Abstract
To date, there is an increasing interest in the nitric oxide (NO) pathway as a potential pharmacological target to treat male lower urinary tract symptomatology (LUTS). In the transition zone of the human prostate, a dense nitrinergic innervation has been shown of the fibromuscular stroma, glandular epithelium and blood vessels. The expression of key proteins of the NO pathway, such as the endothelial and neuronal nitric oxide synthase (eNOS, nNOS), cGMP-degrading phosphodiesterase type 5 (PDE5) and cGMP-binding protein kinase (cGK), has also been demonstrated. The hypothesis that an impaired NO/cGMP-signaling may contribute to the pathophysiology of benign prostatic hyperplasia (BPH) is supported by the results from randomized, placebo-controlled clinical studies, indicating that NO donor drugs and PDE5-inhibitors sildenafil, tadalafil and vardenafil may be useful to treat storage and voiding dysfunctions resulting from LUTS in men. Thus, given a potential role of the NO-pathway in the prostate and/or in other parts of lower urinary tract (e.g. bladder), the enhancement of the NO signaling by NO donor drugs, PDE5 inhibitors or activators of the soluble guanylyl cyclase (sGC) may represent a new therapeutic strategy for the treatment of LUTS. This review serves to focus on the role of NO and the NO-dependent signaling in the control of smooth muscle function in the human prostate. Results from clinical trials in men with LUTS/BPH are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
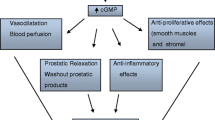
In the past years, NO has been identified as an important non-adrenergic, non-cholinergic neurotransmitter in the lower urinary tract, acting alongside the classical adrenergic and cholinergic systems [1, 2]. To date, the pivotal role for NO in the relaxation of male penile erectile tissue (corpus cavernosum) has been widely accepted [3]. NO has also been suggested to be involved in the micturition process, namely by inhibiting neurotransmission in the urethra and by modulating bladder afferent nerves and related reflex pathways in the spinal cord [4]. Detailed information has also been accumulated on the physiological role of NO in the control of the prostate function in mammals and men, including the regulation of prostate smooth muscle tone, glandular secretory function and local blood flow [5, 6]. NO is synthesized from the amino acid l-arginine by a family of enzymes known as NO synthases (NOS). Three distinct isoforms of NOS have been identified, which were originally named after the tissues in which they were first described [7]: two Ca2+/calmodulin-dependent, constitutive isoforms, the neuronal NOS (nNOS) and endothelial NOS (eNOS), and a Ca2+-independent, inducible NOS (iNOS), which is mainly expressed in macrophages and other tissues following an immunological stimulus. After it has been released from nerve endings or the endothelium, NO diffuses the smooth muscle cell and stimulates the synthesis and accumulation of the second messenger molecule cyclic guanosine monophosphate (cGMP) by the activity of the soluble guanylyl cyclase (sGC). cGMP then phosphorylates specific down-stream targets, such as the protein kinase G (cGK), ion channels and cGMP-binding phosphodiesterases (PDEs), thus leading to the relaxation of smooth muscle cells via the depletion of intracellular (cytosolic) Ca2+ and desensitization of contractile proteins towards Ca2+ [8]. Cyclic GMP is degraded by the activity of PDE isoenzymes catalyzing the hydrolysis of cGMP to the inactive form 5′GMP [9]. The aim of this review is to summarize the current knowledge on the significance of NO and key proteins of the NO pathway, such as the eNOS, nNOS, cGMP-degrading PDE isoenzymes and cGK, in the control of smooth muscle function in the human prostate.
