Abstract
We present an overview of current and emerging lasers for Urology. We begin with an overview of the Holmium:YAG laser. The Ho:YAG laser is the gold standard lithotripsy modality for endoscopic lithotripsy, and compares favorably to standard electrocautery transurethral resection of the prostate for benign prostatic hyperplasia (BPH). Available laser technologies currently being studied include the frequency doubled double-pulse Nd:Yag (FREDDY) and high-powered potassium-titanyl-phosphate (KTP) lasers. The FREDDY laser presents an affordable and safe option for intracorporeal lithotripsy, but it does not fragment all stone compositions, and does not have soft tissue applications. The high power KTP laser shows promise in the ablative treatment of BPH. Initial experiments with the Erbium:YAG laser show it has improved efficiency of lithotripsy and more precise ablative and incisional properties compared to Ho:YAG, but the lack of adequate optical fibers limits its use in Urology. Thulium:YAG fiber lasers have also demonstrated tissue ablative and incision properties comparable to Ho:YAG. Lastly, compact size, portability, and low maintenance schedules of fiber lasers may allow them to shape the way lasers are used by urologists in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the early 1980s lasers have been the subject of intense study and clinical research in Urology. Since that time, various lasers have been used to treat clinical problems including benign prostate hyperplasia (BPH), urolithiasis, stricture disease, ureteropelvic junction (UPJ) obstruction, skin lesions and urogenital malignancy. As research in these areas continues to progress, new laser technologies show promise in Urology. During the last decade some laser technologies have become established as standard modalities widely available to urologists, while others are still under investigation for applications in the field. We present a summary of the state-of-the-art of laser technology in clinical research in urology. In addition, we present an overview of emerging laser technologies, which may find their way into the urologist’s toolbox in the coming years. It is our belief that the Holmium:YAG laser is the dominant and reference laser for lithotripsy or BPH treatment. For this reason, we will start our discussion of lasers with Holmium, and then review other lasers that might emerge as good alternatives, with possibly even better outcomes.
Discussion of individual lasers
Holmium: Yttrium-Aluminum-Garnet laser
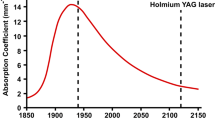
The Holmium:YAG laser is the most widespread and versatile of lasers currently available for urologists. Holmium:YAG lasers are produced by a number of manufacturers. Most Holmium:YAG lasers marketed to urologists operate at a wavelength of 2.1 μm, with a pulse duration of 350 μs. With these characteristics, Holmium:YAG energy is absorbed efficiently in water, implying high safety margin in the aqueous environment in which urologic endoscopy is conducted [1]. It is also transmitted efficiently by low OH-silica fibers, making delivery systems practical for clinical use. There are acceptable low OH-silica fibers ranging from 150 to 940 μm in diameter. The small caliber fibers permit Holmium:YAG use for flexible ureteronephroscopy. The long pulse (350 μs) also implies lack of photoacoustical effects. Indeed, the mechanism of lithotripsy is photothermal [2]. It is effective in lithotripsy, and soft tissue ablative procedures. Thus, the Holmium:YAG laser has been used to treat urinary stone disease [3], BPH [4–6], and UPJ obstruction [7, 8]. In addition, its application in urethral and ureteric stricture disease [9–11] and malignancy [12, 13] continue to be investigated.
Unlike the Nd:YAG laser (λ = 1,064 NM), which lacks the precision of Holmium:YAG, or the Alexandrite (λ = 755) and pulsed dye (λ = 540 nm) lasers, which lacks its ability to treat all stone compositions, the Holmium laser is superior in the endoscopic treatment of urolithiasis. Most ureteroscopy series report a stone free rate greater than 95% with one procedure [3, 14–17]. Its effectiveness in renal calculi is more limited [3, 18, 19], especially for lower pole stones larger than 1 cm [20]. Currently, its main drawback is its potential capacity for collateral soft tissue damage (and thus requirement for direct visualization), long lithotripsy times for certain stone compositions, and some technical challenges posed by stone retropulsion [21–23].
