Abstract
Transurethral resection of bladder tumours (TURBT) using a wire loop remains the gold-standard treatment for bladder tumours, but it is associated with unacceptably high early recurrence rates after first resection. Improvements to standard resection techniques and a range of optical and technological advances offer exciting possibilities for improving outcomes. Early second resection has been shown to reduce recurrence rates, and increase response to intravesical chemotherapy and/or immunotherapy. It should be considered in most high-risk non-muscle invasive cancers (T1; G3; multifocal) being managed by bladder conservation. Newer energy sources, such as laser, may facilitate day case management of bladder tumours using local anaesthesia in select groups of patients. The novel technique of photodynamic diagnosis improves tumour detection, and quality of resection, and is likely to become the standard for initial tumour management. The traditional ‘incise and scatter’ resection technique goes against all oncological surgical principles. En-bloc resection of tumours would be far preferable and demands further development and evaluation. The technique of TURBT needs to evolve to allow first-time clearance of disease and low recurrence rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transurethral resection of bladder tumours (TURBT) using a wire loop and diathermy is considered the gold-standard treatment for tumour removal and for obtaining tissue for histological analysis. The technique, using a resectoscope that a urologist of today would recognise, was first described by Stern and McCarthy in 1931. In this article, we consider the optimisation of the traditional techniques and discuss potential advances in the transurethral operative management of bladder cancer.
Traditional transurethral resection of bladder tumours (TURBT)
The goal of TURBT is to remove all visible tumour and obtain tissue for histological diagnosis with the minimal morbidity to the patient. A wire loop is used via a 26-28F resectoscope to facilitate continuous irrigation and a clear operative field. The introduction of video endoscopy transformed the ergonomics of the procedure and made teaching the techniques more straightforward. It is important that the tumour and its base are resected separately in all cases and sent for histological analysis in separate pots. This gives the pathologist the best chance of accurately assigning a stage and also emphasises to the surgeon the need to resect detrusor muscle for adequate staging.
Nevertheless, there remains evidence that the quality of TURBT is highly variable: in a meta-analysis of EORTC trials, recurrence rates 3 months post-initial resection varied between 0 and 15% for single tumours, and between 7 and 45% for multiple tumours [1]. Clearly, there is a considerable scope for improving the performance of TURBT.
Recurrence could in theory be due to unresected residual tumour either adjacent to or distant from the dominant tumour; implantation of cells shed during surgery; tumours arising due to a field effect; or due to aggressive tumour biology, i.e., high grade.
Modifications of standard resection techniques
Early re-resection
The limitations of primary TURBT have led many urologists, particularly in Germany, to recommend early re-resection as a standard technique in bladder tumour management. Astonishingly, at re-resection residual tumour rates as high as 75% have been reported [2] but most studies report rates around 25–40% [1, 3–9]. In addition to detecting additional tumour, a second-look TURBT may improve medium term disease prognosis with the 3-year recurrence rates in one series reported to be almost half that seen in patients who had a single resection [10].
Residual tumour is most likely in patients with high-grade tumours, T1 tumours, multifocal tumours, or CIS. Early re-resection can be undertaken at 2–6 weeks in these groups if a bladder preservation strategy is being contemplated [1, 3–9]. Early re-resection is of course mandatory if no detrusor muscle is present in the first specimen to ensure zero staging error; or where the initial TURBT was difficult due to large tumour bulk or bleeding, or where the resection was incomplete.
There is also now data accruing that suggests that patients who undergo early second resection have an improved response to intravesical chemotherapy or immunotherapy with BCG. In a randomised study [11] of patients with intermediate risk T1 tumours Divrik et al. noted that recurrence post six doses of mitomycin was 25% in those who underwent re-resection compared with 63% in those who had a single resection. Clearly intravesical chemotherapy does not compensate for an inadequate resection. Herr [12] in a randomised study noted progression rates in patients treated with BCG for high-risk bladder cancer were 7% in those managed with re-resection compared with 34% in patients who had a single resection. Clearly, intravesical chemotherapy does not compensate for an inadequate resection.
Early re-resection is now a very popular strategy in bladder cancer management but in one sense is an admission of failure of technique. A far better strategy would be to clear the disease at the first operation, and the availability of second-look resection should not be seen by urologists as an invitation to do a poor quality initial operation. Second operations are expensive, potentially morbid, and delay definitive treatment. A urologist who has residual tumour rates approaching 0%, who always takes detrusor muscle in the first specimen, and who derives no new information from the second operation is probably doing the operation correctly the first time.
