Abstract
Aim
To evaluate the clinical outcome of high-risk prostate cancer (PC) treated by radical prostatectomy (RP) according to risk factors.
Methods
Patients with stage cT1–T3 PC were stratified in high and low/intermediate risk groups using D’Amico’s criteria: PSA ≥20 ng/ml or Gleason score ≥8 or clinical stage ≥T2c. The Kaplan Meier and Log rank test were used to generate estimates of biochemical free-survival (BFS) and PC specific mortality (PCSM).
Results
We analysed 1,109 patients with a median age of 64.1 years, a mean PSA of 12.8 and a median follow-up of 8.18 years (max. 17.5 years). Overall PSA failures (PSAF) were observed in 23.4%, mortality by all causes in 11.4% and PCSM in 2.9%. The 10-year BFS of the 290 high-risk was 45 versus 75.5% for low/intermediate risk patients and the 10-year PCSM was 10.3 versus 1.4%, respectively. Of the 290 high-risk PC, 25% had organ-confined disease at surgery with 28% PSAF compared to 55% PSAF for non-organ-confined PC irrespective of nodal status. High-risk patients with 1 or ≥2 high risk criteria had 2.6 and 3.86 times increased risk of PSAF compared to low/intermediate risk. Ten-year PCSM for PC individual risk criteria was 4.5% for PSA ≥20, 9.2% for stage ≥T2c and 18.2% for Gleason ≥8.
Conclusion
Patients with high-risk PC treated by RP by experienced surgeons can have a favourable long-term survival. Further substratification should take into account the variable prognostic implication of the different individual risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The optimal management of prostate cancer (PC) remains controversial. However, in the recent years, significant progress has been made in the identification of risk factors for disease progression and cancer death. Albertsen et al. [1] identified high Gleason grade as a major determinant for cancer mortality following conservative management of localised prostate cancer. D’Amico developed three risk categories incorporating serum PSA value and clinical stage to Gleason grading as pre-treatment determinant of treatment failure after radical prostatectomy (RP) and radiation therapy [2]. Cohort studies and population-based studies adjusting for age and co-morbidities have consistently shown a better cancer specific survival in patients treated by RP, particularly in patients with high-risk disease [3–6]. The best evidence of the benefit of surgery over observation was recently provided by the results of the Swedish randomised trial showing a 44% reduction in risk of mortality by cancer at 10 years [7]. Since the surgeons in that study were relatively early in their learning curve of RP, in a country where conservative management was the norm, it is possible that the true potential benefit of RP has been underestimated.
Several definitions of high-risk PC have been published. The most widely studied is the one by D’Amico based on Gleason grade ≥8 or PSA ≥20 or clinical stage ≥T2c [2]. Using the same criteria, two large series showed a 10% 10-year PC mortality rate in patients treated by RP [3, 5]. This is similar to the PCSM of the total cohort of RP patients in the Swedish trial.
In the present study, we report the outcome of a cohort of 1,109 men treated by RP contemporary to the Swedish trial and with similar PC risk factors. We further analyse the contribution of individual risk factors to substratify risk of failure after RP in high-risk patients.
Materials and methods
Patients
We included all patients treated by RP at CHUQ—Hôtel-Dieu de Québec for clinically localised or locally advanced (cT1, T3) PC in whom baseline characteristics and follow up were completely available. Institutional Review Board approval was obtained for data collection and outcome analyses of these patients. We included in the cohort 228 (20.6%) patients treated with neo-adjuvant hormone therapy before RP since no difference in PSAF compared to RP alone was observed in the present study and in several other published studies [8–10]. Patients were followed for recurrence with serum PSA determination and rectal examination every 6 months for the first 5 years and annually thereafter if no recurrence was observed. Recurrence was defined as PSA >0.3 ng/ml or the initiation of a definitive antiandrogen hormone therapy or salvage radiotherapy. Secondary cancer treatment was instituted at the discretion of the treating physician. Patients were stratified as high risk or low/intermediate risk according to D’Amico’s criteria of having or not: a serum PSA ≥20 ng/ml and/or Gleason score ≥8 and/or clinical tumour ≥T2c.
