Abstract
Objectives
We evaluated whether the surgical approach during the implementation of a robotic kidney surgery program influenced perioperative and oncologic outcomes.
Methods
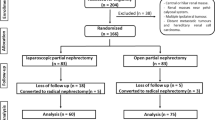
We prospectively evaluated a single institution experience with minimally invasive partial nephrectomy between 2006 and 2010. The study cohort comprised 86 consecutively treated patients who underwent laparoscopic partial nephrectomy (LPN, N = 59) or robotic-assisted (RPN, N = 27) partial nephrectomy by a single surgeon.
Results
There was no difference between the LPN and RPN cohort in terms of gender, age, operative side, American Society of Anesthesiology score, or preoperative estimated glomerular filtration rate (eGFR). An early unclamping technique was used for 22 (82%) patients in the RPN cohort and 6 (10%) patients in the LPN cohort. (P < 0.001). Warm ischemia time was lower in the RPN cohort (mean 18.5 vs. 28.0 min, P = <0.001) as result of majority undergoing early unclamping. There was no difference in operative time, estimated blood loss, length of stay, transfusion rate, positive surgical margin, or postoperative decrease in eGFR. There was no difference in mean eGFR decrease after early unclamping (16%) versus traditional clamping (22%); however, 11 (29%) patients had greater than 50% decrease in eGFR after traditional clamping versus 0 patients after early unclamping (P = 0.014).
Conclusion
Patients undergoing RPN during implementation of a robotic kidney surgery program when compared with LPN appear to have equivalent perioperative outcomes and oncologic efficacy. RPN patients had surgery later in our minimally invasive partial nephrectomy experience, and these results may not be generalizable to laparoscopic and/or robotic naïve surgeons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liberal utilization of cross-sectional imaging has led to an increased detection of incidental small renal masses [1]. Surgical removal remains the optimal treatment for patients who are able to undergo surgery. Recent retrospective studies have shown that partial nephrectomy reduces the risk of progressive renal failure compared with radical nephrectomy [2]. Moreover, partial nephrectomy may also reduce the risk of adverse cardiovascular events and overall mortality in these patients [3, 4]. Recently, the American Urologic Association (AUA) issued guidelines for stage 1 small renal masses recommending surgical excision by partial nephrectomy as a reference standard for the management of clinical T1 renal masses, thus highlighting the importance of preservation of renal function [5].
Laparoscopic partial nephrectomy (LPN) has the potential to offer improved convalescence over open partial nephrectomy; however, a recent multi-institutional study comparing the initial LPN experience with a seasoned open partial nephrectomy experience observed that LPN was associated with increased risk of urologic complications and longer warm ischemia times [6]. As robotics has become more commonplace in urologic surgery, several groups with prior extensive experience with LPN have demonstrated the feasibility of robotic partial nephrectomy (RPN) [7–9]. Our aim was to report our experience of LPN versus RPN during implementation of a robotic kidney surgery program, evaluate the safety of the early unclamping approach and compare perioperative and oncologic outcomes.
Materials and methods
Patient population
All patients were operated on by a single surgeon (AAW) at an academic center between 2006 and 2010. Prior to initiation of the robotic kidney surgery program, the surgeon had performed >100 LPN, >150 laparoscopic radical nephrectomies, >200 laparoscopic prostatectomies, and >75 robotic prostatectomy cases during endourology fellowship training and as an attending surgeon. Upon implementation of the robotic prostatectomy program and performing >75 robotic prostatectomy cases, the surgeon (AAW) discontinued LPN and initiated RPN. Data from all patients undergoing kidney surgery were recorded prospectively in an institutional review board (IRB) approved kidney surgery database.
Complications were recorded using the Clavien classification system [10]. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula was used to estimate glomerular filtration rate (eGFR) preoperatively as well as at least 6 months postoperatively [11]. We applied an objective renal mass scoring system (R.E.N.A.L. Nephrometry Score [12]) to characterize resected lesions. Complexity of the lesions was further evaluated based on total nephrometry score as low (4–6), moderate (7–9), and high (10–12).
