Abstract
Purpose
To assess the feasibility of seeding adipose-derived stem cells (ADSCs) onto bladder acellular matrix grafts (BAMGs) for bladder reconstruction in a rabbit model.
Methods
Autologous ADSCs were isolated, expanded and identified by flow cytometry. In the experimental group, ADSCs were seeded onto BAMGS for reconstructing bladder defects in 12 male rabbits. Unseeded BAMGs were used for bladder reconstruction in the control group of 12 rabbits. Cystography was performed at 4, 12 and 24 weeks after grafts implantation. Following cystography, the animals were killed and grafts were harvested; H&E and immunohistochemical staining were performed with cytokeratin AE1/AE3, smooth muscle α-actin and S-100 markers.
Results
Flow cytometry demonstrated that the ADSCs expressed CD90, CD44, CD105, CD166 and CD34, but not CD45 or CD106. The cells demonstrated good biocompatibility with BAMGs. At 24 weeks, in the experimental group, the reconstructed bladders reached a mean volume of 94.68 ± 3.31% of the pre-cystectomy bladder capacity. Complete regeneration of smooth muscle and nerve tissue was evident. Regenerated SMCs, urothelium and nerve cells stained positively for α-smooth muscle actin, AE1/AE3 and S-100. In the control group, the mean bladder volume was 69.33 ± 5.05% of the pre-cystectomy volume; histologically, the control group was characterized by multi-layered urothelium without evidence for organized muscle or nerve tissue.
Conclusions
These data demonstrate that seeding ADSCs onto BAMGs promote regeneration of smooth muscle and nervous tissue regeneration in a rabbit model. This compound graft was more suitable for bladder reconstruction than BAMG alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Profound developments in the field of tissue engineering bring new hope for bladder reconstruction [1]. Conventional bladder reconstruction, which uses gastrointestinal tissue, is associated with complications such as infection, metabolic disturbances, urolithiasis, perforation and malignancy [2]. Tissue engineering techniques may avoid many of these complications and offer a potential alternative for bladder reconstruction [3].
Historically, approaches to tissue engineering bladder involved seeding acellular matrices with autologous bladder smooth muscle and urothelial cells [4]. However, this approach cannot be applied for patients with invasive bladder cancer or neurogenic bladders as these patients lack a source of normal bladder smooth muscle and urothelial cells [5]. Therefore, alternative sources of tissue are required. Great consideration has been paid to the bone marrow stromal cell (BMSC), due to their versatility. However, such BMSCs can only be obtained by bone marrow aspiration, which is traumatic. Compared with BMSCs, adipose-derived stem cells (ADSCs) can be obtained in relatively large quantities with greater ease. Like BMSCs, they also can differentiate into several mature cell types [6].
Bladder acellular matrix grafts (BAMGs) are collagen-based xenogenetic biomaterials, which exhibit good biocompatibility and support tissue regeneration [7]. Previous investigations demonstrated the feasibility of using BAMGs for bladder reconstruction [8]. Presently, we isolated and cultured ADSCs in vitro and seeded them onto BAMGs to repair bladder defects in a rabbit model. Our present investigation allows us to assess the feasibility of utilizing ADSC-seeded BAMGs for bladder reconstruction. To our knowledge, this is the first report to study whether ADSC-seeded BAMGs can promote bladder tissue regeneration in an animal model.
Materials and methods
Cell culture and flow cytometry analysis
Experimental protocols were approved by the Animal Care and Use Committee at our institution. ADSCs were prepared by collagenase digestion. The adipose tissues were carefully dissected from the back of rabbits (approximately 2 × 2 cm). After rinsing in 0.25% chloromycetin three times, tissues were cut into small pieces and then digested in 0.1% collagenase A (Roche USA) at 37°C for 1 h. The suspension was filtered through a 200-μm nylon mesh to separate single cells from undigested tissue. The isolated ADSCs were cultured in a 10 cm cell culture plate at 37°C with 5% CO2.