Statistical analysis
Descriptive statistics were used to characterise patients at baseline.
The Kaplan–Meier and log–rank test methods were used to generate estimates of BFS and PCSM according to the definition of high-risk versus non-high-risk category, high-risk organ-confined versus non-organ-confined and between non-organ-confined with positive or negative lymph node (LN). Cox regression analysis to define hazard ratio (HR) for PSAF and PCSM in high-risk cancers defined by one or more than one high-risk criteria, was performed.
Results
The cohort included 1,109 patients with cT1–T3 PC (median age 64.1 years) treated by RP between 1989 and 2005. The median follow up was 8.18 years (max 17.5 years). Table 1 describes the pre-operative and pathological characteristics of these patients. Majority (61%) had clinical and pathological T2 stage and serum PSA <10. However, 8.9% had a biopsy Gleason ≥8 but this proportion increased to 21.4% Gleason ≥8 on pathological specimen which also showed seminal vesicle invasion in 17%, extra-capsular extension in 50.4% and lymph node (LN) metastasis in 14.1%.
PSA failure was observed in 260 patients (23.4%) with a median time to failure of 5.24 years. At last follow up, 126 (11.4%) patients had died with a median time to death of 8.14 years. Mortality was attributed to PC in 32 cases (2.9%).
There were 290 patients with clinically high-risk PC defined by PSA ≥20 and/or cT ≥2c and/or Gleason ≥8. In these patients, surgical pathology showed seminal invasion in 37%, extra-capsular extension in 72% and LN invasion in 33%.
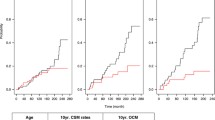
The 10-year BFS of high risk versus low/intermediate risk patients was 45 (95% CI36.8–53.2) versus 75.5% (95% CI 71.2–79.8) (P = 0.0001) and the 10-year PCSM was 10.3 (95% CI 5.8–14.6) versus 1.4% (95% CI 0.3–2.4), respectively (P = 0.0001). The Kaplan–Meier curves are reported in the Fig. 1a, b.
Of the 290 high-risk patients, 25.8% had organ confined disease on the pathology specimen and their 10 year BFS was 69.5% (95% CI 56–82.9), and the 10-year PCSM was 3.8% (95% CI 0–9.0), an outcome very similar to low/intermediate risk patients (Fig. 2a, b). The 74.2% non-organ confined cancers included 118 patients with negative LN and 97 with LN positive. The 10-year BFS was 39.3% (95% CI 26.5–52.1) for LN negative and 33.4% (95% CI 18.8–48.0) for LN positive and the 10-year PCSM was 10.3% (95% CI 2.9–17.6) for LN negative and 15.6% (95% CI 6.4–24.9) for LN positive (P = 0.035) (Fig. 2b).
Of the 290 high-risk patients, 203 presented a single high-risk determinant, PSA ≥20 (41.8%) or cT ≥ (33%) or Gleason ≥8 (25.1%). Totally 76 patients had two and 11 had three. Because of the small number, patients with three risk factors were combined with those with two for further analysis. Cancers with c ≥ T2c alone had the highest 10-year BFS (50.8%) similar to cancers with PSA ≥20 (49.7%) and better than biopsy Gleason ≥8 (40.7%). The highest 10-year PCSM was for high-risk cancers with biopsy Gleason ≥8 (18%), followed by cancers with clinical stage ≥T2c and PSA ≥20 with a PCSM of 9.2 and 4.5%, respectively (Table 2). The log–rank test showed a statistically different BFS between patients with no versus 1 or ≥2 high-risk criteria (P < 0.003) and also between 1 and 2 (P = 0.048) (Fig. 3a). PCSM was not statistically different between 1 and 2 high-risk criteria (P = 0.102) (Fig. 3b). High-risk patients with 1 or ≥2 high-risk criteria have a HR of 2.62 (95% CI 1.99–3.45, P = 0.0001) and 3.86 (95% CI 2.74–5.45, P = 0.0001) of PSAF when compared with the low/intermediate risk PC and 9.2-time risk of PCSM (95% CI 3.82–22.2, P = 0.0001) for ≥2 risk factors and 4.69 times (95% CI 2.03–10.9, P = 0.003) for one risk factor compared to none.
