Abstract
Purpose
To investigate prognostic markers in patients with metastatic renal cell carcinoma (mRCC) undergoing treatment with the tyrosine kinase inhibitors (TKIs) sorafenib (So) or sunitinib (Su).
Patients and methods
Eighty-three patients with mRCC, who were treated at our institution between 2006 and 2009, were evaluated prospectively. Clinical and laboratory parameters were investigated, as well as, treatment-related adverse events. Subclinical hypothyroidism was characterized by serum TSH above the upper limit of normal and both total triiodothyronine (T3) and thyroxine (T4) within normal limits. Clinical hypothyroidism was defined as low serum T3 and T4 together with elevated TSH.
Results
Thirty-one (37.3%) patients received So, and 52 (62.7%) were treated with Su. In univariate analysis, the ECOG status (P < 0.0001) as well as MSKCC criteria (P = 0.003) and response to therapy (P < 0.0001) were associated with progression-free survival (PFS). Twenty-one of 66 (31.8%) evaluable patients developed hypothyroidism during treatment. Of those patients, 8/21 (38.1%) were treated with So and 13/21 (61.9%) with Su. Response rate in this subgroup was 49.2%. Hypothyroidism was associated with a longer PFS (16.0 ± 0.8 months vs. 6.0 ±0.8 months, P = 0.032). Most patients [16/21 (76.2%)] developed abnormal TSH values during the first 4 weeks of treatment. Hormone replacement with l-thyroxine did not have an influence on survival. In multivariate analyses, only the ECOG status (ECOG 0/1 vs. ECOG 2, P = 0.018) and hypothyroidism (P = 0.01) were independent prognostic parameters.
Conclusions
The development of hypothyroidism during treatment might be useful as a predictor of PFS for mRCC patients undergoing treatment with targeted agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all malignant tumors in adults. In Europe, the estimated incidence was 88,400 cases and 39,300 cancer-related deaths in 2008 [1].
The treatment of metastatic renal cell carcinoma (mRCC) has changed enormously during the recent past. After more than a decade of only cytokine therapy, a new era of targeted agents began in 2006 with the approval of the tyrosine kinase inhibitors (TKIs) sorafenib (So) and sunitinib (Su) [2, 3].
The Memorial Sloan-Kettering Cancer Center (MSKCC) score system is widely used to stratify risk groups and identify patients who will likely benefit from treatment with So and Su or other targeted agents. This prognostic model identifies certain variables to be of predictive value in terms of survival, to include the Karnofsky performance status, time from diagnosis to treatment <1 year and also laboratory abnormalities such as low hemoglobin, elevated LDH, and high corrected serum calcium. Although generally used in daily clinical practice, this model was only validated for the treatment with cytokines [4] but not with targeted agents. However, due to data lacking on other prognostic and predictive factors for this latter treatment type, the MSKCC criteria were reluctantly accepted and included in several therapeutic guidelines [5, 6].
Due to little evidence associated with the use of MSKCC criteria in this context, many renal cancer experts emphasize the necessity of determining and validating other molecular and clinical markers that may predict survival with more accuracy. Therefore, our goal in this study was to prospectively evaluate several clinical and laboratory variables that could be of predictive value, in terms of progression-free survival (PFS), in patients with mRCC undergoing treatment with So or Su.
Patients and methods
Patients
Between June 2006 and July 2009, 95 patients with mRCC were treated with either So or Su as first- or second-line therapy (after cytokine failure). All patients included in this study were at least 18 years of age and had histologically confirmed mRCC. Additional requirements were an ECOG performance status of 0–2 and adequate bone marrow, hematologic, liver, pancreatic, renal, and cardiac function prior to the treatment. Patients with severe missing laboratory data and/or inaccurate follow-up information were excluded from further evaluation. The final study population comprised 83 patients (Table 1).
Treatment
Patients received continuous treatment with oral So at a dose of 400 mg, twice daily, in 6-week cycles. Su was administered at 50 mg, once daily, in repeated 6-week cycles of daily therapy for 4 weeks, followed by 2 weeks off treatment. Toxicity was evaluated using National Cancer Institute Common Toxicity Criteria version 3.0. Physical examination, performance status, and laboratory tests were generally assessed on days 0, 15, 45, 75, and 105. Response to therapy was evaluated using RECIST criteria every two cycles by either computed tomography scans or magnetic resonance imaging, while bone scans were usually performed when clinically indicated.
