Abstract
Background
Robotic technology disseminated into urological practice without robust comparative effectiveness data.
Objective
To review the diffusion of robotic surgery into urological practice
Methods
We performed a comprehensive literature review focusing on diffusion patterns, patient safety, learning curves, and comparative costs for robotic radical prostatectomy, partial nephrectomy, and radical cystectomy
Results
Robotic urologic surgery diffused in patterns typical of novel technology spreading among practicing surgeons. Robust evidence-based data comparing outcomes of robotic to open surgery were sparse. Although initial Level 3 evidence for robotic prostatectomy observed complication outcomes similar to open prostatectomy, subsequent population-based Level 2 evidence noted an increased prevalence of adverse patient safety events and genitourinary complications among robotic patients during the early years of diffusion. Level 2 evidence indicated comparable to improved patient safety outcomes for robotic compared to open partial nephrectomy and cystectomy. Learning curve recommendations for robotic urologic surgery have drawn exclusively on Level 4 evidence and subjective, non-validated metrics. The minimum number of cases required to achieve competency for robotic prostatectomy has increased to unrealistically high levels. Most comparative cost-analyses have demonstrated that robotic surgery is significantly more expensive than open or laparoscopic surgery.
Conclusions
Evidence-based data are limited but suggest an increased prevalence of adverse patient safety events for robotic prostatectomy early in the national diffusion period. Learning curves for robotic urologic surgery are subjective and based on non-validated metrics. The urological community should develop rigorous, evidence-based processes by which future technological innovations may diffuse in an organized and safe manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An innovation is an idea, practice, or object that is perceived as new. Diffusion is the process by which innovations are communicated over time among the members of a social system [1]. Innovations diffuse in predictable and reproducible patterns through social constructs as diverse as Iowa farmers planting novel hybrid corn, consumers purchasing the latest electronic devices [1], and surgeons adopting new operative technology [2].
The diffusion of robotics into urological practice began in 2001 with the first published reports of robotic-assisted laparoscopic prostatectomy [3–5]. Thereafter, robotics disseminated relatively rapidly among urologists [6], driven by heavily marketed—yet unproven—benefits related to three-dimensional magnified vision, enhanced ergonomics, tremor filtration, motion scaling, and improved manual dexterity relative to open surgery (http://www.intuitivesurgical.com). Published studies during robot diffusion focused primarily on proof-of-principle concepts, surgical technique, and non-comparative outcomes rather than evidence-based population analyses of safety and efficacy [7–9].
There are, however, two important and under recognized characteristics of the robot diffusion process that bear directly on the public health. First, early claims of reduced length of stay, decreased operative blood loss, and improved outcomes—based on small, single-center surgical case series—outpaced the publication of robust, comparative effectiveness data on oncological and functional efficacy [10–12]. Second, the adoption of robotics occurred without systematic processes to optimize patient safety, define technical competency, establish uniform learning curves, and analyze costs and benefits relative to open surgery [6, 13–15]. Further reflection upon and understanding of these diffusion issues would potentially inform the processes by which urologists assimilate future surgical innovations and thereby improve the public health.
This review synthesizes published data on the dissemination of robotic technology into urological practice, focusing on population and outcomes studies of the three most commonly described robotic-assisted operations: radical prostatectomy, partial nephrectomy, and radical cystectomy. Within this context, we highlight four topics: the process of diffusion, patient safety, the learning curve, and costs.
Diffusion of surgical innovations and comparative outcomes
Surgical innovations, as with any type of innovation, diffuse in predictable patterns, progressing through well-defined stages as they spread through the surgical community. Not all innovations diffuse successfully: variables such as persuasion of practicing surgeons (i.e. marketing), perceived advantage, patient demand, and practicality determine whether any given innovation transitions effectively into broader practice [1, 16]. The “tipping point” or “take-off” is the time point following introduction of an innovation at which diffusion reaches a maximum velocity, beyond which assimilation of the innovation into a community is, to some extent, inevitable. The tipping point occurs when adoption of the innovation reaches a prevalence of 10 to 20 % [1, 2, 16, 17].