Discussion
Treatment decisions for patients with localised PC are today strongly influenced by patient and physician personal preferences and experience because of the paucity of prospective randomised data comparing treatment options. However, the superiority of RP over observation was demonstrated in the Swedish randomised trial showing a PC specific mortality rate of 16% at 10-year for the observation arm compared to 10% for the RP arm [7]. Albertsen et al. in a case series analysis reported a similar 10-year PCSM of 15% for men electing observation but a 6.5% PCSM for those treated by RP [3, 5]. In the present study, men were treated during the same period as those in the Swedish trial at a time where the shift to higher Gleason scoring had not occurred [11]. Our cohort includes slightly less patients with PSA >20 (13.7 vs. 19.9%) but significantly more Gleason ≥8 (8.9 vs. 4%) which, in our series, was the most important single high-risk factor predictive of cancer mortality. The overall 10-year PCSM in the present study was 2.9% compared to the 10% PCSM observed in the Swedish trial. These results suggest that the true impact of RP on 10-year PCSM is substantially higher than that estimated in the randomised Swedish trial. Surgeons’ experience is being increasingly recognised as a major determinant of cancer control [12] and may provide one explanation for the significant difference in 10-year PCSM of 10% in Sweden versus 6.5% in a US population-based cohort and a 2.9% in a high-volume institution.
Two other case series have reported outcomes stratified by the classification scheme of D’Amico et al. based on baseline PSA, biopsy tumour grade and clinical stage. The 10-year PCSM of 10.3% observed in high-risk cancer in the present study is remarkably similar to the 10% PCSM reported in the two other studies [3, 5]. However, the 10-year PCSM of 1.4% for the combined low/intermediate risk in our study is significantly lower than the 10-year PCSM reported for low and intermediate risk groups of 3 and 6% in the Albertsen’s study and 2 and 4% in the D’Amico’s study. The percentage of 10-year PSA free-survival in our series of high-risk cancers was slightly lower at 45% compared to a 59% reported in another larger series using the same high-risk definition [13]. However, the disease appeared more extensive on surgical pathology specimen in our series perhaps reflecting a higher risk population based on combination of risk factors within the high-risk category. As an indication, 33% of our high-risk patients had positive nodes, an incidence that is at the high end of that reported in several series. An intriguing observation is the lack of difference in 10-year BFS between LN positive and LN negative high-risk patients with non-organ confined disease at pathology. This observation deserves further analysis in other series but it may suggest that a more extensive node dissection including the internal iliac chains as routinely performed at our institution may have a therapeutic benefit. Overall, the 10-year cancer specific survival of our LN positive patients is similar to that reported in a large series from the Mayo Clinic (87 vs. 86%) [14].
In an attempt to substratify patients with high-risk disease, we found a significant difference in the prognostic implication of individual risk factors. The PSA ≥20 has the lowest 10-year PCSM (4.5%) followed by cT ≥2c (9.2%) and Gleason score ≥8 (18.2%). The latter 10-year PCSM of 18% compares favourably with the 25% recently reported by Tewari et al. in patients treated by RP in a retrospective series of 453 high-grade prostate cancer [6]. Another interesting finding in the present study is the ability to further substratify high-risk patients based on the combination of risk factors. The presence of two or more risk factors doubled the 10-year PCSM (7.5 vs. 16.1) and there was a trend for even higher risk in patients with all three risk factors although the numbers were too small for statistical analysis.