Holmium is a useful tool for tissue incision and ablation in the treatment of urological disease. In the treatment of urethral stricture disease, post radical retropubic prostatectomy anastamotic strictures [24] and pediatric urethral atresia [25], several small studies have shown its safety and efficacy. Most procedures are relatively free of complications and can be done on an outpatient basis. However, comparative studies have indicated it is no better than direct vision internal urethrotomy with a cold knife in terms of short and long-term outcomes. Retrograde laser endopyelotomy is safe and effective as a minimally invasive, first line treatment. A retrospective review showed 83% success when Ho:YAG endopyelotomy was combined with intraluminal ultrasound in 23 patients with UPJ obstruction. Of the four primary failures, 50% improved with repeat laser endopyelotomy [7]. A recent randomized controlled trial comparing Accucise™ balloon endopyelotomy with Ho:YAG endopyelotomy showed fewer complications and better success rates in the laser arm, but the differences were not statistically significant [26]. This data suggest that laser endopyelotomy is likely equivalent, and possibly superior to Accucise™ balloon endopyelotomy. These results for UPJO must be tempered with the knowledge that laparoscopic pyeloplasty has largely supplanted ureteroscopic UPJ endopyelotomy. The need for a laser to effect an endopyelotomy in this setting is likely to remain limited [27–31].
Holmium:YAG has emerged as a viable alternative to electrocautery TURP in treatment of symptomatic BPH. The two notable techniques are Holmium laser resection of the prostate (HoLRP) and Holmium laser enucleation of the prostate (HoLEP). Controlled trials have shown HoLEP to be equivalent to TURP for BPH while also showing decreased hospital stay, decreased catheterization time, fewer adverse effects, and greater amounts of tissue resection both in the short term and, with followup in up to 4 years [4–6]. It has also been favorably compared to simple prostatectomy for large glands with similar improvement of flow rate, hospital length of stay, blood loss, catheterization time, transfusion and complications [32, 33]. Theoretically, the hemostatic properties of the holmium laser should allow HoLEP or HoLRP to be done even in anticoagulated patients. A recent study suggests that HoLEP in anticoagulated patients is safe and effective with a transfusion rate of 14% in the patients on full anticoagulation undergoing HoLEP [34]. This figure compares to 30% transfusion rate for patients undergoing TURP without withdrawal of anticoagulation [35]. The major limitation in HoLEP is the steep learning curve required to master it. A study of self-taught practitioners showed a learning curve of at least 50 cases [36]. As the procedure continues to be disseminated, the urologist’s familiarity and proficiency with it should increase.
Potassium-Titanyl-Phosphate (KTP) laser
The KTP or “green light” laser (λ = 840 nm) was originally introduced in treatment for BPH in the early 1990s. However, these underpowered lasers did not prove themselves effective for this application [37–39]. Recently, higher powered, 80 W, 532 nm rapid pulse versions of the KTP laser have been studied as an alternative in ablative therapy for BPH. KTP vaporization of the prostate has several advantages. First, the technique is similar to TURP and thus familiar and easy to learn for urologists. Second, the 532 nm wavelength of current laser models is close to the peak absorption of hemoglobin, allowing excellent hemostasis and transmission through water. Lastly, high power KTP lasers produce only 1–2 mm of coagulation zone for safe vaporization [40, 41]. Although long-term data are lacking, short-term data are promising. Several studies show improved symptoms and peak flow rates with minimal intra-operative blood loss [40, 42, 43]. In a study of 139 men undergoing photoselective vaporization of the prostate with a high power KTP laser with 1 year follow up, Te et al. [40] reported improvements in symptom scores, residual volume, quality of life, and maximum flow rates. A randomized controlled trial of 150 men with obstructive voiding showed KTP laser vaporization and TURP equivalent in terms of flow rate and symptom control. The morbidity associated with the KTP laser was less than with TURP [44]. KTP laser prostate ablation can be done as same day surgery with no requirement for catheter drainage in select patients [45, 46]. Some potential drawbacks are irritative voiding symptoms and hematuria. In addition, long operative times for large glands may limit the practicality of this technique [42], although Te et al. [40] showed a mean operative time of 39 min. Lastly, long term data and further randomized studies comparing KTP vaporization of the prostate to TURP or HoLEP are wanting. Overall, preliminary data for high power KTP laser ablation of the prostate seem encouraging, but optimism is tempered by the fact that previously studied vaporization techniques have not endured.