Isoforms of nitric oxide synthase in the prostate
The presence of NOS has been demonstrated in the human prostate by means of biochemical, immunohistochemical and molecular biology methods. Various investigations have been conducted mainly to assess the immunohistochemical distribution of NOS in the human prostate. Burnett et al. [10] reported the localization of nNOS in nerve fibers and ganglia interspersing the transition zone and observed also signals related to the glandular epithelium and subepithelial nerve plexus. Bloch et al. [11], using specific antibodies, evaluated the expression and distribution of nNOS, eNOS, and the nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d), which is considered as a non-specific immunohistochemical marker for NOS. While they detected eNOS in endothelial cells of small vessels supplying the prostate and nNOS in nerve fibers transversing the fibromuscular stroma, they were unable to relate the staining for NADPH-d, observed in the glandular epithelium, to either nNOS or eNOS. The authors concluded that this staining might represent an unidentified isoform of NOS. Sjöstrand et al. [12], who investigated the co-localization of NADPH-d with acetylcholine esterase in the human urogenital tract, also demonstrated NADPH-d activity in the glandular epithelium of the prostate without attempting to correlate this staining to nNOS and eNOS. Hedlund et al. [13] reported NOS-positive nerve terminals in close relation to stromal smooth muscle cells and glandular structures, these signals were well in accordance to the localization of NADPH-d. Nevertheless, in contrast to the findings presented by Burnett et al. and Bloch et al., they did not observe the expression of nNOS in the glandular epithelium. Later, Richter et al. [14], using specific antibodies against eNOS and nNOS in combination with advanced fixation and staining procedures, re-examined the distribution of NOS in the transition zone of the human prostate. They observed signals related to eNOS in endothelial cells of small vessels supplying glandular structures. Interestingly, while the glandular epithelium omitted staining for eNOS, basal cells presented immunoreactivity specific for nNOS in their cytoplasm. Gradini et al. [15], using an immunohistochemical and molecular biology approach (RT-PCR), reported the expression of eNOS in the basal epithelial cells in sections of normal and hyperplastic prostate tissue while nNOS was mainly expressed in the secretory layer of the glandular epithelium. From their findings, the authors suggested a role for eNOS/NO in the control of glandular epithelial cell proliferation.
Bloch et al. [11], using tissue from obstructive patients with bladder outlet obstruction due to BPH, found a reduction in the density of the NADPH-d reaction in nerve fibers when compared to normal prostate tissue. Thus, they speculated that an impairment of the local nitrinergic innervation contributes to the dynamic component of LUTS/BPH. This hypothesis is supported by results from comparative mRNA analysis presenting evidence that the translation/expression of the NOS gene is lower in prostate specimens resected by means of TURP from patients with BPH than in tissue excised from normal prostates. The reduction in the expression of NOS positively correlated with older age (71 ± 7 years vs. 53 ± 7 years) and a greater prostate volume (131 ± 71 gr. vs. 48 ± 11 gr.) [16]. Interestingly, in vitro studies using prostate strips isolated from old rabbits revealed a significant reduction in the NO-mediated relaxation of the tissue when compared with strips from young animals [17]. Until today, the inducible form of NOS, the iNOS, has not been found in normal prostate tissue, however, there are hints that the enzyme is expressed in hyperplastic and malignant tissue [18, 19]. Given the findings mentioned above, one can assume that in the human prostate, the eNOS is related to the maintenance of local vascular perfusion, whereas the nNOS is mainly involved in the control of smooth muscle tone and glandular function, including proliferation of epithelial and subepithelial cells.
Phosphodiesterases and protein kinase G1 (cGK1)
It is assumed that the binding to and activation of cGMP-dependent protein kinase (cGK) is one crucial step in the mechanism mediating the biological actions of cGMP [8]. The major subtype of cGK is the type I of which two isoforms exist: type Iα and Iβ [20]. Only two studies have addressed the expression and localization of isoforms of cGKI in the human prostate: Haynes et al. [21], using molecular biology techniques, detected mRNA transcripts encoding for both isoforms in human prostate tissue. Waldkirch et al. [22] were the first who described by means of immunohistochemistry, the distribution of both cGKIα and Iβ in stromal areas of the transition zone, where the enzymes were found co-localized with its main effector, cGMP. As of today, the clinical significance of the cGKI in the control of prostate function remains to be elucidated.