Equipment
Resectoscope
A novel working element for the resectoscope has recently been described and is currently undergoing clinical evaluation. The device converts the standard in/out linear/axial movement at the handgrip into a side-to-side, bidirectional, lateral rotating motion (Fig. 1). A variety of loops have been designed that facilitate both bladder and prostate tissue resection. Early reports suggest that the device improves the accuracy of resection and may decrease the rates of perforation [13].
Energy source
Bipolar
Bipolar energy has been widely trialled in transurethral resection of the prostate (TURP) [14], and more recently for TURBT [15]. In the case of TURP the main advantage is the reduced rates of TUR syndrome, whereas in TURBT the putative advantages are a reduction in obturator nerve “kicks” and less diathermy artefact in the resected specimen leading to more accurate histology.
Laser
Studies have been performed using both neodyium: YAG and holmium: YAG lasers to both resect and coagulate bladder tumours. The former has a penetration depth of 4–6 mm and is not well suited to resection in the bladder, the latter penetrates to only 0.3–0.4 mm and so is preferable for use in TURBT. The main advantages of holmium resection are: improved haemostasis potentially facilitating catheter-free day case treatment, resection in saline and en-bloc resection [5, 16–18]. Many departments have access to the technology as it is already widely used for TURP and in stone surgery. A disadvantage of holmium treatment is tissue destruction compromising histological reporting of tumour depth.
A valuable potential use for laser in the treatment of bladder cancer is for fulguration of superficial tumours as day cases, especially in those patients in whom formal resection under a general anaesthetic holds significant risk due to patient co-morbidities [19, 20]. The amount of tumour released into the bladder cavity by laser fulguration may be less than by conventional fulguration with diathermy [21].
New techniques
Photodynamic-assisted resection
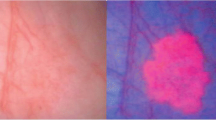
Photodynamic diagnosis (PDD) requires the intravesical instillation of a porphyrin-derived photo sensitizer [5 aminolevulinate (5-ALA) or hexylaminolevulinate (HAL)] to allow the preferential accumulation of the photosensitive metabolite, protoporphyrin IX, in bladder tumour cells. ‘Blue light’ illumination then elicits the emission of red fluorescence from tumour. Colour contrast is improved allowing far easier differentiation of tumour from benign tissue. PDD can improve both the detection of flat CIS and papillary tumours that may be barely visible during standard ‘white light’ cystoscopy. Improved detection of tumour has two potential therapeutic advantages: it might allow, first, more complete resection of tumour, and secondly more appropriate adjuvant treatment. The evidence for the effectiveness of PDD is mounting: in one trial of initial standard TUR versus PDD-assisted TURBT, residual tumour at early ‘white light’ re-resection was 25% in the standard TURBT arm versus 4.5% in the fluorescence-treated arm. This early improvement was maintained in long-term follow-up so that recurrence-free survival at 2 years was 89.6% in the PDD group and 65.9% in the ‘white light’ group [22]. In other trials examining the issue of residual tumour post-TURBT, PDD has been shown to be effective (residual tumour post-PDD 38 vs. 59% post standard TURBT [23]; residual post-PDD 16 vs. 39% post standard TURBT [24]). The benefits of PDD-assisted resection may be greatest in patients with multiple tumours [25].
The published trials of PDD do have limitations: in most, patients with both primary and recurrent tumours are included; in none has TURBT plus one-shot chemotherapy has been used as the control treatment; and many have used 5-ALA as the photosensitizer, whereas the photosensitizer licensed for use in Europe is HAL. The available data suggests that HAL is easier to use than ALA and may improve colour contrast. PDD is less useful in patients who have been treated with intravesical chemotherapy or BCG, and in patients undergoing early re-resection due to false-positive fluorescence secondary to inflammation. PDD is clearly of interest and may well become a standard technique.
