mediate most autonomic signals including thermal stimuli and visceral pain. Compared to controls, no difference was seen in the myelinated fibers, but men with CP/CPPS reported higher pain intensity at lower temperatures indicative of altered C-fiber function [41].
Thermal sensitivity tests given to the perineum and anterior thigh in a much larger group of men with CP/CPPS and controls confirmed pilot data and again showed that men with CP/CPPS were more sensitive to heat in the perineum but not the anterior thigh compared to controls [42]. This indicates alterations in the afferent autonomic nervous system and suggests central sensitization of the nerves. Studies on the efferent side showed that when on 5-min resting supine and standing blood pressure measurements, men with CP/CPPS showed alterations in the heart rate variability compared to controls. When measures of parasympathetic activity decreased in the controls, there was little change in men with CPPS, and sympathetic activity increased in the controls and decreased in men with CP/CPPS [43]. This is a reflection of efferent autonomic activity. Overall heart rate variability has been reported as lower in men with CPPS than controls [44]. A more recent study examined sensory perception thresholds in the perineum, penis, palms, and soles. There were no sensory threshold differences between CPPS men and controls, indicating that there is no peripheral neuropathy [45].
There are subtle signs of neurologic abnormality in men with CPPS also. The segments of the tibial nerve that innervate the toes come from S2 to 3 roots, the same that innervate the pelvis. Men with CPPS are less often able to spread all toes as compared to men without CPPS. This is considered to possibly reflect neural deficits in the sacral roots [46]. One of the unanswered questions in CPPS is the association with cardiovascular disease. Men in the CPCRN study were six times more likely to self-report a history of cardiovascular disease, especially hypertension [14]. A recent report indicates that men with CPPS also have alterations in arterial stiffness and lower reactive hyperemia index, and measures of cardiovascular dysfunction [47]. Whether this is related to autonomic dysfunction and/or localized to the endothelium is unclear.
Neurological abnormalities are postulated to also cause spam in the pelvic floor [48]. Men with CPPS are noted on urodynamic studies to have detrusor sphincter dyssynergy in 73 % of cases. This is normally seen in patients with suprasacral spinal cord lesions [49]. In the study by Zermann et al. [48], CPPS patients were found to have pathological tenderness of the striated pelvic floor muscle and poor to absent function in ability to relax the pelvic floor efficiently with a single or repetitive effort. An EMG study of the pelvic floor in men with CPPS showed that compared to controls, men with CPPS had (1) greater preliminary resting hypertonicity and instability and (2) lowered voluntary endurance contraction amplitude [50]. Men with CPPS also have more tender points in areas outside of the pelvis as compared to controls [51]. This adds to the evidence that CPPS may be a systemic pain syndrome.
Studies of brain anatomy and function have begun to describe changes in men with CPPS. Farmer et al. studied spontaneous pelvic pain, with men lying in the fMRI scanner for 10 min and rating their fluctuations in the absence of any external stimulus. Pain perception was localized to the anterior insula and correlated with the intensity of self-reported pain. Density of gray matter in the anterior insula and anterior cingulate cortex also correlated with pain intensity. There was no decrease in gray matter in this study, but there were differences in the relationship of gray and white matter in men with CPPS compared to controls [52]. It is not known whether changes in the CNS are as a result of the chronic pain or are abnormalities that lead to pain in the first place. The findings in men with CPPS differ from those in other pain conditions including low back pain [53]. The insula is involved in determining the intensity of pain magnitude [54]. A study from Switzerland reported that in a group of 40 men with CPPS, there was a significant reduction in relative gray matter volume in the anterior cingulate gyrus that correlated with overall NIH-CPSI score as well as the pain subscale [55]. The ACC is involved in emotional pain processing [56] and sympathetic activity [57]. Important to keep in mind is that brain anatomy is dynamic and not static. Cortical changes are noted in patients after treatment for back pain [58].