The degradation of cGMP is mediated by phosphodiesterases, a heterogenous group of hydrolytic enzymes. To date, the existence of 11 PDE families has been established. RT-PCR analysis revealed the expression of mRNA transcripts specifically encoding for PDE1, PDE2, PDE4, PDE5, PDE7, PDE8, PDE9 and PDE10 in the different anatomical regions of the human prostate. Using anion exchange chromatography, the hydrolytic activities of PDE4 (cAMP-PDE) and PDE5 (cGMP-PDE) have been identified in cytosolic fractions of prostate tissue isolated from the transition zone [23]. Immunohistochemical studies demonstrated that PDE4 is abundantly present in the fibromuscular stroma and glandular epithelium of the transition zone, whereas PDE5 and so-called dual substrate PDE11 (cAMP-/cGMP-PDE) are mainly associated with glandular epithelial and subepithelial layers. The stainings revealed no immunoreactivity specific for PDE3 (cGMP-inhibited PDE) [24]. Taken together, the presence of function proteins and mediators of the NO pathway, cGKI, cGMP, PDE5 and PDE11, in the transition zone strongly implicates a role for this transduction system in the control of prostate function.
In vitro effects of NO and PDE-inhibitors on isolated prostate tissue and on intracellular levels of cyclic GMP
Numerous in vitro studies have elucidated the effects of endogenous agents known to stimulate the NO-cGMP cascade on the tension of prostate smooth muscle. Takeda et al. [25] demonstrated that electrical field stimulation (EFS) of strip-shaped isolated prostate tissue in the presence of inhibitors of the sympathetic and parasympathetic pathway induced a weak biphasic response consisting of an initial relaxation and subsequent small contraction. The contractile response was significantly increased by the NOS inhibitor l-NAME, indicating the release of a nitrinergic transmitter during EFS. The effect of l-NAME was reversed by l-arginine, known as the major substrate of NOS. In their experiments, the NO donor drug sodium nitroprusside (SNP) induced a significant relaxation of spontaneous baseline tension expressed as grams of tension per cross-sectional area of tissue strips. Similar results were described by Hedlund et al. (1997) [13]. They reported that phasic relaxation of isolated human prostate strip preparations induced by means of EFS was significantly attenuated after pre-treatment of the tissue with the NOS inhibitor l-NOARG. The impact of NO donor drugs, such as SNP, linsidomine (SIN-1), S-nitrosoglutathione (GSNO) and S-nitrosocysteine (SNC), on the tension induced by the alpha-adrenoceptor agonist norepinephrine (NE) or the vasoconstrictory peptide endothelin-1 (ET-1) on isolated human prostate tissue was also investigated. The initial tension of the strips induced by the agonists were dose-dependently reversed by the drugs to a degree of 35–50% [26, 27]. Exposure of isolated human prostate tissue to PDE5 inhibitors also produced a relaxation of the tension induced by NE or ET-1. The reversion of tension was determined within a range from 20 to 55%, these effects were paralleled by a significant enhancement in tissue levels of cGMP [28, 29]. Presumably, in in vivo systems, where the turn-over of NO and cGMP is much higher, PDE5 inhibitors might tend to be much more effective. It has been demonstrated by Haynes et al. [21] that compounds such as PET-cGMP (β-phenyl-1,N2-bromoguanosine-3′5′-cyclic monophosphate) and APT-cGMP (8-[2-aminophenylthio]guanosine-3′,5′-cyclic monophosphate) acting as activators of the cGMP-dependent protein kinase I (cGKI), can also reverse the tension of prostate tissue induced by phenylephrine (PE). Interestingly, they also observed that the relaxation induced by the adenylyl cyclase activator forskolin was equivocal to those induced by SNP and the cGKI activator PET-cGMP. In contrast, Sp-8-bromo-cAMP, an activator of the cAMP-dependent protein kinase A (cAK), did not significantly reduce the tension induced by PE. These findings give rise to the speculation that relaxation of prostate tissue mediated by cAMP may involve the activation of cGKI. This is well in accordance with the hypothesis that a cross-talk between the cGMP- and cAMP-dependent pathways may occur in urogenital tissues [30].
Soluble guanylyl cyclase (sGC) is considered the most important receptor for the signaling molecule NO. Thus, this enzyme represents another potential downstream target for the pharmacological stimulation of the NO/cGMP-signaling. Currently, drugs are available combining the properties of an activator of the sGC (via the release of NO) with those of a PDE5 inhibitor [31, 32]. However, the functional effect of this class of drugs on the human prostate has not yet been examined.