Diffusion graphs, with the prevalence of adoption plotted on the y-axis and time on the x-axis, display S-shaped curves. The steepness of the curve denotes the rapidity with which the innovation is adopted, with swiftly spreading innovations displaying steep positive slopes. Diffusion occurs in five stages, divided by category of adopter: innovators (2.5 % of adopters), early adopters (13.5 %), early majority (34 %), late majority (34 %), and laggards (16 %). The tipping point occurs during the transition from innovators to early adopters [16]. Analyses of the Nationwide Inpatient Sample in the United States have observed that the national tipping points for robotic-assisted radical prostatectomy, partial nephrectomy, and cystectomy occurred in 2005 [10], 2007 [18], and 2010 [19], respectively.
Importantly, diffusion of a surgical innovation usually outpaces the availability of robust clinical evidence supporting its safety and efficacy [12]. This pattern indeed occurred with the robot: the DaVinci robotic platform received clearance as part of the FDA 510(k) process in 2000 (http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/510kClearances/ucm093464.htm); subsequently, robotic surgery diffused widely prior to the publication of well-designed, comparative effectiveness analyses. In fact, 9 years elapsed between FDA approval and initial publication of population-based outcomes of robotic prostatectomy culled from a national analytic cohort—a troubling observation given that, contrary to prior single-institution case series, this study noted increased diagnoses of genitourinary complications, incontinence, and erectile dysfunction [20].
Diffusion and patient safety
Robotic-assisted radical prostatectomy
Initial analyses of patient safety and robotic prostatectomy examined specific complications related to prostatectomy during diffusion of this procedure. The majority of these studies were single-institution case series (Level 3 evidence, www.cebm.net) that observed complication prevalences similar to open prostatectomy series reported in the literature [21–23]. Subsequent meta-analyses (Level 2 evidence), the first of which was performed by Parsons and colleagues in 2008, synthesized safety data from prior institutional series and reported on perioperative transfusion risk and complications. These demonstrated that perioperative blood loss and the prevalence of blood transfusion were significantly less with robotic radical prostatectomy compared to open radical retropubic prostatectomy [24–26].
More robust population-based data, however, later observed that robotic prostatectomy diffusion was in fact associated with diminished patient safety. In a large US cohort, Parsons and colleagues investigated the association of patient safety with the national diffusion of minimally invasive radical prostatectomy, a surrogate measure of robotic prostatectomy [10]. These investigators used patient safety indicators (PSIs)—validated, process-focused outcomes metrics developed by the US Agency for Healthcare Research and Quality—to analyze safety outcomes comparing MIRP to open radical retropubic prostatectomy between 2003 and 2009. They observed a more than twofold increase in the risk of any PSI among patients undergoing minimally invasive radical prostatectomy in the years immediately preceding the tipping point: a pattern that occurred in both teaching and non-teaching hospitals.
Similarly, Hu and colleagues compared effectiveness of minimally invasive to open radical retropubic prostatectomy in the US Medicare cohort for the period 2003 to 2007 and demonstrated that patients undergoing minimally invasive prostatectomy compared to open techniques had increased risk of genitourinary complications [20].
In another study, Hu and colleagues reported a decrease in perioperative complications and shorter lengths of stay, but also an increase in anastomotic strictures among a Medicare cohort of men undergoing minimally invasive versus open radical prostatectomy between 2003 and 2005 [27].
Robotic-assisted partial nephrectomy
Robotic partial nephrectomy accounted for 3.38 % of all robotic procedures performed between 2008 and 2009 and was the fourth most common surgery performed robotically in the United States [28]. Unlike robotic prostatectomy, the diffusion of robotic partial nephrectomy has not been associated with diminished patient safety. In a population-based analysis, Parsons and colleagues reviewed the safety of the diffusion of minimally invasive partial nephrectomy between 1998 and 2009. The “tipping point” occurred in 2007, but patients undergoing minimally invasive partial nephrectomy were 28 % less likely to sustain an adverse safety event as measured by PSIs compared to open technique during this period of rapid diffusion; however, specific comparisons between robotic-assisted and open partial nephrectomy demonstrated comparable likelihood of a PSI occurring [18].