Conclusion
RP performed by experienced surgeons has a low morbidity and offers more than 50–75% reduction in 10-year PCSM compared to observation particularly in younger patients with high-risk disease. The definition of youth must be reassessed as men live healthier and longer. The cumulation of high-risk factors identify men with increased risk of mortality by cancer despite hormone therapy upon PSA failure. These men should be considered for randomised trial evaluating the impact of systemic therapies such as chemotherapy and cancer vaccines.
References
Albertsen PC, Hanley JA, Fine J (2005) 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA 293:2095–2101
D’Amico AV, Whittington R, Malkowicz SB, Fondurulia J, Chen MH, Kaplan I, Beard CJ, Tomaszewski JE, Renshaw AA, Wein A, Coleman CN (1999) Pretreatment nomogram for prostate-specific antigen recurrence after radical prostatectomy or external-beam radiation therapy for clinically localized prostate cancer. J Clin Oncol 17:168–172
Albertsen PC, Hanley JA, Penson DF, Barrows G, Fine J (2007) 13-year outcomes following treatment for clinically localized prostate cancer in population based cohort. J Urol 177:932–9356
Merglen A, Schmidlin F, Fioretta G, Verkooijen HM, Rapiti E, Zanetti R, Mirabell R, Bouchardy C (2007) Short- and long-term mortality with localized prostate cancer. Arch Intern Med 167:1944–1950
D’Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen M-H (2003) Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol 21:2163–2172
Tewari A, Divine G, Chang P, Shemtov MM, Milowsky M, Nanus D, Menon M (2007) Long-term survival in men with high grade prostate cancer: a comparison between conservative treatment, radiation therapy and radical prostatectomy—a propensity scoring approach. J Urol 177:911–915
Bill-Axelson A, Holmberg L, Ruutu M, Häggman M, Andersson S-O, Bratell S, Spangberg A, Busch C, Nortling S, Garmo H, Palmgren J, Adami H-O, Norléen BJ, Johansson JE, for the Scandinavian Prostate Cancer Group Study (2005) Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 352:1977–1984
Meyer F, Bairati I, Bédard C, Lacombe L, Têtu B, Fradet Y (2001) Duration of neoadjuvant deprivation therapy prior to radical prostatectomy and disease-free survival in men with prostate cancer. Urology 58:71–77
Soloway MS, Pareek K, Sharifi R et al (2002) Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol 167:112–116
Klotz L, Goldenberg SL, Jewett M, Barkin J, Chetner M, Fradet Y, Chin J, Laplante S (1999) A CUOG randomized trial of neoadjuvant androgen ablation prior to radical prostatectomy: 36 month post-treatment PSA results. Urology 53:757–763
Albertsen PC, Hanley JA, Barrows GH, Penson DF, Kowalczyk PDH, Sanders MM, Fine J (2005) Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst 97:1248–1253
Vickers AJ, Bianco FJ, Serio AM, Eastham JA, Schrag D, Klein EA, Reuther AM, Kattan MW, Pontes JE, Scardino PT (2007) The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst 99:1171–1177
Yossepowitch O, Eggener SE, Bianco FJ Jr, Carver BS, Serio A, Scardino PT, Eastham JA (2007) Radical prostatectomy for clinically localized, high risk prostate cancer: critical analysis of risk assessment methods. J Urol 178:493–499; discussion 499. Epub 2007 Jun 11
Boorjian SA, Thompson RH Siddiqui S, Bagniewski S, Bergstralh EJ, Karnes RJ, Frank I, Blute ML (2007) Long-term outcome after radical prostatectomy for patients with lymph node positive prostate cancer in the prostate specific antigen era. J Urol 178:864–871
Conflict of interest statement
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lodde, M., Harel, F., Lacombe, L. et al. Substratification of high-risk localised prostate cancer treated by radical prostatectomy. World J Urol 26, 225–229 (2008). https://doi.org/10.1007/s00345-008-0252-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-008-0252-5