Effects of drugs interfering with the NO pathway in patients with LUTS associated with BPH
The role of the NO-mediated pathway in the treatment of symptoms of LUTS/BPH has already been addressed by some open-label studies and, more recently, three placebo-controlled clinical trials. Klotz et al. [33] were the first who investigated in an open-label study the effects of an orally administered nitrate (isosorbide dinitrate 60–120 mg/day) on the micturition function in 32 patients with ischemic heart disease. Fifteen of these patients concomitantly suffered from untreated, moderate to severe LUTS with a mean International Prostate Symptom Score (IPSS) of 18.2 ± 3.6, a peak flow rate (Q max) of 9.8 ± 2.4 and a postvoidal residual urine (PVR) of 70 ± 20 ml. After 12 weeks of treatment, an improvement of symptoms was reported in 10 of 15 patients. Mean IPSS (−7.3 ± 4.2) and PVR (−20 ml) had significantly decreased while Q max had increased by +3.0 ml/s. No significant changes in micturition parameters were found in the asymptomatic patients.
Sairam et al. [34], in an open-label study, examined the effects of the PDE5 inhibitor sildenafil (VIAGRA™) in patients presenting with ED and LUTS. From the 112 patients enrolled in the study, 20 subjects complained of LUTS, from these, 32% had moderate to severe symptoms (IPSS > 7). After 12 weeks of treatment with sildenafil, there was an overall improvement in the IPSS and LUTS-specific quality of life (QoL) score. All patients who had severe LUTS showed a moderate improvement of the disease, 60% of those who initially presented with moderate LUTS showed only a mild symptomatology. The authors concluded that treatment with sildenafil appears to improve urinary symptom scores. Using a similar protocol, Mulhall et al. [35] also assessed the impact of sildenafil in 48 patients with ED and LUTS. Thirty-two men (68%) with an IPSS >10 were enrolled and treated with sildenafil (100 mg on demand for 12 weeks, mean drug intake: 2 ± 0.6 times/week). Urinary symptom scores were improved in 63% of the patients, 37% reported an improvement of at least five points in the IPSS-score, which was associated with an improvement in QoL (mean improvement: 1.4 points). These results were confirmed by Ying et al. [36], who also reported changes from baseline in IPSS in 32 men with ED and LUTS who had received sildenafil for at least 24 weeks.
In a pilot study, Kaplan et al. [37] examined the efficacy and safety of combining sildenafil and the alpha1-adrenoceptor antagonist alfuzosin in men suffering from LUTS/BPH and ED. Sixty-two men (mean age 63 years) with previously untreated LUTS/BPH/ED were randomized to alfuzosin (10 mg/day), sildenafil (25 mg/day) or the combination of both drugs. Changes from baseline in IPSS, voiding diary, Q max, PVR, and the International Index of Erectile Function (IIEF) were assessed after 12 weeks of treatment. IPSS significantly improved in all treatment groups. When compared to those groups who were on alfuzosin (−16%) or sildenafil (−17%), the mean change in IPSS was greater in the group who received both drugs (−24%). Significant reductions in frequency and nocturia were observed in men who received alfuzosin or both alfuzosin and sildenafil. These patients also demonstrated significant changes in Q max (alfuzosin alone: +1.1 ml/s, alfuzosin and sildenafil: +2.0 ml/s) and PVR (alfuzosin: −23 ml, alfuzosin and sildenafil: −21 ml, baseline PVR volume 50 ml). No significant improvement was noted in the sildenafil group. The authors concluded that the combination of sildenafil with alfuzosin is safe and effective to improve both voiding and sexual function in men with LUTS and ED. McVary et al. [38] assessed in a randomized, double-blind, placebo-controlled trial, the effects of sildenafil given for 12 weeks in 366 men suffering from ED (IIEF ≤25) and LUTS secondary to BPH (IPSS ≥12). Patients received 50 mg sildenafil at bed time or up to 1 h before sexual activity for at least 2 weeks, followed by 100 mg sildenafil once daily for 10 weeks. Primary outcome measures were changes in the erectile function domain of the IIEF, secondary outcome measures were changes in all other domains of the IIEF and IPSS including QoL, BPH Impact Index (BPH II) and Q max. Sildenafil significantly improved