En-bloc resection
The basic principle of all oncological surgery is to remove the tumour en-bloc by dissecting through normal tissue to prevent scatter of malignant cells. This principle is completely ignored during conventional TURBT where an ‘incise and scatter’ technique is the norm, showering tumour cells around the bladder. A breast surgeon cutting a breast carcinoma into 20 or so pieces before dispersing them throughout the remaining breast would be sacked, but a bladder cancer surgeon does this every day. Over 100 years ago, Albarran and Imbert postulated that floating tumour cells implanted into the bladder might cause recurrence. Boyd and Burnand’s [26] clinical observations that recurrence commonly occurs in the dome of the bladder supported this as do more modern molecular studies demonstrating multifocal tumours to be monoclonal [27]. Why do urologists continue to cut into bladder tumours?
Several studies have examined the feasibility of en-bloc resection using a variety of techniques. Saito [28] describes a technique using the knife electrode for tumours in the bladder and holmium laser for tumours at the bladder neck, while Ukai et al. [29] used a modified loop that was cut in half and trimmed and bent to form a ‘J loop’. In both studies, the tumour was circumscribed and then sculpted out until muscle was visualised. They both reported no complications and good quality pathology specimens. These studies are attractive in their simplicity, use of existing equipment, clinical safety and adequacy of pathology but have not commented on the essential outcome of recurrence rates.
At Guys hospital, we are very interested in the technique of en-bloc resection. Clearly, it is not possible for every broad-based tumour but with a range of novel instruments it is feasible for many tumours. We have coined the term “sand wedge resection” because of its resemblance to a bunker shot in golf; the sand wedge lifts the ball up on a cushion of sand without the ball itself being struck by the club [30]. En-bloc resection is deserving of more formal evaluation in a trial.
Conclusion
The technique of TURBT needs to evolve. Residual tumour rates and recurrence rates post surgery are unacceptably high. Meticulous attention to good quality initial resection is paramount and should be combined with early re-resection where appropriate. The technology of PDD offers a great potential for improving surgical clearance, and also for better selection of patients for adjuvant therapy. The instruments used to perform TURBT are very limited and modifications in design should lead to higher quality, more complete, surgery.
References
Brausi M, Collette L, Kurth K, van der Meijden AP, Oosterlinck W, Witjes JA et al (2002) Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol 41(5):523–531. doi:10.1016/S0302-2838(02)00068-4
Herr H (1999) The value of a second transurethral resection in evaluating patients with bladder tumours. J Urol 162:74–76. doi:10.1097/00005392-199907000-00018
Schwaibold H, Sivalingam S, May F, Hartung R (2006) The value of a second transurethral resection for T1 bladder cancer. BJU Int 97:1199–1201. doi:10.1111/j.1464-410X.2006.06144.x
Herr H (2005) Surgical factors in the treatment of superficial and invasive bladder cancer. Urol Clin North Am 32:157–164. doi:10.1016/j.ucl.2005.02.003
Holzbierelein JM, Smith JA (2000) Surgical management of noninvasive bladder cancer (stages Ta/T1/CIS). Urol Clin North Am 27(1):15–24. doi:10.1016/S0094-0143(05)70230-5
Soloway M Optimal transurethral resection of bladder tumours. Optimal therapy for patients with high risk superficial bladder cancer—controversy and consensus. MEDICINE Publishing Foundation 1997; Symposium Series 37:31–35
Oosterlinck W, Lobel B, Jaske G et al (2002) The European Association of Urology (EAU) Working Group on Oncological Urology. Guidelines on bladder cancer. Eur Urol 41:105–112. doi:10.1016/S0302-2838(01)00026-4
Nieder A, Brausi M, Lamm D et al (2005) Management of stage T1 tumours of the bladder: international consensus panel. Urology 66:108–125. doi:10.1016/j.urology.2005.08.066
Sedelaar J, Witjes A (2007) Technique of TUR of bladder tumours: value of repeat TUR and random biopsies. Eur Urol Suppl 5:139–144