KTP lasers have also been studied in the treatment of urethral stricture disease. Schmidlin et al. [47] performed a prospective study of 20 patients using the KTP laser to treat a variety of urethral strictures with reasonable short-term outcomes. Eighty-one percentage had symptomatic and urodynamic success at 3 and 6 months with only one of three failures requiring re-treatment [47]. Still, further studies and long-term data are lacking, and like the holmium laser, it is unlikely this will supplant internal urethrotomy for the time being.
Frequency doubled double-pulse Nd:Yag (FREDDY) laser
One of the first laser systems to be used in the treatment of urinary stone disease was the pulsed dye laser that had a short pulse duration and fragmented stones via a photoacoustic mechanism. This laser had the advantage of less collateral tissue damage during lithotripsy, reducing the reliance on direct visualization. However, its failure to fragment calcium oxalate monohydrate, cystine, and brushite stones led to its abandonment for other lasers. The FREDDY laser operates via a similar photoacoustic mechanism, which is safe for tissues, but does so at a significant cost savings compared to Holmium. By incorporating a KTP crystal into the resonator of a Nd:YAG laser, the FREDDY laser produces two pulses, one at 532 nm and another at 1,064 nm, simultaneously. Laser light at 532 nm (green spectrum) initiates plasma formation at the stone surface, while light at a wavelength of 1,064 nm heats the preformed plasma, causing expansion and contraction, which fragments calculi, using pulse durations of 0.3–1.5 μs [48–50].
In vitro experiments show the FREDDY laser capable of lithotripsy and animal model studies show little to no effect on normal tissues [51–53]. One human in vivo study of FREDDY laser effects on ureteric urothelium pre-cystectomy showed only minimal edema after 300 pulses of 120 mJ each [54]. When compared with other lasers, 2,000 pulses of the FREDDY laser failed to perforate human ureteric tissue ex vivo, while Ho:YAG lasers required an average of only two pulses [55]. Although Holmium seems to be “dangerous” in these experiments, it is important to note that the experiments were conducted with the laser fiber oriented at a right angle to the ureteral mucosa and in contact, whereas in clinical circumstances, the laser fiber is generally in the direction of the ureteral lumen (oriented parallel to mucosa) and not in contact. Clinical experience shows the perforation rate from Ho:YAG use is low [3, 14, 56].
A study of 50 patients using FREDDY laser lithotripsy showed overall 95% immediate stone free rates in treatment of ureteral calculi with no complications [57]. Stark et al. [58] showed an 87% combined stone free rate for kidney, ureteric and bladder stones, with no complications. A study of 21 patients showed 100% stone free rates in kidney and ureteric stones, but a 57% stone free rate for bladders stones using the laser [54]. Of interest, the theoretical risk due to absorption of hemoglobin at 532 nm did not prove to be clinically relevant. This high safety margin is likely due to the low total energy of 20 mJ emitted at 532 nm [50].
The FREDDY laser was approved by the FDA in November of 2001. Unfortunately, several studies have shown the FREDDY laser ineffective in the treatment of “hard” urinary calculi, such as those for which the pulsed dye laser was also ineffective [59, 60]. Even so, its low cost, and intrinsic safety to soft tissues make it a reasonable alternative in the treatment of calcium phosphate stones, especially in clinical situations where visualization is limited.
Erbium:YAG laser
The Erbium:YAG laser has already been used clinically in dentistry, otolaryngology, and ophthalmology [61–65]. Its use in urology is still under investigation, but several of its properties make it a viable alternative to the Ho:YAG laser.