For cytometry analysis, third passage ADSCs (approximately 2 × 105 cells) were incubated with 10 μl of individual IO test monoclonal primary antibodies coupled to either phycoerythrin (PE) or fluorescein isothiocyanate (FITC in 50 μl PBS for 30 min in the dark at room temperature. Then cells were washed twice with 1 ml PBS, resuspended with PBS, diluted in 200 μl PBS and analyzed with a FACScan flow cytometer (Becton–Dickinson). Data were acquired using CellQuest Pro and analyzed using FlowJo software. Antibodies for CD34, CD44, CD166, CD90, CD45, CD105 and CD106 were obtained from BD Biosciences Franklin Lakes, NJ, USA.
Preparing the BAMGs
Bladders from another 14 male New Zealand white rabbits were obtained and rinsed with PBS. To promote cell lysis, isolated bladders were soaked for 12 days at 37°C in 0.2% Triton X-100 (Sigma) and 0.1% (v/v) ammonium hydroxide [9]. The solution was refreshed every 3 days. The resultant matrix was then washed thoroughly with distilled water and lyophilized for 24 h. Ethylene oxide was used for sterilization before seeding.
Seeding on ADSCs onto BAMGs
Third passage ADSCs were seeded onto the mucosal side of BAMGs by placing a cell suspension (3 × 107 cells/ml) onto the matrix. Four milliliter of culture medium was added 4 h after cell seeding. The medium was changed daily for 7 days.
Bladder reconstruction
A defect in the dome of the bladder wall, representing approximately 1.5 × 1.5 cm2 of the bladder surface area (about 40–60% of the bladder surface), was created by surgical excision in 24 rabbits. The seeded BAMG was sutured as a patch onto the dome of the bladder using a running 5–0 Vicryl suture in 12 rabbits (experimental group). Unseeded BAMGs were used in the remaining 12 rabbits (control group). Multiple nonabsorbable marking sutures were placed at the margin of the graft in order to rule out the variation of tissue harvest. A small piece of perivesical fat was wrapped over the ventral bladder wall in order to cover the graft. The catheter was left indwelling and fastened with sutures to provide bladder drainage for 14 days postop.
Measurement of bladder volumes
Bladder volumes were measured using a 7 Fr double-lumen transurethral catheter. The bladder was emptied and then filled with a pre-warmed saline solution at constant rate. Maximal bladder capacity was defined as the volume of infusion, which triggered the first leakage of urine [10].
Histology and immunohistochemistry
Four animals in each group were killed at 4, 12 and 24 weeks post-implantation. Before being euthanized, cystography was performed and the bladder capacity was measured. Then, the entire bladder was removed and its entire mucosal surface was evaluated. 5 μm sections of formalin-fixed, paraffin wax-embedded tissue were stained with H&E. Epidermal cell layers were identified immunohistochemically based on their reactivity with the AE1/AE3 antibody (Sigma, St. Louis, MO). Similarly, smooth muscle cells in the regenerating bladder were identified by reactivity to the α-smooth muscle (α-SM) actin monoclonal antibody (Sigma, St. Louis, MO). Antibodies to S-100 (Sigma, St. Louis, MO) was used as neural cell markers.
Results
After incubation for 2–3 weeks, the cell population covered approximately 90% of the culture plate. ADSCs became fusiform and exhibited spindle shaped morphologies (Fig. 1). Flow cytometry demonstrated expression of CD90 (88.10%), CD44 (99.40%), CD34 (35.29%), CD166 (66.96%) and CD105 (35.55%). There was no significant expression of CD45 (0.86%) or CD106 (1.60%).
Histological examination demonstrated that BAMGs were completely acellular. Masson’s trichrome staining demonstrated that BAMGs consisted mainly of a collagenous extracellular matrix. After seeding, the compound grafts were maintained in vitro for 7 days to allow for cell adhesion. H&E staining showed that ADSCs grew well and formed multilayers on the scaffolds (Fig. 1).
All rabbits in each group survived til killing with the exception of one rabbit in the control group which died due to infection secondary to bladder leakage. Preoperatively, similar bladder capacities were recorded in the control and experimental groups (49.83 ± 1.89 vs. 49.73 ± 0.25 ml). At 4 weeks postoperatively, the capacity decreased to 36.83 ± 1.34 ml (73.88 ± 4.25% of original capacity) in the experimental group and 24.5 ± 1.51 ml (49.17 ± 2.34%) in the control group. At 24 weeks after implantation, the capacity increased to 47.17 ± 1.68 ml (94.68 ± 3.31% of the original capacity) in the experimental group and 34.5 ± 2.81 ml (69.33 ± 5.05% of original capacity) in the control group. Furthermore, cystography revealed the better shape of reconstructed bladders in the experimental group (Fig. 2).