Treatment
LPN was performed via a transperitoneal approach [8]. We used two laparoscopic bulldog clamps for renal artery occlusion only and laparoscopic ultrasound to visualize the lesion in all cases. After extirpation of the tumor, the collecting system and obvious open vessels were oversewn with either 3–0 polyglactin or 3–0 V-lock (Covidien, Mansfield, MA) sutures with preplaced absorbable clips (Lapra-Ty, Ethicon, Cincinnati, OH). Hemostatic agents were used during our early LPN experience (first 50 patients) and included a cellulose rolled bolster and topical fibrin placed in the base of the surgical bed prior to cortical reconstruction. More recently, the resection bed was closed primarily without bolsters or hemostatic agents using a sliding renorrhaphy technique [13]. Additionally, an early unclamping technique was implemented as described by Dr. Gill’s group [14]. RPN was performed via a transperitoneal approach with or without early unclamping of the renal artery. In those patients undergoing early unclamping, the bulldog clamp was removed immediately after resection of the tumor and placement of a running suture at the base of the resection bed to close collecting system and open vessels. All additional reconstructions were done “off clamp”. A Jackson–Pratt drain and foley catheter were placed in all patients. The bedside assistant for the RPN cases was a senior urology resident or fellow (postgraduate years 3 through 7). Operative time for all cases was from veress needle insertion and ended at placement of the dressing. For the RPN cohort, robot docking and console times were not separately recorded.
Partial nephrectomy specimens were evaluated by one of three fellowship trained pathologists with expertise in genitourinary cancers. A positive surgical margin was defined as tumor extending to the inked parenchymal cut edge of tissue. All specimens, whether obtained by laparoscopic or robotic procedures, were treated identically.
Statistical methods
Descriptive statistics were used to characterize the clinical characteristics of the study cohort at baseline and are shown in Table 1 stratified by the surgical procedure. A Mantel–Haenszel chi-square metric was used to compare the distributions of baseline categorical clinical characteristics across treatment modalities. In cases where the sample size was small (N < 5), a Fisher’s exact test was used. For the continuous covariates including the calculated eGFR and age at the time of partial nephrectomy, medians and their distributions were compared using a Wilcoxon two-sample test across surgical procedures. Two-sided P-values <0.05 were considered statistically significant. SAS version 9.2 (SAS Institute, Cary, North Carolina) was used for all calculations.
Results
Comparison of the distribution of baseline patient and tumor characteristics according to surgical procedure
The study cohort comprised 86 consecutively treated patients who underwent laparoscopic partial nephrectomy (LPN, N = 59) or robotic-assisted partial nephrectomy (RPN, N = 27). The majority of the LPN were performed from 2006 to 2009, and the RPN performed from 2009 to 2010. As shown in Table 1, there was no difference between the LPN and RPN cohort in regard to gender (P = 0.622), age (P = 0.684), operative side (P = 1.00), American Society of Anesthesiology score (P = 0.565), or eGFR (P = 0.294). An early unclamping technique was used for 22 (82%) patients in the RPN cohort and 6 (10%) patients in the LPN cohort (P < 0.001).
Perioperative outcomes
Table 2 illustrates perioperative outcomes between the two groups. Mean operative times were similar between the two groups at approximately 4 h. Mean warm ischemia time was significantly shorter in the RPN group (18.5 vs. 28.0 min, P < 0.001). Estimated blood loss was not significantly different between the two groups. Although there were three patients who received transfusions in the LPN group and none in the RPN group, this was not significant. One patient in the LPN group required conversion to open partial nephrectomy due to excessive bleeding from the resection bed and incomplete renal artery clamping. No conversions occurred in the RPN group.
There were no intraoperative complications in either group (Table 2). There were fewer adverse events in the RPN group; however, this was not significant (19% vs. 27%, P = 1.00). Complications in the LPN group included 4 Clavien grade I (pneumonia, L median nerve palsy, ileus, urinary tract infection), 4 Clavien grade IIa (hematuria, ileus, retroperitoneal bleed, wound infection), and 4 Clavien grade IIb (angioembolization, clot retention in 3 patients) complications. Complications in the RPN group included 4 Clavien grade I (ileus, urinary tract infection, uvular edema, rash) and 2 Clavien grade IIa (angina, fever) complications. There was no significant difference in postoperative decrease in eGFR (23% LPN vs. 14% RPN; P = 0.506). Furthermore, there was no difference in mean eGFR decrease after early unclamping versus traditional clamping, respectively (16 vs. 22%, P = 0.340). A total of 11 (29%) patients had a greater than 50% decrease in eGFR after traditional clamping versus 0 patients after early unclamping (P = 0.014).