In a comprehensive meta-analysis of published data comparing robotic to laparoscopic partial nephrectomy, Aboumarzouk and colleagues reported that there were no differences in estimated perioperative blood loss or complications and concluded that robotic partial nephrectomy is a safe and feasible alternative to laparoscopic partial nephrectomy [29]. Similarly, Long and colleagues demonstrated that robotic partial nephrectomy was associated with a lower rate of conversion to radical nephrectomy compared to laparoscopic partial nephrectomy [30].
Robotic radical cystectomy
Robotic radical cystectomy was first reported in 2003 [31] and in 2009 was the 12th most common procedure performed robotically in the United States [28]. As with partial nephrectomy, Level 3 and Level 2 evidences suggest that the diffusion of robotic cystectomy has been associated with comparable, if not superior, safety outcomes to open cystectomy [32–35]. A national cohort analysis reported fewer inpatient complications, deaths, and lower parenteral nutrition use among patients undergoing robotic radical cystectomy compared to open radical cystectomy [36], while another analysis by Cohen and colleagues demonstrated that minimally invasive radical cystectomy was associated with decreased odds of any PSI compared to open radical cystectomy [19].
Diffusion and the learning curve
In surgery, the learning curve is the minimum number of cases required to achieve competency for a given procedure [37]. Three general observations summarize the existing literature regarding learning curves and robotic urologic surgery. First, definitions of learning curves have drawn exclusively on expert opinion (Level 4 evidence). Second, surgical competency metrics have varied widely and included operative time, vaguely defined “outcomes similar to open,” and surgical margin status. Finally, the published minimum number of cases for robotic prostatectomy has increased steadily and substantially (Table 1) [9, 37–44]. Indeed, some investigators have published more than once on this topic, upwardly modifying their initial estimates of minimum competency after achieving higher volumes within their own case series.
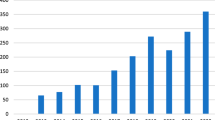
Currently, the most prevalent definition of competency for robotic prostatectomy mirrors that of open prostatectomy: oncological efficacy as defined by biochemical recurrence-free survival [44, 45]. Advocates of this metric cite volume-outcomes data demonstrating statistically significant decreases in rates of positive surgical margins and biochemical recurrence with continuous increases in surgical volume [46]. Concomitantly, published estimates for the minimum number of cases required to achieve competency for robotic prostatectomy have steadily and substantially increased (Fig. 1), with numerous investigators citing these data as evidence that radical prostatectomy patients should be referred to “high-volume” centers [47, 48] (Fig. 2).
Calls for patients to undergo prostate surgery exclusively in regionalized, “high-volume” centers raise several important issues. First, and perhaps foremost, advocates for regionalization have yet to propose practical methods by which regionalization would be structured and funded. Without a publicly subsidized delivery system to insure equitable access for patients in lower socioeconomic brackets, regionalization could severely exacerbate existing health care disparities for those without the resources to travel to metropolitan areas in which “high-volume” centers are located [48–51].
Second, only an extremely small percentage of practicing urologists would, by current metrics, qualify as “high volume.”[47] With over 60,000 prostatectomies performed each year in the United States [10], it is unclear how such a large number of procedures would be limited to such a small number of urologists clustered in geographically narrow regions. Third, every “high-volume” surgeon, for any surgical procedure, enters practice as a lower volume surgeon—and yet “high-volume” surgeons have not outlined specific methods for training lower volume surgeons to safely achieve minimum competency metrics. Fourth, robust definitions of “high volume” remain elusive, as do recommendations for which professional organizations would be charged with defining “high volume” and credentialing qualified surgeons. Finally, the clinical significance and benefits of incremental, statistically significant improvements in biochemical-free survival with increasing case volume remain unproven: from a clinical standpoint, there may very well be a cut-point of diminishing returns beyond which additional numbers of cases lack real clinical benefit.