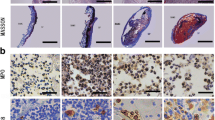
Cystographies of bladders reconstructed 4 weeks postoperatively. a The control group, b the experimental group 24 weeks postoperatively, c the experimental group and d the experimental group. Cystography demonstrated an improvement in both the shape and capacity of bladders reconstructed with seeded matrices
At macroscopic inspection, the unresolvable reference sutures were easily distinguishable at all time points. There were no diverticulum and the reconstructed bladders were covered by soft, vascularized connective tissue at 4 weeks postoperatively. The thickness of the grafted segment in both groups was similar to native bladder tissue after 24 weeks. However, shrinkage of the grafts occurred in many control rabbits, which accounts for the observed decrease in bladder volumes.
All retrieved bladder tissues were studied by routine histology and immunohistochemistry. There were no significant differences in the H&E appearance of the experimental and control tissues at 4 weeks postoperatively. Multilayered urothelium could be observed at implanted sites in each group by 4 weeks. There was evidence of several smooth muscle cells around the graft site. At 24 weeks in the control group, the smooth muscle cells at the periphery of the graft were organized, but still distinguishable from normal bladder tissue. In the experimental group, the bladder graft demonstrated a similar appearance to native bladder tissue histologically as there were organized smooth muscle tissue, neo-angiogenesis and proliferation of neural cells. Furthermore, immunohistochemical staining was positive for α-smooth muscle actin, confirming the presence of well-differentiated smooth muscle cells. The entire urothelial cell layers stained positively with anti-pancytokeratins AEl/AE3. Positive staining for S-100 demonstrates the presence of mature neural cells (Fig. 3).
Histological features of the transplanted grafts. Four weeks postoperatively, native bladder tissue (blue arrow) and in the graft (yellow arrow). a The control group, b the experimental group 24 weeks postoperatively, c in the control group there is no evidence of organized bladder tissue regeneration, d in the experimental group, the grafts had formed a multilayer epithelium with organized smooth muscle cells. Immunohistochemistry of the transplanted grafts. e Staining with cytokeratin AE1/AE3. f α-SM actin. g S-100 (arrows)
Discussion
Tissue engineering offers a viable alternative for functional bladder reconstruction. Atala et al. [1] used autologous, tissue-engineered bladder for cystoplasty in seven patients with myelomeningoceles. Their encouraging results bring new hope for bladder reconstruction.
In previous reports, investigators seeded autologous bladder smooth muscle and urothelial cells onto acellular matrices or synthetic scaffolds. Oberpenning et al. [11] reconstructed functional bladders with PGA meshes seeded with both autologous bladder smooth muscle and urothelial cells in a canine model. Yoo et al. [12] used seeded collagen matrices for bladder reconstruction, also in a canine model. However, such seed cells are difficult to harvest and culture in vitro [13]. In addition, such cells cannot be harvested from patients with invasive bladder cancer or neuropathic bladders. Therefore, alternative cell sources are required for such patients.
Several other alternative cell sources have been investigated, including embryonic stem cells, bone marrow stem cells (BMSCs), muscle-derived cells and adult stem cells from other tissues [14, 15]. Since adult stem cells, which can differentiate into several mature cell types, can be harvested and cultured easily with a high proliferative rate in vitro, they are one of the most popular seed cells for tissue engineering. However, harvest of stem cells is often associated with trauma at the donor site.
Adipose tissue is derived from embryonic mesodermal precursors and contains multipotent progenitor cells capable of differentiating into mesenchymal tissue [16]. Compared to other kinds of adult stem cells, ADSCs can be procured easily. Adipose tissue contains 100–1,000 times more pluripotent cells on a per-cubic centimeter basis than bone marrow [17]. As multipotential cells, they can differentiate into other mature cell types including cardiac [18], neural [19] and smooth muscle [20]. In addition, Rodriguez et al. [21] have demonstrated that ADSCs are immunoprivileged both in vitro and in vivo. They may have potential as immunoprivileged universal donor cells for allogeneic transplant. For these reasons, we selected ADSCs as the source of seed cells in our study.