Pathologic findings were similar between the 2 groups as seen in Table 2. The rate of positive surgical margins was less for the RPN group; however, this was not significant (4 vs. 12%, P = 0.426).
We performed a further analysis of our first 10 RPN and last 17 RPN cases in order to better discern a learning curve effect (Table 3). Total nephrometry scores, complexity of lesions, estimated blood loss, length of stay, positive surgical margins, and perioperative complications were similar throughout our RPN experience. Mean operative times were significantly longer earlier in our RPN series (255 vs. 220 min, P = 0.043) and longer than the LPN cohort (255 vs. 221 min, P = 0.022). Furthermore, warm ischemia times were significantly greater for the first 10 RPN versus last 17 RPN cases (24 vs. 15 min, P = 0.002) owing to early unclamping utilized later in our RPN experience (50 vs. 100%, P = 0.003). There was no difference in warm ischemia time for the first 10 RPN cases versus the LPN cohort (24 vs. 28 min, P = 0.295), respectively.
Comment
This study was performed to determine whether implementation of a robotic kidney surgery program by an experienced laparoscopic kidney surgeon resulted in any change in perioperative and oncologic outcomes. We found in our early experience that patients undergoing the robotic when compared to the laparoscopic approach had equivalent short-term results. Furthermore, RPN with early unclamping is a safe technique with similar perioperative outcomes as LPN, resulting in fewer patients losing renal function.
Several points require further consideration. First, the vast majority of RPN patients (22/27 or 82%) in this study had early unclamping of the renal artery. Gill et al. described this technique in his vast experience with LPN and was able to reduce his warm ischemia time from 31 to 14 min without a significant increase in blood loss or margin rate [14]. We have adopted his early unclamping technique for RPN which has in turn reduced our warm ischemia time significantly and likely resulted in fewer patients in the RPN cohort losing renal function as measured by eGFR. In fact, none of our patients undergoing RPN with early unclamping EU had a warm ischemia time of longer than 25 min. It has been demonstrated that there is a significant decrease in eGFR when the warm ischemia time was longer than 30 min [15]. We found no difference in warm ischemia time, blood loss, length of stay, and no perioperative complications were noted between the LPN cohort and our first 10 RPN cases. Thus, we were able to safely initiate a RPN program without adding additional risks to the patient. Although reducing warm ischemia time is a worthy goal, more important is that surgeons, whether through open or minimally invasive techniques, attempt partial nephrectomy for all reasonably located small renal masses. It is worth mentioning again that the current AUA consensus panel on the subject clearly states that partial nephrectomy should be considered in all patients with clinical T1 renal masses [5].
Overall, we observed relatively few complications in this early cohort of patients undergoing RPN, although it is important to note that the learning curve for LPN has been observed to last through hundreds of minimally invasive partial nephrectomy procedures [15]. Similar to other series of RPN [8, 9], our study is a comparison of RPN patient outcomes after a surgeon has extensive experience with LPN. It is still unclear whether the addition of the robotic system offers improvements that shorten the learning curve for a laparoscopic naïve surgeon, as has been observed in robotic radical prostatectomy. The possibility of catastrophic blood loss is much greater during partial nephrectomy, and therefore we surmise that our familiarity with LPN provided the necessary experience for safe RPN. It is worth mentioning that as seen in the robotic prostatectomy literature, the level of expertise of the assistant may affect surgical outcomes [16]. Rogers et al. [17] have suggested the use of the fourth arm of the DaVinci robot and TilePro to maximize console surgeon independence from the assistant when performing RPN. We have found that assistants must be familiar not only with the robotic system, but with advanced laparoscopic maneuvers such as placing and removing laparoscopic bulldog clamps, aggressively clearing operative field of blood during tumor resection, rapid introduction and retrieval of sutures, and placement of laparoscopic clips.