It is worth noting that unrealistically high learning curves for surgical procedures potentially expose the urological community to risk. These are data that exist within the public domain and to which key stakeholders in these processes—including but not limited to the federal government, insurers, hospital administrators, representatives of other medical specialties, attorneys, and patients—have access. Future innovations in urologic surgery will demand uniform and transparent processes for assessing surgical competency. Without consensus building and the development of reproducible, robust metrics for surgeon credentialing, urologic surgeons will potentially cede credibility and authority to other key stakeholders.
Learning curve estimates for robotic partial nephrectomy and cystectomy also draw on Level 4 evidence and variable definitions of competence. Using metrics such as operative time, perioperative complication prevalence, and perioperative blood loss, investigators have reported learning curves of 15 to 30 cases to achieve minimum competency for robotic partial nephrectomy [52, 53]. Based on the International Robotic Cystectomy Consortium database, Hayn and colleagues defined the learning curve for robotic cystectomy to be 30 cases based on the number of cases required for a surgeon to achieve a lymph node yield of ≥20, a positive surgical margin prevalence <5 %, and an operative time <6.5 h [54].
Cost
Most of the comparative cost analysis literature is consistent and unequivocal: robotic surgery is, on average, more expensive than open or laparoscopic surgery for all procedures [13, 55–58]. In a systematic cost comparison, Bolenz and colleagues demonstrated that robotic prostatectomy increased cost of care by $2,698 per patient in a high-volume hospital and identified increased surgical supply and operating room costs as the underlying causes. Disposable robotic equipment, increased operative time, and large outlays in capital investment and maintenance costs increase the cost of robotic prostatectomy compared to open or laparoscopic radical prostatectomy [13]. Current costs for the robot, which include a $1.5 million capital cost and a $150,000 annual maintenance contract, add $1,000 per case relative to open prostatectomy, even if the robot is used for 300 prostatectomies per year [58].
Still, Yu and colleagues reported that higher robotic prostatectomy volume hospitals were associated with lower costs compared to low-volume hospitals [57], while Scales and colleagues have suggested that the cost of robotic prostatectomy may approach those of open radical prostatectomy in hospitals performing 10 to 14 robotic prostatectomies per week or more than 500 cases annually [59]. It is worth noting, though, that the cost of the learning curve has been estimated to be $217,034 per surgeon due to increased operative times [60].
Similar to the comparative cost-analyses of robotic prostatectomy, several analyses have observed that robotic partial nephrectomy and cystectomy are more expensive than their open counterparts. Robotic partial nephrectomy costs as much as $1,600 more per patient than open [28, 55], although a more recent analysis suggested that costs of robotic partial nephrectomy might be approaching those of open partial nephrectomy [61]. Robotic cystectomy costs as much as $3,797 more per patient [55, 56, 62].
While direct in-hospital costs are consistently higher for robotic prostatectomy, such analyses do not quantify the costs associated with readmissions or complications following surgery. Chung et al. demonstrated that patients undergoing robotic radical prostatectomy had a significantly lower risk of 90-day readmission than patients undergoing either open radical retropubic prostatectomy [61], and thus, 90-day costs associated with robotic prostatectomy would be less than that of open radical retropubic prostatectomy. A cost-utility analysis performed by O’Malley et al. [62] also demonstrated that while robotic prostatectomy was incrementally $2264.35 more expensive than each open radical prostatectomy performed, robotic prostatectomy was associated with an incremental gain of 0.093 quality-adjusted life years (QALYs) over 1 year with shorter median durations of incontinence (1.47 vs. 5.26 months) and erectile dysfunction (5.79 vs. 14.46 months). Longer-term cost-analyses quantifying the value of such QALYs are necessary to determine whether robotic prostatectomy is cost effective.