LUTS, mean IPSS decreased by 6.3 points versus 1.93 in the placebo group. Interestingly, patients with severe LUTS (−8.6 ± − 2.4) experienced greater improvement in IPSS than those with moderate LUTS (−3.6 ± − 1.7). A significant reduction of bladder storage symptoms and an increase in the BPH II and QoL were also noted in the treatment arm while no significant changes in Q max were registered. From the later findings, the authors concluded that extraprostatic pathophysiological mechanisms related to an impairment of the activity of the NO-system might be involved in the etiology of LUTS. The efficacy and safety of tadalafil (CIALIS™) were also investigated in a randomized, double-blind, placebo-controlled study in men with moderate to severe LUTS, secondary to BPH. Following a 4-week placebo run-in phase, 281 men were randomized to 5 mg tadalafil for 6 weeks, followed by dose escalation to 20 mg for another 6 weeks or placebo for 12 weeks. Primary end point was the change in IPSS after 6 and 12 weeks of treatment. Secondary efficacy endpoints included patient IPSS, QoL, BPH II and the LUTS Global Assessment Question (GAQ, Has the treatment you have been taking since your last visit improved your urinary symptoms?), as well as parameters from uroflowmetric measurements (Q max). After 6 weeks and 12 weeks, tadalafil showed significant improvement in those with LUTS, the respective mean change from baseline in IPSS was −2.8 in tadalfil group versus −1.2 in the placebo group (6 weeks) and −3.8 (verum) versus −1.7 (placebo). Mean subscores related to irritative and obstructive symptoms also significantly improved in patients who had received tadalafil. Except for BPH II, after 6 weeks, all assessments of the disease-related QoL had significantly improved after treatment with tadalafil. In contrast, no significant changes in uroflowmetric values were observed. The authors concluded that tadalafil once daily is well tolerated with clinically meaningful and significant symptomatic improvement of LUTS associated with BPH [39].
In an 8-week randomized, double-blind, placebo-controlled, multi-centre study Stief et al. [40] examined the efficacy of vardenafil (LEVITRA™) in a cohort of 222 men (aged 45–64 years) presenting with LUTS/BPH (IPSS ≥12). Patients were randomized either to vardenafil (10 mg twice daily) or placebo. Efficacy outcome included changes in peak urinary flow rate, as well as scores from the IPSS and UROLIFE QoL 9 (a nine-item BPH-specific quality-of-life questionnaire). After treatment, a decrease of 5.9 points in the IPSS was observed in the vardenafil group versus 3.6 points in the placebo group. Significant changes in the IPSS sub-scores for storage and voiding symptoms and an improvement in the UROLIFE QoL were also noted in the vardenafil group. Since baseline values were already close to normal, Q max and PVR volume did not change significantly with treatment. The authors concluded that vardenafil might offer a valuable new option for the treatment of LUTS associated with BPH.
The clinical efficacy of NO donor drugs and PDE5-inhibitors on male LUTS might be explained by their potential relaxant effect on the smooth muscle of the prostate, urethra and bladder/bladder neck, as well as on pelvic vessels supplying the prostate and bladder [41–44]. Based on preliminary data derived from in vitro studies on human and animal tissues, the chronic administration of NO donor drugs and PDE5-inhibitors may also induce anti-proliferative and/or apoptotic effects in the prostate, thus corresponding with a clinical efficacy in terms of progression of LUTS [45–47]. The lack of effect of sildenafil and tadalafil on mean Q max might be explained by the following arguments: (1) theoretically, the majority of patients were not obstructive at the time of their enrolment into the respective protocols. This is supported by the results from pressure-flow studies demonstrating that approximately 40% of men with LUTS/BPH do not have BOO [48, 49]. (2) Patients may have had a low density of prostate smooth muscle (PSM). This hypothesis is in accordance with the results from a study by Shapiro et al. [50] demonstrating that the increase in Q max induced by the alpha-adrenoceptor antagonist terazosin significantly correlated with the PSM density. Table 1.