As ADSCs phenotype similar to fibroblasts, it is difficult to characterize ADSCs based solely on their morphology in culture. In our study, flow cytometry analysis demonstrated that ADSCs from rabbits expressed high levels of stem cell-related antigens (CD90, CD29, CD44, CD34 and CD166), but did not express the CD106 or CD45. Our results were similar to previous reports, which suggested that they are a distinct population of cells of mesenchymal origin [22].
The process by which we decellularize BAMGs resulted in a biomaterial with compositional and structural characteristics similar to native urinary collagenous extracellular matrix. BAMGs contain several growth factors that are not present in synthetic grafts, including fibroblast growth factor, transforming growth factor-b and vascular endothelial growth factor [23], which may promote cellular growth and differentiation. Therefore, we believed that BAMGs may be an ideal matrix for bladder reconstruction in our study.
Since urothelium is associated with high reparative capability, we simplified the experimental procedure by no urothelial cells seeded onto the surface of BAMG. Multilayered urothelium could be observed at the sites of implantation in each group. Thus, these results indicate that the bladder urethelial layer can regenerate independently. Most of the implanted free grafts had a well-developed urothelial layer but an abnormal muscular layer [24]. Our results indicate that the unseeded matrices cannot be used to fully reconstruct the 1.5 × 1.5 cm2 defect in the bladder (representing 40–60% of the total bladder surface area). This finding is in agreement with previous reports in the literature [25].
Bladder volumes were smaller in the control group. We hypothesize that the collagen matrix is likely resorbed over time, as has been reported with other types of collagen matrices, such as SIS [26]. In the experimental group, the compound graft showed similar bladder volume to the normal. We believe that the transplanted cells can help to form a well-organized muscular layer, which can maintain the normal architectural framework of the bladder.
In our study, the merit of ADSC-seeded BAMGs is readily apparent. Nevertheless, the role of ADSCs in smooth muscle regeneration has not yet been elucidated. A fascinating new theory has been advocated that suggests that patterns of adult stem cell differentiation depend on the host niche. For example, ADSCs were found to differentiate into smooth muscle cells when injected into the bladder wall [27]. An alternative theory postulates that seeded adult stem cells simply assist in proliferation of existing smooth muscle cells [28]. In our study, we excised 40–60% of the bladder surface in order to avoid the regeneration of smooth muscle cells from the native bladder tissue. However, cell-labeling techniques may be the best way to identify whether the smooth muscle cells regenerated from ADSCs or native smooth muscle cells. We are presently pursuing this avenue of research.
A neuronal network is crucial to create a tissue engineered bladder with effective function. In the study group, mature neural cells, which stained positively with the S-100 antibody, could be observed in the renconstructed bladder. Since S-100 is a marker for mature neural cells including gliocytes, dendritic cells and Schwann cells, our results suggested that proliferation of these neural cells is likely necessary for recovery of bladder functionality. Growth factors secreted by ADSCs may stimulate neural cell regeneration [29].
In our study, we focused on regeneration of bladder urothelium, smooth muscle and neural cells, which is certainly relevant to recovery of bladder function. However, future directions of research may include functional studies of the regenerating bladder. In addition, studies with long-term follow-up in a larger animal model are also merited.
Conclusions
Adipose-derived stem cells demonstrate good biocompatibility with BAMGs. Our results suggest that seeding BAMGs with ADSCs promotes regeneration of smooth muscle and neural tissue. ADSCs may be an excellent cell sources for tissue engineering.