One of the unifying hypotheses of chronic pain syndromes is that of central sensitization. Original theories of nervous system function believed that the CNS was directly determined by the duration, intensity, and location of a stimulus. However, as described by Woolf in [59], it is now widely recognized that there can also be sensitization of the nerves such that even after a stimulus is terminated, there is continued nerve activity in the absence of stimulation or which requires a very low level of nociceptive stimulation to continue. One of the hallmarks of central sensitization is also an expansion of the field of pain to areas of non-injured tissue [60]. We commonly see this phenomenon in men with CPPS, in which pain in one area, for instance the testis or from epididymitis, eventually leads to pain in the area between the umbilicus and mid-thigh. Experimental evidence for central remodeling is provided by the finding that chemical irritation of rat prostate and bladder causes c-fos expression at spinal cord levels L6 and S1 along with plasma extravasation at the identical L6 and S1 dermatomes, underscoring the overlap of afferent nerve fiber distribution [61].
Psychosocial factors
The same biological nociceptive process will result in a different pain experience in different individuals on the basis of the psychosocial context of that process. Assessing quality of life with the 12-item short-form (SF-12) instrument, [62], the mental component summary score for CPPS patients is lower than that observed in the most severe subgroups of congestive heart failure (CHF) and diabetes [63]. In the CPCRN study, men with CP/CPPS self-reported a history of anxiety or depression twice as often as age-matched controls with no pain [14]. The factors that contributed most to the reduced quality of life were pain intensity and quality of life subscale of the NIH-CPSI [64]. Further detailed investigation of psychological variables in this cohort showed that helplessness/catastrophizing predicted overall pain along with urinary symptoms and depression [65]. The helplessness subscale of catastrophizing also predicts the mental subscale scores on the SF-12 in these patients [66]. Catastrophizing can mediate ethnic differences in pain response, pointing out the importance of the social context on the pain experience [67]. It is postulated that catastrophizing may activate central attention centers and inhibit the suppression of significant pain leading to chronic pain [68].
Another important modifier of the pain experience in men with CP/CPPS is spousal support. Lower perceptions of spousal support also contribute to lower mental component scores on the SF-12 in the CPCRN study [66]. Pain and disability are greater at higher levels of solicitous responses by spouses; the less the spouse tries to distract the patient from the pain, such as by trying to get them involved in other activities, the greater the pain and disability connection [66]. Spousal response also changes the physiology of the patients. In category III prostatitis patients, the degree of spousal concern and support as well as effort to distract the patient from his symptoms correlates with lower seminal plasma IL-6 and IL-10 concentrations [37]. The term “stress prostatitis” was used in the late 1980s to describe CP/CPPS given the common association between psychological stress and symptoms. Later studies have confirmed this association. Ullrich and colleagues used measures of perceived stress in a study of men with CPPS and found that greater perceived stress during the 6 months after the health care visit was associated with greater pain intensity (p = .03) and disability (p = .003) at 12 months, even after controlling for age, symptom duration, and pain and disability during the first 6 months [69].
Early adverse experiences may contribute to later symptoms of chronic pain. In the Boston Area Community Health Survey (BACH), men who reported having experienced sexual, physical, or emotional abuse were more likely (OR 1.7–3.3) to report symptoms of CP/CPPS in the survey [70]. It is known that stress has physiological consequences, as in addition to many other stimuli such as cytokines, bacterial toxins, and hypoxia, mast cells release their contents in response to stress [71]. Chronic stress also changes DNA by epigenetic modifications, in which biochemical changes alter the DNA and affect function [72]. Some of these changes are reversible and some are not [72].
Endocrine abnormalities
Also effecting both immune and nervous system functions are endocrine factors. Men with CP/CPPS are more likely to have other chronic pain conditions such as irritable bowel syndrome, fibromyalgia, and chronic fatigue syndrome [73]. One of the common findings in chronic pain conditions is alterations in the hypothalamic–pituitary–adrenal axis [74, 75]. Similar findings have been reported in CP/CPPS. On awakening, serum cortisol levels rise; there is a significantly greater cortisol rise in men with CPPS compared to controls [76]. Men with CPPS also have a lower baseline ACTH level and blunted ACTH rise in response to stress than men without symptoms [76]. One report has indicated reduced activity of CYP21A2 (P450c21), the enzyme that converts progesterone to corticosterone and 17-hydroxyprogesterone to 11-deoxycortisol [77].