Conclusions
Evidence-based data comparing robotic to open surgery are limited but suggest an increased prevalence of adverse patient safety events occurring for robotic prostatectomy early in the national diffusion period. Safety outcomes for partial nephrectomy and cystectomy are comparable or superior to open surgery. Learning curves for robotic urologic surgery are subjective and based on non-validated metrics. Robotic surgery is significantly more expensive than open or laparoscopic surgery. The urological community should develop rigorous, evidence-based processes by which future technological innovations may diffuse in an organized and safe manner.
References
Rogers EM (2003) Diffusion of innovations, 5th edn. Free Press, New York
Barkun JS, Aronson JK, Feldman LS, Maddern GJ, Strasberg SM, Balliol Collaboration, Altman DG, Barkun JS, Blazeby JM, Boutron IC, Campbell WB, Clavien PA, Cook JA, Ergina PL, Flum DR, Glasziou P, Marshall JC, McCulloch P, Nicholl J, Reeves BC, Seiler CM, Meakins JL, Ashby D, Black N, Bunker J, Burton M, Campbell M, Chalkidou K, Chalmers I, de Leval M, Deeks J, Grant A, Gray M, Greenhalgh R, Jenicek M, Kehoe S, Lilford R, Littlejohns P, Loke Y, Madhock R, McPherson K, Rothwell P, Summerskill B, Taggart D, Tekkis P, Thompson M, Treasure T, Trohler U, Vandenbroucke J (2009) Evaluation and stages of surgical innovations. Lancet 374(9695):1089–1096
Binder J, Kramer W (2001) Robotically-assisted laparoscopic radical prostatectomy. BJU Int 87(4):408–410
Abbou CC, Hoznek A, Salomon L, Lobontiu A, Saint F, Cicco A, Antiphon P, Chopin D (2000) Remote laparoscopic radical prostatectomy carried out with a robot. Report of a case. Prog Urol 10(4):520–523
Pasticier G, Rietbergen JB, Guillonneau B, Fromont G, Menon M, Vallancien G (2001) Robotically assisted laparoscopic radical prostatectomy: feasibility study in men. Eur Urol 40(1):70–74
Zorn KC, Gautam G, Shalhav AL, Clayman RV, Ahlering TE, Albala DM, Lee DI, Sundaram CP, Matin SF, Castle EP, Winfield HN, Gettman MT, Lee BR, Thomas R, Patel VR, Leveillee RJ, Wong C, Badlani GH, Rha KH, Eggener SE, Wiklund P, Mottrie A, Atug F, Kural AR, Joseph JV (2009) Members of the Society of Urologic Robotic Surgeons. Training, credentialing, proctoring and medicolegal risks of robotic urological surgery: recommendations of the society of urologic robotic surgeons. J Urol 182(3):1126–1132
Wolfram M, Bräutigam R, Engl T, Bentas W, Heitkamp S, Ostwald M, Kramer W, Binder J, Blaheta R, Jonas D, Beecken WD (2003) Robotic-assisted laparoscopic radical prostatectomy: the Frankfurt technique. World J Urol 21(3):128–132
Menon M, Shrivastava A, Tewari A, Sarle R, Hemal A, Peabody JO, Vallancien G (2002) Laparoscopic and robot assisted radical prostatectomy: establishment of a structured program and preliminary analysis of outcomes. J Urol 168(3):945–949
Parsons JK, Palazzi-Churas K, Stroup S et al (2012) Patient safety and the diffusion of minimally invasive radical prostatectomy. J Urol 187:S E24–S E25
Cadeddu JA, Gautam G, Shalhav AL (2010) Robotic prostatectomy. J Urol 183(3):858–861
Barry MJ, Gallagher PM, Skinner JS, Fowler FJ Jr (2012) Adverse effects of robotic-assisted laparoscopic versus open retropubic radical prostatectomy among a nationwide random sample of medicare-age men. J Clin Oncol 30(5):513–518
Bolenz C, Gupta A, Hotze T, Ho R, Cadeddu JA, Roehrborn CG, Lotan Y (2010) Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur Urol 57(3):453–458