Study endpoints
The identification of prognostic factors predicting response to therapy, and PFS were the primary endpoints of the study.
We evaluated clinical parameters such as gender, age, kind of treatment (So versus Su), ECOG performance status, MSKCC risk groups, prior nephrectomy, histologic subtype, number of metastatic sites, time between diagnosis and treatment <1 year, presence of bone metastases, and brain metastases. Furthermore, we assessed response to therapy to be a surrogate parameter for PFS.
We investigated laboratory data at baseline, but also changes during treatment (hemoglobin, calcium, LDH, leukocytes, neutrophile granulocytes, thrombocytes, creatinine, uric acid, and alkaline phosphatase). Beginning in February 2007, we also evaluated TSH (if elevated, also T3 and T4).
Finally, common treatment-related adverse events were evaluated, including severe reduction in weight, arterial hypertension, and hand–foot syndrome.
Definition of hypothyroidism
The biochemical diagnosis of subclinical hypothyroidism was determined in accordance with guidelines of the American Thyroid Association (ATA) and the American Association of Clinical Endocrinologists (AACE) as follows: subclinical hypothyroidism was considered as serum TSH above the upper limit of normal, with total triiodothyronine (T3) and thyroxine (T4) within normal limits. Clinical hypothyroidism was defined as low serum T3 and T4 together with elevated TSH. Patients with overt hypothyroidism and those with symptoms compatible with hypothyroidism (e.g., fatigue, cold intolerance, constipation, or weight gain) received thyroid hormone replacement therapy with l-thyroxine.
Data analysis
For statistical analysis, the Statistical Package for the Social Sciences for Windows, version 18.0 (SPSS Inc, Chicago, II, USA) was used. The Kaplan–Meier method was used to derive the PFS, and the log-rank test was employed to compare curves for two or more groups. Univariable Cox regression analyses were performed to identify differences with regard to predictors of response to therapy. For multivariate analysis of prognostic factors, a Cox regression analysis was performed. All proportional hazard assumptions were correlated using the Grambsch–Therneau test. The p values were two-sided, and a P value <0.05 was considered to indicate significant differences between groups.
Results
Of the 83 patients analyzed, 56 (67.5%) were men and 27 (32.5%) were women. Median age was 63 ± 10.3 years (range 30–84 years) (Table 1).
Thyroid function during treatment
In total, 66 out of 83 (79.5%) patients were eligible for evaluation. Twenty-one of 66 (31.8%) patients developed subclinical or clinical hypothyroidism, meeting the criteria mentioned earlier. Of those patients, 8/21 (38.1%) were treated with So and 13/21 (61.9%) with Su. Most patients [16/21 (76.2%)] developed abnormal TSH values during the first 4 weeks of treatment. No complete response (CR) was observed. A total of 9/21 (42.9%) patients responded with a partial response (PR) as best treatment response, while stable disease (SD) was present in 7/21 (33.3%) patients. Progressive disease (PD) occurred in 5/21 (23.8%) patients. Best treatment response was independent from type of treatment (So vs. Su) in this subgroup.
Response to therapy
The ECOG status correlated with response to therapy ([ECOG 0; CR: 1 (2.1%), PR: 20 (41.7%), SD: 24 (50.0%), and PD: 3 (6.2%)], [ECOG 1; CR: 0 (0%), PR: 1 (3.8%), SD: 11 (42.3%), and PD: 14 (53.9%)], [ECOG 2; CR: 0 (0%), PR: 1 (16.7%), SD: 1 (16.7%), and PD: 4 (66.6%)] P < 0.0001).