Genetic predisposition to CP/CPPS
The idea of genetic differences that may predispose CP/CPPS patients to develop the condition has been explored in several targets. Differences in the DNA sequence, or polymorphisms, have been identified in the promoter regions of several cytokines. Polymorphisms in the genes or promoters for IL-10 AA and TNF-α are associated with low IL-10 [78] or TNF-α production [79]. In one study, significantly more men with CPPS expressed the IL-10 AA genotype compared to controls (11 of 36, 31 % vs. 33 out of 272, 12 %; p = .007) [80]. All eight IIIa patients had the low TNF-α production genotype. There was no difference in the TNF-α genotype in the 22 IIIb patients versus 272 controls but all 8 of the IIIa patients had the low TNF-α genotype. The cytokine polymorphisms also correlated with the clinical response to treatment with the antioxidant quercetin. Differences have been reported in the frequency of three alleles near the phosphoglycerate kinase (PGK) gene, between CPPS patients and controls [81]. The alleles differed in the number of short tandem repeats (STRs). The PGK1 gene in the region assessed has been found to be associated with familial prostate cancer, hypospadias, and androgen insensitivity. Another gene in the same region of the X chromosome, Xq11–Xq13, is the androgen receptor [82]. This finding raises the possibility of androgen insensitivity or dysfunction in the pathogenesis of CPPS. Dinucleotide sequences have also been found to be common in genes for neural development [83]. Investigation of SNPs in the gene for manganese superoxide dismutase has reported significant differences between men with CP/CPPS and controls but not between category IIIa and IIIb CP/CPPS [84]. The enzyme levels of manganese superoxide dismutase and glutathione peroxidase were higher in control subjects than prostatitis patients, indicating lower antioxidant status in men with CP/CPPS.
Conclusion
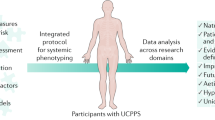
It was much easier to discuss mechanisms in prostatitis when all symptoms were thought to come from an infection. We now know that CP/CPPS is nothing if not a chronic pain syndrome. The complexity of chronic pain conditions is becoming apparent. There are a myriad of possible phenotypes that may be seen [85]. Figure 1 outlines the author’s current perspective on the development of CP/CPPS. It is unlikely that a given insult such as infection, trauma, or even extreme stress will cause symptoms in all individuals. Men who develop CP/CPPS probably have some risk factor or factors that predispose them to developing chronic pain. The common denominator is then neurologic alteration. The neurological insult is modified by immune and endocrine factors; deficits in these areas can contribute to the chronic state. Finally, the biological insult must be put into the psychosocial context to produce each patient’s pain experience. Much like we are also responsible for dermatologic lesions in the genitourinary organs, we are responsible for treating a chronic pain condition that occurs in our area of interest. We must become on some level a pain doctor.
References
Krieger JN, Nyberg L Jr, Nickel JC (1999) NIH consensus definition and classification of prostatitis. JAMA 282:236–237
Schaeffer AJ, Landis JR, Knauss JS, Propert KJ, Alexander RB, Litwin MS, Nickel JC, O’Leary MP, Nadler RB, Pontari MA, Shoskes DA, Zeitlin SI, Fowler JE Jr, Mazurick CA, Kishel L, Kusek JW, Nyberg LM, Chronic Prostatitis Collaborative Research Network Group (2002) Demographic and clinical characteristics of men with chronic prostatitis: the national institutes of health chronic prostatitis cohort study. J Urol 168:593–598
Nickel JC, Alexander RB, Schaeffer AJ, Landis JR, Knauss JS, Propert KJ, Chronic Prostatitis Collaborative Research Network Study Group (2003) Leukocytes and bacteria in men with chronic prostatitis/chronic pelvic pain syndrome compared to asymptomatic controls. J Urol 170:818–822