A limitation of our study was that it was underpowered to discover small differences in perioperative outcomes between LPN and RPN. The majority of clinically relevant outcomes were similar between the groups, including blood loss, hospital stay, and margin rate. There was a non-significant trend toward decrease positive surgical margins in the RPN group. This may be due to a multitude of factors including enhanced visualization of the robotic system, improved dexterity during resection, or smaller lesions in the RPN group. However, the most likely explanation is that the RPN patients had surgery later in our minimally invasive partial nephrectomy experience, with minor technical nuances and more aggressive resection contributing to the difference. Ultimately, in order to evaluate true comparative effectiveness of partial nephrectomy techniques, comparisons of oncologic outcomes, prospective patient-reported quality of life and direct costs between LPN, RPN and open partial nephrectomy will be necessary.
Conclusion
Patients undergoing RPN during implementation of a robotic kidney surgery program when compared with LPN appear to have equivalent perioperative outcomes and similar oncologic efficacy. These results are especially important as partial nephrectomy remains the gold standard of care for small renal masses and utilization of robotics continues to be on the rise. LPN and RPN remain challenging procedures, and in particular, RPN requires a highly skilled bedside assistant. RPN patients had surgery later in our minimally invasive partial nephrectomy experience, and these results may not be generalizable to laparoscopic and/or robotic naïve surgeons. In addition, a majority of our RPN patients had early unclamping, which needs to be taken into account when interpreting postoperative renal function between surgical approaches. Further studies in comparative effectiveness are needed to validate these initial results.
References
Kunkle DA, Egleston BL, Uzzo RG (2008) Excise, ablate or observe: the small renal mass dilemma–a meta-analysis and review. J Urol 179:1227–1233 (discussion 1233–1234)
Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, Scardino PT, Russo P (2006) Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 7:735–740
Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, Blute ML (2008) Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol 179:468–471 (discussion 472–473)
Huang WC, Elkin EB, Levey AS, Jang TL, Russo P (2009) Partial nephrectomy versus radical nephrectomy in patients with small renal tumors–is there a difference in mortality and cardiovascular outcomes? J Urol 181:55–61 (discussion 61–62)
Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, Faraday MM, Kaouk JH, Leveillee RJ, Matin SF et al (2009) Guideline for management of the clinical T1 renal mass. J Urol 182:1271–1279
Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR Jr, Frank I, Permpongkosol S, Weight CJ, Kaouk JH et al (2007) Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol 178:41–46
Gettman MT, Blute ML, Chow GK, Neururer R, Bartsch G, Peschel R (2004) Robotic-assisted laparoscopic partial nephrectomy: technique and initial clinical experience with DaVinci robotic system. Urol 64:914–918
Wang AJ, Bhayani SB (2009) Robotic partial nephrectomy versus laparoscopic partial nephrectomy for renal cell carcinoma: single-surgeon analysis of >100 consecutive procedures. Urol 73:306–310
Haber GP, White WM, Crouzet S, White MA, Forest S, Autorino R, Kaouk JH: Robotic versus laparoscopic partial nephrectomy: single-surgeon matched cohort study of 150 patients. Urol 76: 754-8
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Kutikov A, Uzzo RG (2009) The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 182:844–853
Taneja SS, Dakwar G, Godoy G (2009) Simplified reconstruction after laparoscopic partial nephrectomy using a single-pass suturing technique. J Endourol 23:589–591 (discussion 591–592)
Nguyen MM, Gill IS (2008) Halving ischemia time during laparoscopic partial nephrectomy. J Urol 179:627–632 (discussion 632)
Gill IS, Kamoi K, Aron M, Desai MM: 800 Laparoscopic partial nephrectomies: a single surgeon series. J Urol. 183:34-41
Lee DI, Eichel L, Skarecky DW, Ahlering TE (2004) Robotic laparoscopic radical prostatectomy with a single assistant. Urol 63:1172–1175
Rogers CG, Laungani R, Bhandari A, Krane LS, Eun D, Patel MN, Boris R, Shrivastava A, Menon M (2009) Maximizing console surgeon independence during robot-assisted renal surgery by using the fourth arm and TilePro. J Endourol 23:115–121
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, S.B., Kacker, R., Alemozaffar, M. et al. Robotic partial nephrectomy versus laparoscopic partial nephrectomy: a single laparoscopic trained surgeon’s experience in the development of a robotic partial nephrectomy program. World J Urol 31, 793–798 (2013). https://doi.org/10.1007/s00345-011-0648-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-011-0648-5