Secin FP (2011) The learning curve of robotic assisted laparoscopic radical prostatectomy: what is the evidence? Arch Esp Urol 64(8):830–838
Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, Guazzoni G, Guillonneau B, Menon M, Montorsi F, Patel V, Rassweiler J, Van Poppel H (2009) Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol 55(5):1037–1063
Wilson CB (2006) Adoption of new surgical technology. BMJ 332(7533):112–114
Gladwell M (2000) The tipping point: how little things can make a big difference. Little, Brown, New York
Parsons JK, Palazzi K, Chang DC, and Stroup S (2012) Patient safety and the diffusion of surgical innovations: a national analysis of laparoscopic partial nephrectomy 2012. Surgical Endoscopy 2012
Cohen SA, Parsons JK, Palazzi KL, Jaehoon Cho, Kader AK (2012) Minimally invasive cystectomy is associated with improved peri-operative patient safety and decreased length of stay compared to open cystectomy. J Urol 187:710 Abstract 1759
Hu JC, Gu X, Lipsitz SR, Barry MJ, D’Amico AV, Weinberg AC, Keating NL (2009) Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA 302(14):1557–1564
Agarwal PK, Sammon J, Bhandari A, Dabaja A, Diaz M, Dusik-Fenton S, Satyanarayana R, Simone A, Trinh QD, Baize B, Menon M (2011) Safety profile of robot-assisted radical prostatectomy: a standardized report of complications in 3317 patients. Eur Urol 59(5):684–698
Rabbani F, Yunis LH, Pinochet R, Nogueira L, Vora KC, Eastham JA, Guillonneau B, Laudone V, Scardino PT, Touijer K (2010) Comprehensive standardized report of complications of retropubic and laparoscopic radical prostatectomy. Eur Urol 57(3):371–386
Parsons JK, Bennett JL (2008) Outcomes of retropubic, laparoscopic, and robotic-assisted prostatectomy. Urology 72(2):412
Kang DC, Hardee MJ, Fesperman SF, Stoffs TL, Dahm P (2010) Low quality of evidence for robot-assisted laparoscopic prostatectomy: results of a systematic review of the published literature. Eur Urol 57(6):930–937
Novara G, Ficarra V, Rosen RC, Artibani W, Costello A, Eastham JA, Graefen M, Guazzoni G, Shariat SF, Stolzenburg JU, Van Poppel H, Zattoni F, Montorsi F, Mottrie A, Wilson TG (2012) Systematic review and meta-analysis of perioperative outcomes and complications after robot-assisted radical prostatectomy. Eur Urol 62(3):431–452
Hu JC, Wang Q, Pashos CL, Lipsitz SR, Keating NL (2008) Utilization and outcomes of minimally invasive radical prostatectomy. J Clin Oncol 26(14):2278–2284
Anderson JE, Chang DC, Parsons JK, Talamini MA (2012) The first national examination of outcomes and trends in robotic surgery in the United States. J Am Coll Surg 215(1):107–114 discussion 114–6
Aboumarzouk OM, Stein RJ, Eyraud R, Haber GP, Chlosta PL, Somani BK, Kaouk JH (2012) Robotic Versus Laparoscopic Partial Nephrectomy: A Systematic Review and Meta-Analysis. Eur Urol (Epub ahead of print)
Long JA, Yakoubi R, Lee B, Guillotreau J, Autorino R, Laydner H, Eyraud R, Stein RJ, Kaouk JH, Haber GP (2012) Robotic versus laparoscopic partial nephrectomy for complex tumors: comparison of perioperative outcomes. Eur Urol 61(6):1257–1262
Menon M, Hemal AK, Tewari A, Shrivastava A, Shoma AM, El-Tabey NA, Shaaban A, Abol-Enein H, Ghoneim MA (2003) Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int 92(3):232–236
Nix J, Smith A, Kurpad R, Nielsen ME, Wallen EM, Pruthi RS (2010) Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol 57(2):196–201