Leskinen MJ, Vainionp R, Syrjnen S, Leppilahti M, Marttila T, Kylml T, Tammela TL (2003) Herpes simplex virus, cytomegalovirus, and papillomavirus DNA are not found in patients with chronic pelvic pain syndrome undergoing radical prostatectomy for localized prostate cancer. Urology 61:397–401
Leskinen MJ, Rantakokko-Jalava K, Manninen R, Leppilahti M, Marttila T, Kylmala T, Tammela TL (2003) Negative bacterial polymerase chain reaction (PCR) findings in prostate tissue from patients with symptoms of chronic pelvic pain syndrome (CPPS) and localized prostate cancer. Prostate 55:105–110
Shoskes DA, Shahed AR (2000) Detection of bacterial signal by 16S rRNA polymerase chain reaction in expressed prostatic secretions predicts response to antibiotic therapy in men with chronic pelvic pain syndrome. Tech Urol 6:240–242
Karatas OF, Turkay C, Bayrak O, Cimentepe E, Unal D (2010) Helicobacter pylori seroprevalence in patients with chronic prostatitis: a pilot study. Scand J Urol Nephrol 44:91–94
Wood HM, Shoskes DA (2006) The role of nanobacteria in urologic disease. World J Urol 24:51–54
Geramoutsos I, Gyftopoulos K, Perimenis P, Thanou V, Liagka D, Siamblis D, Barbalias G (2004) Clinical correlation of prostatic lithiasis with chronic pelvic pain syndromes in young adults. Eur Urol 45:333–337
Shoskes DA, Thomas KD, Gomez E (2005) Anti-nanobacterial therapy for men with chronic prostatitis/chronic pelvic pain syndrome and prostatic stones: preliminary experience. J Urol 173:474–477
Zhou Z, Hong L, Shen X, Rao X, Jin X, Lu G, Li L, Xiong E, Li W, Zhang J, Chen Z, Pan J, Song B (2008) Detection of nanobacteria infection in type III prostatitis. Urology 71:1091–1095
Collins MM, Meigs JB, Barry MJ, Walker Corkery E, Giovannucci E, Kawachi I (2002) Prevalence and correlates of prostatitis in the health professionals follow-up study cohort. J Urol 167:1363–1366
Weidner W, Schiefer HG, Krauss H, Jantos C, Friedrich HJ, Altmannsberger M (1991) Chronic prostatitis: a thorough search for etiologically involved microorganisms in 1,461 patients. Infection 19:S119–S125
Pontari MA, McNaughton-Collins M, O’leary MP, Calhoun EA, Jang T, Kusek JW, Landis JR, Knauss J, Litwin MS, CPCRN Study G (2005) A case-control study of risk factors in men with chronic pelvic pain syndrome. BJU Int 96:559–565
Johnson JR (2003) Microbial virulence determinants and the pathogenesis of urinary tract infection. Infect Dis Clin North Am 17:261–278
Ivanov IB, Gritsenko VA, Kuzmin MD (2010) Phenotypic differences between coagulase-negative staphylococci isolated from seminal fluid of healthy men and men suffering from chronic prostatitis syndrome. Int J Androl 33:563–567
Ivanov IB, Gritsenko VA, Kuzmin MD (2009) Phenotypic differences between coryneform bacteria isolated from seminal fluid of healthy men and men with chronic prostatitis syndrome. Asian J Androl 11:517–520
Ivanov IB, Gritsenko VA, Kuzmin MD (2008) Distribution of secretory inhibitor of platelet microbicidal protein among urethral isolates with its correlation with prostatitis. Asian J Androl 10:189–192
Ivanov IB, Gritsenko VA, Kuzmin MD (2006) Staphylococcal secretory inhibitor of platelet microbicidal protein is associated with prostatitis source. J Med Microbiol 55:1645–1648
Ivanov IB, Kuzmin MD, Gritsenko VA (2009) Microflora of the seminal fluid of healthy men and men suffering from chronic prostatitis syndrome. Int J Androl 32:462–467
Rudick CN, Berry RE, Johnson JR, Johnston B, Klumpp DJ, Schaeffer AJ, Thumbikat P (2011) Uropathogenic Escherichia coli induces chronic pelvic pain. Infect Immun 79:628–635
Kikutani H, Makino S (1992) The murine autoimmune diabetes model: NOD and related strains. Adv Immunol 51:285–322
True LD, Berger RE, Rothman I, Ross SO, Krieger JN (1999) Prostate histopathology and the chronic prostatitis/chronic pelvic pain syndrome: a prospective biopsy study. J Urol 162:2014–2018
Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS (2008) The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the reduce trial. Eur Urol 54:1379–1384
Schaeffer AJ, Knauss JS, Landis JR, Propert KJ, Alexander RB, Litwin MS, Nickel JC, O’Leary MP, Nadler RB, Pontari MA, Shoskes DA, Zeitlin SI, Fowler JE Jr, Mazurick CA, Kusek JW, Nyberg LM, Chronic Prostatitis Collaborative Research Network Study Group (2002) Leukocyte and bacterial counts do not correlate with severity of symptoms in men with chronic prostatitis: the national institutes of health chronic prostatitis cohort study. J Urol 168:1048–1053
Schenk JM, Kristal AR, Neuhouser ML, Tangen CM, White E, Lin DW, Kratz M, Thompson IM (2010) Biomarkers of systemic inflammation and risk of incident, symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol 171:571–582
Nadler RB, Koch AE, Calhoun EA, Campbell PL, Pruden DL, Bennett CL, Yarnold PR, Schaeffer AJ (2000) IL-1beta and TNF-alpha in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J Urol 164:214–218
Hochreiter WW, Nadler RB, Koch AE, Campbell PL, Ludwig M, Weidner W, Schaeffer AJ (2000) Evaluation of the cytokines interleukin 8 and epithelial neutrophil activating peptide 78 as indicators of inflammation in prostatic secretions. Urology 56:1025–1029
Desireddi NV, Campbell PL, Stern JA, Sobkoviak R, Chuai S, Shahrara S, Thumbikat P, Pope RM, Landis JR, Koch AE, Schaeffer AJ (2008) Monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha as possible biomarkers for the chronic pelvic pain syndrome. J Urol 179:1857–1861
Thumbikat P, Shahrara S, Sobkoviak R, Done J, Pope RM, Schaeffer AJ (2010) Prostate secretions from men with chronic pelvic pain syndrome inhibit proinflammatory mediators. J Urol 184:1536–1542
Rudick CN, Schaeffer AJ, Thumbikat P (2008) Experimental autoimmune prostatitis induces chronic pelvic pain. Am J Physiol Regul Integr Comp Physiol 294:R1268–R1275
Done JD, Rudick CN, Quick ML, Schaeffer AJ, Thumbikat P (2012) Role of mast cells in male chronic pelvic pain. J Urol 187:1473–1482
Alexander RB, Brady F, Ponniah S (1997) Autoimmune prostatitis: evidence of T cell reactivity with normal prostatic proteins. Urology 50:893–899
Motrich RD, Maccioni M, Riera CM, Rivero VE (2007) Autoimmune prostatitis: state of the art. Scand J Immunol 66:217–227
Kouiavskaia DV, Southwood S, Berard CA, Klyushnenkova EN, Alexander RB (2009) T-cell recognition of prostatic peptides in men with chronic prostatitis/chronic pelvic pain syndrome. J Urol 182:2483–2489
Hou DS, Long WM, Shen J, Zhao LP, Pang XY, Xu C (2012) Characterisation of the bacterial community in expressed prostatic secretions from patients with chronic prostatitis/chronic pelvic pain syndrome and infertile men: a preliminary investigation. Asian J Androl 14:566–573
Miller LJ, Fischer KA, Goralnick SJ, Litt M, Burleson JA, Albertsen P, Kreutzer DL (2002) Nerve growth factor and chronic prostatitis/chronic pelvic pain syndrome. Urology 59:603–608
Birder L, de Groat W, Mills I, Morrison J, Thor K, Drake M (2010) Neural control of the lower urinary tract: peripheral and spinal mechanisms. Neurourol Urodyn 29:128–139
Watanabe T, Inoue M, Sasaki K, Araki M, Uehara S, Monden K, Saika T, Nasu Y, Kumon H, Chancellor MB (2011) Nerve growth factor level in the prostatic fluid of patients with chronic prostatitis/chronic pelvic pain syndrome is correlated with symptom severity and response to treatment. BJU Int 108:248–251
Nickel JC, Atkinson G, Krieger JN, Mills IW, Pontari M, Shoskes DA, Crook TJ (2012) Preliminary assessment of safety and efficacy in proof-of-concept, randomized Clinical trial of tanezumab for chronic prostatitis/chronic pelvic pain syndrome. Urology 80:1105–1110
Lee JC, Yang CC, Kromm BG, Berger RE (2001) Neurophysiologic testing in chronic pelvic pain syndrome: a pilot study. Urology 58:246–250
Yang CC, Lee JC, Kromm BG, Ciol MA, Berger RE (2003) Pain sensitization in male chronic pelvic pain syndrome: why are symptoms so difficult to treat? J Urol 170:823–826