Free electron laser experiments showing that urinary calculi absorb energy maximally at 2.9 μm led to the conclusion that Er:YAG (λ = 2.90–2.94 μm) may be more effective than Ho:YAG in treating urolithiasis [66]. Further studies showed that the Er:YAG laser (pulse duration 275 μs) fragments stones by a photothermal mechanism and has vapor bubble dynamics similar to Ho:YAG lasers. Like Holmium, Erbium produces minimal acoustic transients [67]. In vitro experiments comparing Erbium and Holmium laser lithotripsy showed that Er:YAG lasers fragmented stones three to five times more efficiently than a Ho:YAG laser. This efficiency applied both to calcium oxalate monohydrate and cystine stones [68]. Erbium lasers produce a smoother, deeper ablation crater as compared to Holmium lasers which translates to a larger volume of stone ablated, and greater efficiency of the erbium laser [69]. Although the vapor bubble dynamics between these lasers are similar, the vapor bubble geometry produced by the Er:YAG laser is torpedo shaped, as compared to the pear shaped bubble of the Ho:Yag laser, which may improve erbium energy transmission and, ultimately, ablation efficiency [65].
Due to its increased precision and efficiency, the Er:YAG laser is under study as a tool to treat ureteric and urethral strictures. The Er:YAG laser produces more efficient and precise incisions than the Ho:YAG laser [70]. In animal ureteric experiments, the Er:YAG and Chromium-Thulium-Erbium:YAG lasers produce less deep tissue damage than Chromium-Thulium-Holmium:YAG lasers [71]. In vivo experiments on animal urethra and bladder neck show that Er:YAG lasers produce less collateral damage, granulation tissue, and incision depth at 14 days than Ho:YAG lasers [72]. However, holmium showed improved hemostasis over erbium post soft tissue incision. Although hemostatic property has implications for prostatic ablation or resection, hemostasis could be affected by defocusing the erbium laser. Further studies are needed.
Erbium lasers have yet to find practical use in urology chiefly due to the lack of clinically useful laser fibers. Low OH-silica fibers used in current holmium lasers absorb significant energy during erbium laser operation, especially at their input ends. This can lead to thermal degradation to the fiber, and damage to the laser. Fluoride fibers have been used successfully, but these fibers tend to be brittle and have a hygroscopic structure. Single crystal sapphire fibers allow low energy losses, but only at power outputs of less than 200 mJ. In addition, their cost is prohibitive and they risk damage at their output ends due to high peak energy densities. Lastly, sapphire fibers small enough for current urological endoscopes are not, as yet, commercially available [68]. Hybrid fibers consisting of germanium oxide fibers with silica tips have shown some promise, but are still under development [73]. Another alternative fiber currently being studied is a hollow silica fiber. Initial studies showed good strength and flexibility and the ability to transmit radiation sufficient to fragment a urinary calculus [74]. However, these fibers produce hot spots and irregular craters. The viability of the Erbium laser will largely depend on the development of suitable fiber technology.
Thulium lasers
Thulium lasers (λ = 2 μm) have been studied clinically in otolaryngology [75], ophthalmology [76, 77], and neurology [78]. Like other mid infrared lasers, Thulium lasers operate at wavelengths close to the peak absorption of water, making them a logical choice for soft and hard tissue ablation. When combined with fiber laser technology, thulium lasers may prove valuable in the treatment of urological disease. A fiber laser utilizes the fiber itself, usually doped with a rare earth metal such as thulium, as the amplification medium, and is pumped by a diode laser. This setup has numerous advantages. Fiber lasers have an excellent spatial beam quality compared to Ho:YAG lasers and precise tissue incision capabilities. Since they are diode pumped, they have the ability to operate in short (Q-switched) or long pulse mode, suggesting more versatility for applications in lithotripsy and tissue ablation. In addition, these lasers feature tunable wavelength technology, allowing adjustment of laser output for differential tissue incision depth, and to more closely match the peak absorption of water, the latter increasing soft tissue safety. The lack of airspaces in the laser construction increases laser efficiency and allows for decreased size and weight of the apparatus itself. These lasers are small and portable, without the need for a water-cooling system, and require minimal maintenance. It raises the possibility of any urologist carrying their laser in their briefcase and taking it to any operating room, and plugging the fiber into the wall and treating stones or soft tissue. The practical implications are obvious.