Furthermore, MSKCC criteria ([low risk; CR: 0 (0%), PR: 5 (35.7%), SD: 9 (64.3%), and PD: 0 (0%)], [intermediate risk; CR: 1 (1.9%), PR: 14 (27.5%), SD: 22 (43.1%), and PD: 14 (27.5%)], [high risk; CR: 0 (0%), PR: 0 (0%), SD: 0 (0%), and PD: 5 (100%)] P = 0.002) as well as an elevated LDH (×1.5) before treatment (normal; CR: 0 (0%), PR: 19 (34.0%), SD: 25 (44.6%), and PD: 12 (21.4%)], [>normal; CR: 1 (7.7%), PR: 1 (7.7%), SD: 5 (38.5%), and PD: 6 (46.1%)] (P = 0.032) were predictors of response.
Progression-free survival
Univariate analyses
We did not see a significant difference in PFS with regard to gender, age, kind of treatment, number of metastatic sites, time between diagnosis and treatment <1 year, or presence of bone metastases and brain metastases. Aberrant laboratory values, before or during treatment, for calcium, LDH, leukocytes, neutrophile granulocytes, thrombocytes, creatinine, uric acid, and alkaline phosphatase also failed to show a prognostic impact on PFS. Furthermore, treatment-related adverse events such as severe reduction in weight, arterial hypertension, and hand–foot syndrome were not prognostic factors.
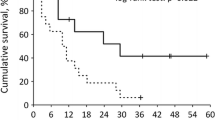
A bad ECOG status was associated with poor survival (ECOG 0: 11.0 ± 2.2 months, ECOG 1: 4.0 ± 0.3 months, and ECOG 2: 3.0 ± 1.6 months) (P < 0.0001) (Fig. 1a). Similarly, MSKCC criteria correlated with PFS (low risk: 12.0 ± 1.7 months, intermediate risk: 6.0 ± 1.0 months, and high risk: 3.0 ± 0.8 months) (P = 0.003) (Fig. 1b). Best response to therapy was a surrogate parameter for PFS, as well (CR: 21.0 ± 0.0 months, PR: 10.0 ± 1.0 months, SD: 13.0 ± 3.3 months, and PD: 4.0 ± 0.1 months (P < 0.0001) (Fig. 1c). On the other hand, development of hypothyroidism was associated with a positive prognosis (16.0 ± 0.8 months vs. 6.0 ± 0.8 months, P = 0.032) (Fig. 1d). No significant difference was found between So and Su in this subgroup. Hormone replacement with l-thyroxine did not have an influence on survival in these patients.
Although not statistically significant, several parameters tended to have an influence on PFS. Patients with prior nephrectomy tended to have a longer PFS compared with those who were not treated with a resection of the primary tumor (9.0 ± 1.3 months vs. 4.0 ± 1.0 months, P = 0.056). The histologic subtype (clear cell vs. others) was also associated with PFS (10.0 ± 2.0 months vs. 6.0 ± 1.0 months, P = 0.052), albeit statistically not significant. Furthermore, baseline anemia tended to correlate with a worse PFS (8.0 ± 0.8 months vs. 19.0 ± 6.2 months, P = 0.079).
Multivariate analysis
In multivariate analysis, only the ECOG status (ECOG 0/1 vs. ECOG 2, P = 0.018) and the development of hypothyroidism during treatment (P = 0.01) were independent prognostic parameters (Table 2).
Discussion
Recent advances in our understanding of the biology of mRCC and the role of angiogenesis of this tumor entity led to the development and approval of TKIs such as So and Su in 2006 [2, 3]. Data regarding clinical or biologic surrogate parameters predicting survival are rare, but urgently needed to select patient groups and offer alternative treatments when oncological benefit seems unlikely. Until the identification of such markers, selection of patients will rely upon baseline clinical and pathological characteristics like the MSKCC criteria. Although used in all TKI phase III trials [2, 3, 7], there are several limitations associated with the use of MSKCC criteria for prognostic purposes. Specifically, all phase III studies predominantly include patients with low or intermediate risk, i.e., populations with a different composition than that of the population used to develop the MSKCC model [8]. Furthermore, the MSKCC system has been validated as a predictor of overall survival, while PFS has been the major end point in nearly all TKI trials.