Yilmaz U, Liu YW, Berger RE, Yang CC (2007) Autonomic nervous system changes in men with chronic pelvic pain syndrome. J Urol 177:2170–2174
Cho DS, Choi JB, Kim YS, Joo KJ, Kim SH, Kim JC, Kim HW (2011) Heart rate variability in assessment of autonomic dysfunction in patients with chronic prostatitis/chronic pelvic pain syndrome. Urology 78:1369–1372
Yilmaz U, Ciol MA, Berger RE, Yang CC (2010) Sensory perception thresholds in men with chronic pelvic pain syndrome. Urology 75:34–37
Yilmaz U, Rothman I, Ciol MA, Yang CC, Berger RE (2005) Toe spreading ability in men with chronic pelvic pain syndrome. BMC Urol 5:11
Shoskes DA, Prots D, Karns J, Horhn J, Shoskes AC (2011) Greater endothelial dysfunction and arterial stiffness in men with chronic prostatitis/chronic pelvic pain syndrome—a possible link to cardiovascular disease. J Urol 186:907–910
Zermann DH, Ishigooka M, Doggweiler R, Schmidt RA (1999) Neurourological insights into the etiology of genitourinary pain in men. J Urol 161:903–908
Blaivas JG, Sinha HP, Zayed AA, Labib KB (1981) Detrusor-external sphincter dyssynergia. J Urol 125:542–544
Hetrick DC, Glazer H, Liu YW, Turner JA, Frest M, Berger RE (2006) Pelvic floor electromyography in men with chronic pelvic pain syndrome: a case-control study. Neurourol Urodyn 25:46–49
Berger RE, Ciol MA, Rothman I, Turner JA (2007) Pelvic tenderness is not limited to the prostate in chronic prostatitis/chronic pelvic pain syndrome (CPPS) type IIIA and IIIB: comparison of men with and without CP/CPPS. BMC Urol 7:17
Farmer MA, Chanda ML, Parks EL, Baliki MN, Apkarian AV, Schaeffer AJ (2011) Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol 186:117–124
Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV (2011) Brain morphological signatures for chronic pain. PLoS ONE [Electronic Resource] 6:e26010
Baliki MN, Geha PY, Apkarian AV (2009) Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol 101:875–887
Mordasini L, Weisstanner C, Rummel C, Thalmann GN, Verma RK, Wiest R, Kessler TM (2012) Chronic pelvic pain syndrome is associated with reduction of relative gray matter volume in the anterior cingulate cortex compared to healthy controls. J Urol 188:2233–2237
Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA (2010) Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology 139(48–57):e2
Luu P, Posner MI (2003) Anterior cingulate cortex regulation of sympathetic activity. Brain 126:2119–2120
Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS (2011) Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci 31:7540–7550
Woolf CJ (1983) Evidence for a central component of post-injury pain hypersensitivity. Nature 306:686–688
Woolf CJ (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152:S2–S15
Ishigooka M, Zermann DH, Doggweiler R, Schmidt RA (2000) Similarity of distributions of spinal c-Fos and plasma extravasation after acute chemical irritation of the bladder and the prostate. J Urol 164:1751–1756
Ware J Jr, Kosinski M, Keller SD (1996) A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233
McNaughton Collins M, Pontari MA, O’Leary MP, Calhoun EA, Santanna J, Landis JR, Kusek JW, Litwin MS, Chronic Prostatitis Collaborative Research Network (2001) Quality of life is impaired in men with chronic prostatitis: the chronic prostatitis collaborative research network. J Gen Intern Med 16:656–662
Tripp DA, Curtis Nickel J, Landis JR, Wang YL, Knauss JS, CPCRN Study G (2004) Predictors of quality of life and pain in chronic prostatitis/chronic pelvic pain syndrome: findings from the national institutes of health chronic prostatitis cohort study. BJU Int 94:1279–1282
Tripp DA, Nickel JC, Wang Y, Litwin MS, McNaughton-Collins M, Landis JR, Alexander RB, Schaeffer AJ, O’Leary MP, Pontari MA, Fowler JE Jr, Nyberg LM, Kusek JW, National Institutes of Health-Chronic Prostatitis Collaborative Research Network (NIH-CPCRN) Study Group (2006) Catastrophizing and pain-contingent rest predict patient adjustment in men with chronic prostatitis/chronic pelvic pain syndrome. J Pain 7:697–708