Conclusion
The importance of the NO/cyclic GMP-pathway in the control of PSM function has been demonstrated and a link is suggested between an impairment of the local nitrinergic innervation and on-set and development of BPH/LUTS. There is evidence from preliminary clinical studies that NO donor drugs and PDE5-inhibitors, such as sildenafil (VIAGRA™), tadalafil (CIALIS™) and vardenafil (LEVITRA™), assumed to act by relaxing prostate or/and bladder smooth muscle, can improve male LUTS. Thus, said compounds as well as other members of this growing family of drugs, such as avanafil, udenafil (DA 8159) and lodenafil, which are currently in the phase of clinical development, might represent useful pharmacological tools for the treatment of storage and voiding dysfunctions secondary to BPS [50, 51, 52].
References
Moncada S, Palmer RMJ, Higgs EA (1991) Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev 43:109–142
Andersson KE, Persson K (1994) Nitric oxide synthase and nitric oxide-mediated effects in lower urinary tract smooth muscles. World J Urol 12:274–280
Andersson KE (2001) Pharmacology of penile erection. Pharmacol Rev 53:417–450
Andersson KE, Persson K (1995) Nitric oxide synthase and the lower urinary tract: possible implications for physiology and pathophysiology. Scand J Urol Nephrol 175(Suppl):43–53
Hedlund P (2005) Nitric oxide/cGMP-mediated effects in the outflow region of the lower urinary tract - is there a basis for pharmacological targeting of cGMP? World J Urol 23:362–367
Andersson KE (2007) LUTS treatment: future treatment options. Neurourol Urodyn 26:928–933
Förstermann U, Closs EI, Pollack JS, Nakane M, Schwarz P, Gath I, Kleinert H (1994) Nitric oxide synthase isoenzymes: characterization, purification, molecular cloning and functions. Hypertension 23:1112–1131
Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP (2000) Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol 184:409–420
Ückert S, Hedlund P, Andersson KE, Truss MC, Jonas U, Stief CG (2006) Update on phosphodiesterase (PDE) isoenzymes as pharmacological targets in urology: present and future. Eur Urol 50:1194–1207
Burnett AL, Maguire MP, Chamness SL, Ricker DD, Takeda M, Lepor H, Chang TSK (1995) Characterization and localization of nitric oxide synthase in the human prostate. Urology 45:435–439
Bloch W, Klotz T, Loch C, Schmidt G, Engelmann U, Addicks K (1997) Distribution of nitric oxide synthase implies a regulation of circulation, smooth muscle tone, and secretory function in the human prostate by nitric oxide. Prostate 33:1–8
Sjöstrand NO, Ehren I, Eldh J, Wiklund NP (1998) NADPH-diaphorase in glandular cells and nerves and its relation to acetylcholinesterase-positive nerves in the male reproductive tract of man and guinea-pig. Urol Res 26:181–188
Hedlund P, Ekstrom P, Larsson B, Alm P, Andersson KE (1997) Heme oxygenase and NO-synthase in the human prostate - relation to adrenergic, cholinergic and peptide-containing nerves. J Auton Nerv Syst 63:115–126
Richter K, Heuer O, Ückert S, Stief CG, Jonas U, Wolf G (2004) Immunocytochemical distribution of nitric oxide synthases in the human prostate. J Urol 171(Suppl 4):347 (abstract)
Gradini R, Realacci M, Ginepri A, Naso G, Santangelo C, Cela O, Sale P, Berardi A, Petrangeli E, Gallucci M, di Silverio F, Russo MA (1999) Nitric oxide synthases in normal and benign hyperplastic human prostate: immunohistochemistry and molecular biology. J Pathol 189:224–229
Luo J, Dunn T, Ewing C, Sauvageot J, Chen Y, Trent J, Isaacs W (2002) Gene expression signature of benign prostatic hyperplasia revealed by cDNA microarray analysis. Prostate 51:189–200