In our study, we assessed clinical and laboratory baseline parameters and hypothetical surrogate parameters during treatment with the question of predicting PFS. At the beginning of the study, we did not define hypothyroidism to be assessed as a possible predictor. However, when evidence arose that the development of hypothyroidism was a likely side effect of treatment with TKIs, the assessments of TSH, T3, and T4 were also included in our analysis, beginning in February 2007. In multivariate analysis, only the ECOG status and the development of hypothyroidism were identified as independent predictors of PFS.
To date, there are several hypotheses to explain TKI-induced hypothyroidism, including reduced synthesis of thyroid hormones due to inhibition of thyroid peroxidase activity and progressive depletion of functional reserves [9], inhibition of thyroid uptake of iodine [10], and drug-induced atrophy of the thyroid through inhibition of gland vascularity or thyroiditis [11]. However, the mechanism responsible for the development of hypothyroidism in patients treated with Su or So is still unclear and deserves further prospective evaluation [12, 13].
In 2008, Wolter et al. prospectively evaluated thyroid function in 40 patients with mRCC treated with Su. Thirteen (32.5%) patients developed hypothyroidism requiring hormone substitution [14]. The authors identified a statistically significant PFS and overall survival advantage for the group of patients with sunitinib-induced thyroid dysfunction. In a subsequent study, the same authors evaluated 59 patients with either mRCC or gastrointestinal stromal tumor treated with Su and identified 16 (27.1%) patients with hypothyroidism requiring hormone replacement [15]. Similar to our study, the development of hypothyroidism was associated with early TSH elevation in most cases, generally within 4 weeks of treatment. Hormone replacement usually resulted in normalization of laboratory values and improvement of clinical symptoms. These results suggest that all patients undergoing TKI treatment should receive thyroid function tests before treatment starts and regularly after, e.g., every 4 weeks, for at least 4 cycles or when clinically indicated.
Recently, Schmidinger et al. were the first to show a correlation between the appearance of subclinical hypothyroidism and the median duration of survival [16]. All hypothyroid patients were diagnosed with increased TSH within the first 8 weeks of treatment. Similar to our study, hormone replacement did not have an influence on survival in these patients.
In recent years, much effort has been made to establish clinical or biologic markers as surrogate parameters for favorable survival [17–20]. Interestingly, in 2008, Rini et al. presented a correlation between diastolic blood pressure (dbp) ≥90 mmHg and survival during treatment with the TKI axitinib (Ax) [21]. Although not validated yet, this phenomenon is under intense investigation, and future nomograms creating risk groups will possibly implement dbp in the treatment with Ax. The implementation of thyroid function laboratory values into routine blood samples seems to be another possibility to identify patients who will likely benefit from treatment with So or Su. As most of our patients developed hypothyroidism within the first 4 weeks of treatment, the appearance of hypothyroidism-associated clinical symptoms might strangely lead to enhanced patient motivation to continue therapy despite adverse events, when this information is imparted to the patient. Furthermore, within our patient group, clinical benefit seemed to last even after normalization of peripheral hormone levels due to hormone replacement. The application of l-thyroxine did therefore not undermine the antitumor efficacy of So and Su.
In summary, subclinical or clinical hypothyroidism affects 1/3 of patients being treated with So or Su within 4–8 weeks after start of treatment and might play a role as a prognostic factor. Due to the low number of patients investigated in our single-center study, we recommend further testing, preferably within prospective clinical multicenter trials also including other targeted agents, e.g., pazopanib. Once validated, hypothyroidism might be useful as a clinical predictor during targeted therapy.