Nickel JC, Tripp DA, Chuai S, Litwin MS, McNaughton-Collins M, Landis JR, Alexander RB, Schaeffer AJ, O’Leary MP, Pontari MA, White P, Mullins C, Nyberg L, Kusek J, NIH-CPCRN Study G (2008) Psychosocial variables affect the quality of life of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome. BJU Int 101:59–64
Hsieh AY, Tripp DA, Ji LJ, Sullivan MJ (2010) Comparisons of catastrophizing, pain attitudes, and cold-pressor pain experience between Chinese and European Canadian young adults. J Pain 11:1187–1194
Seminowicz DA, Davis KD (2006) Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain 120:297–306
Ullrich PM, Turner JA, Ciol M, Berger R (2005) Stress is associated with subsequent pain and disability among men with nonbacterial prostatitis/pelvic pain. Ann Behav Med 30:112–118
Hu JC, Link CL, McNaughton-Collins M, Barry MJ, McKinlay JB (2007) The association of abuse and symptoms suggestive of chronic prostatitis/chronic pelvic pain syndrome: results from the boston area community health survey. J Gen Intern Med 22:1532–1537
Spanos C, Pang X, Ligris K, Letourneau R, Alferes L, Alexacos N, Sant GR, Theoharides TC (1997) Stress-induced bladder mast cell activation: implications for interstitial cystitis. J Urol 157:669–672
Babenko O, Golubov A, Ilnytskyy Y, Kovalchuk I, Metz GA (2012) Genomic and epigenomic responses to chronic stress involve miRNA-mediated programming. PLoS ONE [Electronic Resource] 7:e29441
Rodriguez MA, Afari N, Buchwald DS (2009) National Institute of diabetes and digestive and kidney diseases working Group on urological chronic pelvic, pain. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol 182:2123–2131
Di Franco M, Iannuccelli C, Valesini G (2010) Neuroendocrine immunology of fibromyalgia. Ann N Y Acad Sci 1193:84–90
McEwen BS, Kalia M (2010) The role of corticosteroids and stress in chronic pain conditions. Metab, Clin Exp 59:S9–S15
Anderson RU, Orenberg EK, Chan CA, Morey A, Flores V (2008) Psychometric profiles and hypothalamic-pituitary-adrenal axis function in men with chronic prostatitis/chronic pelvic pain syndrome. J Urol 179:956–960
Dimitrakov J, Joffe HV, Soldin SJ, Bolus R, Buffington CA, Nickel JC (2008) Adrenocortical hormone abnormalities in men with chronic prostatitis/chronic pelvic pain syndrome. Urology 71:261–266
Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV (1997) An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet 24:1–8
Kroeger KM, Carville KS, Abraham LJ (1997) The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol 34:391–399
Shoskes DA, Albakri Q, Thomas K, Cook D (2002) Cytokine polymorphisms in men with chronic prostatitis/chronic pelvic pain syndrome: association with diagnosis and treatment response. J Urol 168:331–335
Riley DE, Krieger JN (2002) X Chromosomal short tandem repeat polymorphisms near the phosphoglycerate kinase gene in men with chronic prostatitis. Biochim Biophys Acta 1586:99–107
Riley DE, Krieger JN (2001) Short tandem repeat polymorphism linkage to the androgen receptor gene in prostate carcinoma. Cancer 92:2603–2608
Riley DE, Krieger JN (2009) Embryonic nervous system genes predominate in searches for dinucleotide simple sequence repeats flanked by conserved sequences. Gene 429:74–79
Arisan ED, Arisan S, Kiremit MC, Tigli H, Caskurlu T, Palavan-Unsal N, Ergenekon E (2006) Manganese superoxide dismutase polymorphism in chronic pelvic pain syndrome patients. Prostate Cancer Prostatic Dis 9:426–431
von Hehn CA, Baron R, Woolf CJ (2012) Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73:638–652
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pontari, M.A. Etiology of chronic prostatitis/chronic pelvic pain syndrome: psychoimmunoneurendocrine dysfunction (PINE syndrome) or just a really bad infection?. World J Urol 31, 725–732 (2013). https://doi.org/10.1007/s00345-013-1061-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-013-1061-z