Aikawa K, Yokota T, Okamura H, Yamaguchi O (2001) Endogenous nitric oxide-mediated relaxation and nitrinergic innervation in the rabbit prostate: the changes with aging. Prostate 48:40–46
Klotz T, Bloch W, Volberg C, Engelmann U, Addicks K (1998) Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer 82:1897–1903
Baltaci S, Orhan D, Gögüs C, Türkölmez K, Tulunay Ö, Gögüs O (2001) Inducible nitric oxide synthase expression in benign prostatic hyperplasia, low- and high-grade prostatic intraepithelial neoplasia and prostatic carcinoma. BJU Int 88:100–103
Vaandrager AB, de Jonge HR (1996) Signalling by cGMP-dependent protein kinases. Mol Cell Biochem 157:23–30
Haynes JM, Cook ALM (2006) Protein kinase G-induced activation of KATP channels reduces contractility of human prostate tissue. Prostate 66:377–385
Waldkirch ES, Ückert S, Langnäse K, Richter K, Jonas U, Wolf G, Andersson KE, Stief CG, Hedlund P (2007) Immunohistochemical distribution of cyclic GMP-dependent protein kinase-1 in human prostate tissue. Eur Urol 52:495–502
Ückert S, Küthe A, Jonas U, Stief CG (2001) Characterization and functional relevance of cyclic nucleotide phosphodiesterase isoenzymes of the human prostate. J Urol 166:2484–2490
Ückert S, Oelke M, Stief CG, Andersson KE, Jonas U, Hedlund P (2006) Immunohistochemical distribution of cAMP- and cGMP-phosphodiesterase (PDE) isoenzymes in the human prostate. Eur Urol 49:740–745
Takeda M, Tang R, Shapiro E, Burnett AL, Lepor H (1995) Effects of nitric oxide on human and canine prostates. Urology 45:440–446
Kedia G, Ückert S, Scheller F, Chigogidze T, Managadze L, Jonas U, Truss MC (2006) In vitro functional responses of isolated normal human prostatic tissue to compounds interacting with the cyclic guanosine monophosphate pathway. Urology 67:1292–1297
Kedia G, Ückert S, Kedia M, Truss MC, Chigogidze T, Jonas U, Managadze L (2006) In vitro effects of drugs interfering with the cAMP- and cGMP-pathway on the tension induced by endothelin-1 of isolated human prostate tissue. Georgian Med News 131:7–13
Ückert S, Sormes M, Kedia G, Gratzke C, Stief CG, Scheller F, Jonas U (2006) Effects of PDE inhibitors on the tension induced by norepinephrine and accumulation of cyclic nucleotides in isolated human prostate tissue. Urologe 45(Suppl 1):25–26 (abstract)
Kedia G, Sormes M, Ückert S, Scheller F, Jonas U (2007) Contraction-relaxation studies on isolated human prostate tissue: the role of endothelin 1, phosphodiesterase inhibitors and cyclic nucleotides. Eur Urol 6(Suppl 2):34 (Abstract)
Stief CG, Ückert S, Becker AJ, Harringer W, Truss MC, Forssmann WG, Jonas U (2000) Effects of sildenafil on cAMP and cGMP levels in isolated human cavernous and cardiac tissue. Urology 55:146–150
Friebe A, Müllershausen F, Smolenski A, Walter U, Schultz G, Koesling D (1998) YC-1 potentiates nitric oxide- and carbon monoxide-induced GMP effects in human platelets. Mol Pharmacol 54:962–967
Müllershausen F, Russwurm M, Friebe A, Koesling D (2004) Inhibition of phosphodiesterase type 5 by the activator of nitric oxide-sensitive guanylyl cyclase BAY 41–2272. Circulation 109:1711–1713
Klotz T, Mathers MJ, Bloch W, Nayal W, Engelmann U (1999) Nitric oxide based influence of nitrates on micturition in patients with benign prostatic hyperplasia. Int Urol Nephrol 31:335–341
Sairam K, Kulinskaya E, McNicholas TA, Boustead GB, Hanbury DC (2002) Sildenafil influences lower urinary tract symptoms. BJU Int 90:836–839
Mulhall JP, Guhring P, Parker M, Hopps C (2006) Assessment of the impact of sildenafil citrate on lower urinary tract symptoms in men with erectile dysfunction. J Sex Med 3:662–667
Ying J, Yao D, Jiang Y, Ren X, Xu M (2004) The positive effect of sildenafil on LUTS from BPH while treating ED. Zhonghua Nan Ke Xue 10:681–683