Grimm M, Ackermann R, Vogeli T (2003) Effect of routine repeat transurethral resection for superficial bladder cancer: a long term observational study. J Urol 170:433. doi:10.1097/01.ju.0000070437.14275.e0
Divrik RT, Yildirim U, Zorlu F, Ozen H (2006) The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumours of the bladder who received intravesical mitomycin: a prospective, randomised clinical trial. J Urol 175(5):1641–1644. doi:10.1016/S0022-5347(05)01002-5
Herr HW (2005) Restaging transurethral resection of high risk superficial bladder cancer improves the initial response to bacillus Calmette–Guerin therapy. J Urol 174(6):2134–2137. doi:10.1097/01.ju.0000181799.81119.fc
Pantuck A, Baniel J, Kirkali Z et al (2007) A novel resectoscope for transurethral resection of bladder tumours and the prostate. J Urol 178:2331–2336. doi:10.1016/j.juro.2007.08.042
Ho H, Cheng C (2008) Bipolar transurethral resection of prostate: a new reference standard? Curr Opin Urol 18:50–55
Wang DS, Bird V, Leonard V et al (2004) Use of bipolar energy for transurethral resection of bladder tumours: pathological considerations. J Endourol 18:578–582
Fraundoerfer M, Cresswell M, Gilling P, Kabalin J (1997) Bladder tumour resection with the holmium laser. BJU Int 80(Suppl 2):39
Das A, Gilling P, Fraundorfer M (1998) Holmium laser resection of bladder tumours (HoLRBT). Tech Urol 4:12–14
Muraro G, Grifoni R, Spazzafumo L (2005) Endoscopic therapy of superficial bladder cancer in high-risk patients: holmium laser versus transurethral resection. Surg Technol Int 14:222–226
Morten J, Lund L, Bisballe S (2004) Holmium: YAG laser vaporisation of recurrent papillary tumours if the bladder under local anaesthesia. BJU Int 94:322–325. doi:10.1111/j.1464-410X.2004.04882.x
Syed H, Biyani C, Bryan N, Brough S, Powell C (2001) Holmium: YAG laser treatment of recurrent superficial bladder carcinoma: initial clinical experience. J Endourol 15:625–627. doi:10.1089/089277901750426427
See WA, Chapman WH (1987) Tumour cell implantation following neodymium-YAG bladder injury: a comparison to electrocautery injury. J Urol 137(6):1266–1269
Filbeck T, Pichlmeier U, Knuechel R, Wieland WF, Roessler W (2002) Clinically relevant improvement of recurrence-free survival with 5-aminolevulinic acid induced fluorescence diagnosis in patients with superficial bladder tumors. J Urol 168(1):67–71. doi:10.1016/S0022-5347(05)64833-1
Kriegmair M, Zaak D, Stepp H, Stepp H, Baumgartner R, Knuechel R et al (1999) Transurethral resection and surveillance of bladder cancer supported by 5-aminolevulinic acid-induced fluorescence endoscopy. Eur Urol 36(5):386–392. doi:10.1159/000020019
Riedl CR, Daniltchenko D, Koenig F, Simak R, Loening SA, Pflueger H (2001) Fluorescence endoscopy with 5-aminolevulinic acid reduces early recurrence rate in superficial bladder cancer. J Urol 165(4):1121–1123
Babjuk M, Soukup V, Petrik R, Jirsa M, Dvoracek J (2005) 5-aminolaevulinic acid-induced fluorescence cystoscopy during transurethral resection reduces the risk of recurrence in stage Ta/T1 bladder cancer. BJU Int 96(6):798–802. doi:10.1111/j.1464-410X.2004.05715.x
Boyd P, Burnand K (1974) Site of bladder tumour recurrence. Lancet 2:1290–1292. doi:10.1016/S0140-6736(74)90145-7
Hafner C, Knuechel R, Zanardo L et al (2001) Evidence for oligoclonality and tumour spread by intraluminal seeding in multifocal urothelial carcinomas of the upper and lower urinary tract. Oncogene 20:4910–4915. doi:10.1038/sj.onc.1204671
Saito S (2001) Transurethral en bloc resection of bladder tumours. J Urol 166:2148–2150. doi:10.1016/S0022-5347(05)65523-1
Ukai R, Kawashita E, Ikeda H (2000) A new technique for transurethral resection of superficial bladder tumour in 1 piece. J Urol 163:878–879. doi:10.1016/S0022-5347(05)67824-X
Ray E, O’Brien TS (2007) Should urologists be spending more time on the golf course? BJU Int 100:728–729. doi:10.1111/j.1464-410X.2007.06876.x
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilby, D., Thomas, K., Ray, E. et al. Bladder cancer: new TUR techniques. World J Urol 27, 309–312 (2009). https://doi.org/10.1007/s00345-009-0398-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-009-0398-9