Animal studies of hard and soft tissue laser ablation with the Thulium:YAG laser show promise. Thiesen et al. [79] demonstrated the tissue cutting ability of a Th:YAG laser on pig liver with coagulation zones of 0.8 mm or less. In another study, a Th:YAG fiber laser could effectively ablate soft tissue in vitro, including muscle, cartilage and liver [80]. Accuracy, energy efficiency, and minimal collateral damage were seen with the laser in Q-switch mode versus continuous wave (CW) mode. In fact, zones of collateral damage were six times less during Q-switch mode versus CW mode [80]. This concern has negative implications for the Th:YAG laser’s applicability to lithotripsy, since operating in Q-switch mode causes more retropulsion and decreases fragmentation efficiency [81].
In vitro studies of Thulium fiber laser lithotripsy show it fragments both hard (calcium oxalate monohydrate) and soft (uric acid) urinary calculi. However, at settings used in these studies, the fragmentation efficiency was inferior to published data on Ho:YAG lasers and FREDDY lasers for uric acid stones. Additional findings were the charring of the laser fiber tip, damage to the fiber tip after lithotripsy and requirement to re-cleave tips to complete experiments [82]. If fibers char, and need to be re-cleaved intra-operatively, this laser system will likely not show any advantage over Holmium.
A recent study described Th:YAG laser ablation of canine prostate, urethra and bladder neck. Tissue was vaporized at a rate of 0.21 ± 0.02 g/min with thermal coagulation zones of 500–2,000 μm, suggesting potential for hemostasis. Incisions in the urethra produced coagulation zones of 400–600 μm [83]. These coagulation depths are comparable to those produced by Ho:YAG lasers [84]. The authors argue that a higher power laser could be used to deliver more energy in a shorter pulse duration, thus reducing collateral tissue damage and better efficiency. The Thulium lasers used in these experiments, however, were not powered high enough to make efficient and accurate incision and ablation clinically feasible without fiber degradation or unacceptable collateral tissue damage [83].
As higher powered models become available, studies are being conducted to further assess higher powered thulium lasers in prostate ablation. Further canine prostate ablation studies using a higher power 110 W Thulium laser provided ablation rates of 0.83 ± 0.11 g/min with 500–2,000 μm coagulation zones [85]. A clinical study revealed that Thulium laser prostate resection is safe and effective. A 50 W 2.01 μm Thulium laser was used to resect the prostate glands of 30 men. The authors cited minimal blood loss, 1–3 days of catheterization, with acceptable post op laboratory values, improved International Prostate Symptom (IPSS) scores and maximum flow rates, and no new cases of impotence or retrograde ejaculation [86]. Initial results are promising, but further clinical studies are needed to assess long-term outcomes, equivalency to currently available technology, and cost effectiveness. A commercial Thulium laser (RevoLix™) has recently become available and published data are awaited.
Conclusions
While the use of lasers in urology is already widespread, refinements to existing technology and new innovations will undoubtedly increase the role of lasers in the specialty. We are likely to see the well-established Ho:YAG laser to continue to be used in lithotripsy and prostate resection and enuceation. Other currently available technologies like the high power KTP laser will continue to be used, but their permanence in the practice of urology depends on awaited long-term data. Currently, high-powered KTP lasers are being used on a large scale, but further refinements in lasers and parameters are likely as clinical and research experience increases. The FREDDY laser may prove useful for some urolithiasis applications, particularly impacted stones, or stones in parts of the upper tract where visualization is limited. Its main attractive feature is it costs less than Holmium. Like other short pulse laser counterparts, the limitations of FREDDY include inability to fragment some stone compositions and lack of soft tissue ablation. The increased lithotripsy efficiency of the Er:YAG laser, combined with its accurate incisional and ablative properties will likely make it a candidate for the treatment of urolithiasis and genitourinary stricture disease when suitable laser fibers can be found. As the technology matures, Th:YAG fiber lasers are likely to find broad utility in urology. Their tunable wavelength and superior incision, ablation and hemostasis properties will make them as powerful and versatile as the Ho:YAG laser in urinary stone and soft tissue disease, with some minor advancements in commercially available models. Lastly, fiber laser technology has the potential to make the large, heavy laser setups of today obsolete, allowing urologists to carry the lasers of their choice to multiple sites.