Kaplan SA, Gonzalez RR, Te AE (2007) Combination of alfuzosin and sildenafil is superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. Eur Urol 51:1717–1723
McVary KT, Monnig W, Camps JL, Young JM, Tseng LJ, van den Ende G (2007) Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol 177:1071–1077
McVary KT, Roehrborn CG, Kaminetsky JC, Auerbach SM, Wachs B, Young JM, Esler A, Sides GD, Denes BS (2007) Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol 177:1401–1407
Stief CG, Porst H, Neuser D, Beneke M, Ulbrich E (2008) A randomised, placebo-controlled study to assess the efficacy of twice-daily vardenafil in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol. 53:1236–1244
Tinel H, Stelte-Ludwig B, Hütter J, Sandner P (2006) Pre-clinical evidence for the use of phosphodiesterase-5 inhibitors for treating benign prostatic hyperplasia and lower urinary tract symptoms. BJU Int 98:1259–1263
Filippi S, Morelli A, Sandner P, Fibbi B, Mancina R, Marini M, Gacci M, Vignozzi L, Vanneli GB, Carini M, Forti G, Maggi M (2007) Characterization and functional role of androgen-dependent PDE5 activity in the bladder. Endocrinology 148:1019–1029
Yono M, Yamamoto Y, Yoshida M, Ueda S, Latifpour J (2007) Effects of doxazosin on blood flow and mRNA expression of nitric oxide synthase in the spontaneously hypertensive rat genitourinary tract. Life Sci 81:218–222
Pinggera GM, Schuster A, Frauscher F, Bartsch G, Strasser H (2004) Sildenafil citrate causes a 3-fold increase in periurethral prostatic blood flow. J Urol 171(Suppl 4):355 (abstract)
Guh JH, Hwang TL, Ko FN, Chueh SC, Lai MK, Teng CM (1998) Antiproliferative effect in human prostatic smooth muscle cells by nitric oxide donor. Mol Pharmacol 53:467–474
Adolfsson PI, Ahlstrand C, Varenhorst E, Svensson SP (2002) Lysophosphatidic acid stimulates proliferation of cultured muscle cells from human BPH tissue: sildenafil and papaverine generate inhibition. Prostate 51:50–58
Deng CH, Chen HR, Qiu SP, Liu JZ, Zheng KL, Mei H (2004) Effect of nitric oxide donor and alpha1-receptor antagonist on proliferation/apoptosis of hyperplastic prostatic stromal cells in vitro. Zhonghua Wai Ke Za Zhi 42:201–204
Reynard JM, Yang Q, Donovan JL, Peters TJ, Schäfer W, de la Rosette JJ, Dabhoiwala NF, Osawa D, Lim AT, Abrams P (1998) The ICS-BPH study: uroflowmetry, lower urinary tract symptoms and bladder outlet obstruction. Br J Urol 82:619–623
Oelke M, Baard J, Wijkstra H, de la Rosette JJ, Jonas U, Höfner K (2008) Age and bladder outlet obstruction are independently associated with detrusor overactivity in patients with benign prostatic hyperplasia. Eur Urol (Epub ahead of print)
Shapiro E, Hartanto V, Lepor H (1992) The response to alpha-blockade in benign prostatic hyperplasia is related to the percent area density of prostate smooth muscle. Prostate 21:297–307
Ückert S, Mayer ME, Stief CG, Jonas U (2007) The future of the oral pharmacotherapy of male erectile dysfunction - things to come. Expert Opin Emerg Drugs 12:219–228
de Nucci G, Lorenzetti R, Okuyama CE, Baracat JS, Donato JL, Antunes E, Teixeira CE (2007) Pharmacological characterization of the novel phosphodiesterase type 5 (PDE5) inhibitor lodenafil carbonate on human and rabbit corpus cavernosum. Urology 70(Suppl 3A):105 (abstract)
Conflict of interest statement
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kedia, G.T., Ückert, S., Jonas, U. et al. The nitric oxide pathway in the human prostate: clinical implications in men with lower urinary tract symptoms. World J Urol 26, 603–609 (2008). https://doi.org/10.1007/s00345-008-0303-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-008-0303-y