References
Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB (2006) Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367:1241–1246
Kaefer M, Hendren WH, Bauer SB, Goldenblatt P, Peters CA, Atala A et al (1998) Reservoir calculi a comparison of reservoirs constructed from stomach and other enteric segments. J Urol 160:2187–2190
Chung SY (2006) Bladder tissue-engineering: a new practical solution? Lancet 367:1215–1216
Zhang Y, Kropp BP, Lin HK, Cowan R, Cheng EY (2004) Bladder regeneration with cell-seeded small intestinal submucosa. Tissue Eng 10:181–187
Dozmorov MG, Kropp BP, Hurst RE, Cheng EY, Lin HK (2007) Differentially expressed gene networks in cultured smooth muscle cells from normal and neuropathic bladder. J Smooth Muscle Res 43:55–72
Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE et al (2005) Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 54:132–141
Kim BS, Atala A, Yoo JJ (2008) A collagen matrix derived from bladder can be used to engineer smooth muscle tissue. World J Urol 26:307–314
Probst M, Piechota HJ, Dahiya R, Tanagho EA (2000) Homologous bladder augmentation in dog with the bladder. BJU Int 85:362–371
Fu Q, Deng CL, Liu W, Cao YL (2007) Urethral replacement using epidermal cell-seede tubular acellular bladder collagen matrix. BJU Int 99:331
Jack GS, Zhang R, Lee M et al (2009) Urinary bladder smooth muscle engineered from adipose stem cells and a three dimensional synthetic composite. Biomaterials 30:3259–3270
Oberpenning F, Meng J, Yoo JJ, Atala A (1999) De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol 17:149–155
Yoo JJ, Meng J, Oberpenning F, Atala A (1998) Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology 51:221–225
Becker C, Jakse G (2007) Stem cells for regeneration of urological structure. Eur Urol 51:1217–1228
Zhang Y, Lin HK, Frimberger D, Epstein RB, Kropp BP (2005) Growth of bone marrow stromal cells on small intestinal submucosa: an alternative cell source for tissue engineered bladder. BJU Int 96:1120–1125
Yokoyama T, Huard J, Pruchnic R, Yoshimura N, Qu Z, Cao B et al (2001) Muscle-derived cell transplantation and differentiation into lower urinary tract smooth muscle. Urology 57:826–831
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
Fraser JK, Wulur I, Alfonso Z, Hedrick MH (2006) Fat tissue: an underappreciated source of stem cell for biotechnology. Trends Biotechnol 24:150–154
Rangappa S, Fen C, Lee EH, Bongso A, Sim EK (2003) Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg 75:775–779
Ashjian PH, Elbarbary AS, Edmonds B, DeUgarte D, Zhu M, Zuk PA et al (2003) In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plast Reconstr Surg 111:1922–1931
Rodríguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ (2006) Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. PNAS 103:12167–12172
Rodriguez AM, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne JY, Wdziekonski B et al (2005) Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. Exp. Med 201:1397–1405
Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A et al (2006) Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem cells 24:376–385
Chun SY, Lim GJ, Kwon TG, Kwak EK, Kim BW, Atala A et al (2007) Identification and characterization of bioactive factors in bladder submucosa matrix. Biomaterials 28:4251–4256
Falke G, Caffaratti J, Atala A (2000) Tissue engineering of the bladder. World J Urol 18:36–43
Nuininga JE, van Moerkerk H, Hanssen A, Hulsbergen CA, Oosterwijk-Wakka J, Oosterwijk E et al (2004) A rabbit model to tissue engineer the bladder. Biomaterials 25:1657–1661
Lai JY, Chang PY, Lin JN (2005) Bladder autoaugmentation using various biodegradable scaffolds seeded with autologous smooth muscle cells in a rabbit model. J Pediatr Surg 40:1869–1873
Gregory SJ, Fernando GA, Zhang R, Alfonso ZC, Patricia AZ et al (2005) Processed lipoaspirate cells for tissue engineering of the lower urinary tract: implication for the treatment of stress urinary incontinence and bladder reconstruction. J Urol 174:2041–2045
Shukla D, Box GN, Edwards RA, Tyson DR (2008) Bone marrow stem cells for urologic tissue engineering. World J Urol 26:341–349
Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE et al (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 16:1292–1298
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Wd., Xu, Ym., Feng, C. et al. Bladder reconstruction with adipose-derived stem cell-seeded bladder acellular matrix grafts improve morphology composition. World J Urol 28, 493–498 (2010). https://doi.org/10.1007/s00345-010-0508-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-010-0508-8