Styn NR, Montgomery JS, Wood DP, Hafez KS, Lee CT, Tallman C, He C, Crossley H, Hollenbeck BK, Weizer AZ (2012) Matched comparison of robotic-assisted and open radical cystectomy. Urology 79(6):1303–1308
Gondo T, Yoshioka K, Nakagami Y, Okubo H, Hashimoto T, Satake N, Ozu C, Horiguchi Y, Namiki K, Tachibana M (2012) Robotic versus open radical cystectomy: prospective comparison of perioperative and pathologic outcomes in Japan. Jpn J Clin Oncol 42(7):625–631
Ng CK, Kauffman EC, Lee MM, Otto BJ, Portnoff A, Ehrlich JR, Schwartz MJ, Wang GJ, Scherr DS (2010) A comparison of postoperative complications in open versus robotic cystectomy. Eur Urol 57(2):274–281
Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Nguyen PL, Choueiri TK, Kibel AS, Hu JC (2012) Comparative analysis of outcomes and costs following open radical cystectomy versus robot-assisted laparoscopic radical cystectomy: results from the US Nationwide Inpatient Sample. Eur Urol 61(6):1239–1244
Herrell SD, Smith JA Jr (2005) Robotic-assisted laparoscopic prostatectomy: what is the learning curve? Urology 66(5):105–107
Ahlering TE, Skarecky D, Lee D, Clayman RV (2003) Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: initial experience with laparoscopic radical prostatectomy. J Urol 170(5):1738–1741
Atug F, Castle EP, Srivastav SK, Burgess SV, Thomas R, Davis R (2006) Positive surgical margins in robotic-assisted radical prostatectomy: impact of learning curve on oncologic outcomes. Eur Urol 49(5):866–871 discussion 871–2
Zorn KC, Orvieto MA, Gong EM, Mikhail AA, Gofrit ON, Zagaja GP, Shalhav AL (2007) Robotic radical prostatectomy learning curve of a fellowship-trained laparoscopic surgeon. J Endourol 21(4):441–447
Shah A, Okotie OT, Zhao L, Pins MR, Bhalani V, Dalton DP (2008) Pathologic outcomes during the learning curve for robotic-assisted laparoscopic radical prostatectomy. Int Braz J Urol 34(2):159–162 discussion 163
Jaffe J, Castellucci S, Cathelineau X, Harmon J, Rozet F, Barret E, Vallancien G (2009) Robot-assisted laparoscopic prostatectomy: a single-institutions learning curve. Urology 73(1):127–133
Freire MP, Choi WW, Lei Y, Carvas F, Hu JC (2010) Overcoming the learning curve for robotic-assisted laparoscopic radical prostatectomy. Urol Clin North Am 37(1):37–47 Table of Contents
Sooriakumaran P, John M, Wiklund P, Lee D, Nilsson A, Tewari AK (2011) Learning curve for robotic assisted laparoscopic prostatectomy: a multi-institutional study of 3,794 patients. Minerva Urol Nefrol 63(3):191–198
Nelson JB (2012) Robotic-assisted radical prostatectomy: who is benefiting? Oncology (Williston Park) 26(7):622, 624, 626
Badani KK, Kaul S, Menon M (2007) Evolution of robotic radical prostatectomy: assessment after 2,766 procedures. Cancer 110(9):1951–1958
Savage CJ, Vickers AJ (2009) Low annual caseloads of United States surgeons conducting radical prostatectomy. J Urol 182(6):2677–2679
Vickers AJ, Bianco FJ, Serio AM, Eastham JA, Schrag D, Klein EA, Reuther AM, Kattan MW, Pontes JE, Scardino PT (2007) The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst 99(15):1171–1177
Trinh QD, Sun M, Sammon J, Bianchi M, Sukumar S, Ghani KR, Jeong W, Dabaja A, Shariat SF, Perrotte P, Agarwal PK, Rogers CG, Peabody JO, Menon M, Karakiewicz PI (2012) Disparities in access to care at high-volume institutions for uro-oncologic procedures. Cancer 118(18):4421–4426. doi:10.1002/cncr.27440