References
Ferlay J, Parkin DM, Steliarova-Foucher E (2010) Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 46:765–781
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM (2007) Target study group sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124
Mekhail TM, Abou-Jawde RM, Boumerhi G, Malhi S, Wood L, Elson P, Bukowski R (2005) Validation and extension of the memorial sloan-kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 23:832–841
Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, Patard JJ, Mulders PF, Sinescu IC (2010) European association of urology guideline group eau guidelines on renal cell carcinoma: the 2010 update. Eur Urol 58:398–406
Motzer RJ, Agarwal N, Beard C, Bolger GB, Boston B, Carducci MA, Choueiri TK, Figlin RA, Fishman M, Hancock SL, Hudes GR, Jonasch E, Kessinger A, Kuzel TM, Lange PH, Levine EG, Margolin KA, Michaelson MD, Olencki T, Pili R, Redman BG, Robertson CN, Schwartz LH, Sheinfeld J, Wang J (2009) NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Canc Netw 7:618–630
Sternberg CN, Davis ID, Mardiak J, SZczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, Zarbá JJ, Chen M, McCann L, Pandite L, Roychowdhury DF, Hawkins RE (2010) Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 28:1061–1068
Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M (2002) Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20:289–296
Wong E, Rosen LS, Mulay M, Vanvugt A, Dinolfo M, Tomoda C, Sugawara M, Hershman JM (2007) Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid 17:351–355
Mannavola D, Coco P, Vannucchi G, Bertuelli R, Carletto M, Casali PG, Beck-Peccoz P, Fugazzola L (2007) A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J Clin Endocrinol Metab 92:3531–3534
Desai J, Yassa L, Marqusee E, George S, Frates MC, Chen MH, Morgan JA, Dychter SS, Larsen PR, Demetri GD, Alexander EK (2006) Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med 145:660–664
Torino F, Corsello SM, Longo R, Barnabai A, Gasparini G (2009) Is hypothyroidism a clinically relevant toxicity of tyrosine kinase inhibitors? Thyroid 19:539–540
Surks MI, Hollowell JG (2007) Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 92:4575–4582
Wolter P, Stefan C, Decallonne B, Dumez H, Fieuws S, Wildiers H, Clement P, Debaere D, Van Oosterom A, Schöffski P (2008) Evaluation of thyroid dysfunction as a candidate surrogate marker for efficacy of sunitinib in patients (pts) with advanced renal cell cancer(RCC). J Clin Oncol suppl 26:abstract 5126
Wolter P, Stefan C, Decallonne B, Dumez H, Bex M, Carmeliet P, Schöffski P (2008) The clinical implications of sunitinib-induced hypothyroidism: a prospective evaluation. Br J Cancer 99:448–454
Schmidinger M, Vogl UM, Bojic M, Lamm W, Heinzl H, Haitel A, Clodi M, Kramer G, Zielinski CC (2010) Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer (Epub ahead of print)
Patel PH, Chadalavada RS, Ishill NM, Patil S, Reuter VE, Motzer RJ, Chaganti RS (2008) Hypoxia-inducible factor (HIF)1a and 2a levels in cell linies and human tumor predicts response to sunitinib in renal cell carcinoma (RCC). J Clin Oncol suppl 26:abstract 5008
Hutson TE, Davis ID, Machiels JH, de Souza PL, Baker K, Bordogna W, Westlund R, Crofts T, Pandite L, Figlin RA (2008) Biomarker analysis and final efficacy results of a phase II renal cell carcinoma trial with pazopanib(GW786034). J Clin Oncol suppl 26:abstract 5046
Kontovinis LF, Papazisis KT, Touplikioti P, Andreadis C, Mouratidou D, Kortsaris AH (2009) Sunitinib treatment for patients with clear-cell metastatic renal cell carcinoma: clinical outcomes and plasma angiogenesis markers. BMC Cancer 9:82
Han KS, Jung DC, Choi HJ, Jeong MS, Cho KS, Joung JY, Seo HK, Lee KH, Chung J (2010) Pretreatment assessment of tumor enhancement on contrast-enhanced computed tomography as a potential predictor of treatment outcome in metastatic renal cell carcinoma patients receiving antiangiogenic therapy. Cancer 116:2332–2342
Rini BI, Schiller JH, Fruehauf JP, Cohen EE, Tarazi JC, Rosbrook B, Ricart AD, Olszanski AJ, Kim S, Spano J (2008) Association of diastolic blood pressure (dBP) >90 mmHg with overall survival (OS) in patients treated with axitinib (AG- 013736). J Clin Oncol suppl 26:abstract 3543
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riesenbeck, L.M., Bierer, S., Hoffmeister, I. et al. Hypothyroidism correlates with a better prognosis in metastatic renal cancer patients treated with sorafenib or sunitinib. World J Urol 29, 807–813 (2011). https://doi.org/10.1007/s00345-010-0627-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-010-0627-2