Liu JH, Zingmond DS, McGory ML, SooHoo NF, Ettner SL, Brook RH, Ko CY (2006) Disparities in the utilization of high-volume hospitals for complex surgery. JAMA 296(16):1973–1980
Ward MM, Jaana M, Wakefield DS, Ohsfeldt RL, Schneider JE, Miller T, Lei Y (2004) What would be the effect of referral to high-volume hospitals in a largely rural state? J Rural Health 20(4):344–354
Mottrie A, De Naeyer G, Schatteman P, Carpentier P, Sangalli M, Ficarra V (2010) Impact of the learning curve on perioperative outcomes in patients who underwent robotic partial nephrectomy for parenchymal renal tumours. Eur Urol 58(1):127–132
Haseebuddin M, Benway BM, Cabello JM, Bhayani SB (2010) Robot-assisted partial nephrectomy: evaluation of learning curve for an experienced renal surgeon. J Endourol 24(1):57–61
Hayn MH, Hussain A, Mansour AM, Andrews PE, Carpentier P, Castle E, Dasgupta P, Rimington P, Thomas R, Khan S, Kibel A, Kim H, Manoharan M, Menon M, Mottrie A, Ornstein D, Peabody J, Pruthi R, Palou Redorta J, Richstone L, Schanne F, Stricker H, Wiklund P, Chandrasekhar R, Wilding GE, Guru KA (2010) The learning curve of robot-assisted radical cystectomy: results from the International robotic cystectomy consortium. Eur Urol 58(2):197–202
Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Hu JC (2010) Use, costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J Urol 187(4):1392–1398
Barbash GI, Glied SA (2010) New technology and health care costs–the case of robot-assisted surgery. N Engl J Med 363(8):701–704
Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Nguyen PL, Hu JC (2012) Hospital volume, utilization, costs and outcomes of robot-assisted laparoscopic radical prostatectomy. J Urol 187(5):1632–1637
Lotan Y (2012) Is robotic surgery cost effective no. Curr Opin Urol 22(1):66–69
Scales CD Jr, Jones PJ, Eisenstein EL, Preminger GM, Albala DM (2005) Local cost structures and the economics of robot assisted radical prostatectomy. J Urol 174(6):2323–2329
Steinberg PL, Merguerian PA, Bihrle W 3rd, Seigne JD (2008) The cost of learning robotic-assisted prostatectomy. Urology 72(5):1068–1072
Anderson JE, Parsons JK, Chang DC, Talamini MA (2012) Hospital costs and lengths of stay related to robot-assisted versus open radical and partial nephrectomy for kidney cancer in the United States. J Robotic Surg 6:19–22
Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Nguyen PL, Choueiri TK, Kibel AS, Hu JC (2012) Comparative analysis of outcomes and costs following open radical cystectomy versus robot-assisted laparoscopic radical cystectomy: results from the US Nationwide Inpatient Sample. Eur Urol 61(6):1239–1244
Chung SD, Kelle JJ, Huang CY, Chen YH, and Lin HC (2012) Comparison of 90-day re-admission rates between open retropubic radical prostatectomy(RRP), laparoscopic RP(LRP), and robot-assisted laparoscopic prostatectomy (RALP). BJU Int (Epub 2012 Apr 30)
O’Malley SP, Jordan E (2007) Review of a decision by the Medical Services Advisory Committee based on health technology assessment of an emerging technology: the case for remotely assisted radical prostatectomy. Int J Technol Assess Health Care. 2007 Spring; 23(2):286-91
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mirheydar, H.S., Parsons, J.K. Diffusion of robotics into clinical practice in the United States: process, patient safety, learning curves, and the public health. World J Urol 31, 455–461 (2013). https://doi.org/10.1007/s00345-012-1015-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-012-1015-x