References
Jansen ED, van Leeuwen TG, Motamedi M, Borst C, Welch AJ (1994) Temperature dependence of the absorption coefficient of water for midinfrared laser radiation. Lasers Surg Med 14(3):258–268
Vassar GJ, Chan KF, Teichman JM, Glickman RD, Weintraub ST, Pfefer TJ, Welch AJ (1999) Holmium: YAG lithotripsy: photothermal mechanism. J Endourol 13(3):181–190
Sofer M, Watterson JD, Wollin TA, Nott L, Razvi H, Denstedt JD (2002) Holmium:YAG laser lithotripsy for upper tract urinary calculi in 598 patients. J Urol 167:31–34
Gilling PJ, Cass CB, Cresswell MD, Malcolm AR, Fraundorfer MR (1996) The use of the holmium laser in the treatment of benign prostatic hyperplasiaL. J Endourol 10:459–461
Westenberg A, Gilling P, Kennett K, Frampton C, Fraundorfer M (2004) Holmium laser resection of the prostate versus transurethral resection of the prostate: results of a randomized trial with 4-year minimum long-term followup. J Urol 172(2):616–619
Tan AH, Gilling PJ, Kennett KM, Frampton C, Westenberg AM, Fraundorfer MR (2003) A randomized trial comparing holmium laser enucleation of the prostate with transurethral resection of the prostate for the treatment of bladder outlet obstruction secondary to benign prostatic hyperplasia in large glands (40 to 200 grams). J Urol 170(4 Pt 1):1270–1274
Giddens JL, Grasso M (2000) Retrograde ureteroscopic endopyelotomy using the holmium:YAG laser. J Urol 164(5):1509–1512
Biyani CS, Cornford PA, Powell CS (2000) Ureteroscopic endopyelotomy with the Holmium:YAG laser. mid-term results. Eur Urol 38(2):139–143
Matsuoka K, Inoue M, Iida S, Tomiyasu K, Noda S (2002) Endoscopic antegrade laser incision in the treatment of urethral stricture. Urology 60(6):968–972
Watterson JD, Sofer M, Wollin TA, Nott L, Denstedt JD (2002) Holmium: YAG laser endoureterotomy for ureterointestinal strictures. J Urol 167(4):1692–1695
Singal RK, Denstedt JD, Razvi HA, Chun SS (1997) Holmium:YAG laser endoureterotomy for treatment of ureteral stricture. Urology 50(6):875–880
Jonler M, Lund L, Bisballe S (2004) Holmium:YAG laser vaporization of recurrent papillary tumours of the bladder under local anaesthesia. BJU Int 94(3):322–325
Syed HA, Biyani CS, Bryan N, Brough SJ, Powell CS (2001) Holmium:YAG laser treatment of recurrent superficial bladder carcinoma: initial clinical experience. J Endourol 15(6):625–627
Teichman JM, Rao RD, Rogenes VJ, Harris JM (1997) Ureteroscopic management of ureteral calculi: electrohydraulic versus holmium:YAG lithotripsy. J Urol 158(4):1357–1361
Razvi HA, Denstedt JD, Chun SS, Sales JL (1996) Intracorporeal lithotripsy with the holmium:YAG laser. J Urol 156(3):912–914
Wu CF, Shee JJ, Lin WY, Lin CL, Chen CS (2004) Comparison between extracorporeal shock wave lithotripsy and semirigid ureterorenoscope with holmium:YAG laser lithotripsy for treating large proximal ureteral stones. J Urol 172(5 Pt 1):1899–1902
Lam JS, Greene TD, Gupta M (2002) Treatment of proximal ureteral calculi: holmium:YAG laser ureterolithotripsy versus extracorporeal shock wave lithotripsy. J Urol 167(5):1972–1976
Ilker Y, Ozgur A, Yazici C (2005) Treatment of ureteral stones using Holmium:YAG laser. Int Urol Nephrol 37(1):31–34
Albala DM, Assimos DG, Clayman RV, Denstedt JD, Grasso M, Gutierrez-Aceves J, Kahn RI, Leveillee RJ, Lingeman JE, Macaluso JN Jr, Munch LC, Nakada SY, Newman RC, Pearle MS, Preminger GM, Teichman J, Woods JR (2001) Lower pole I: a prospective randomized trial of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy for lower pole nephrolithiasis-initial results [erratum appears in J Urol 2002 Apr;167(4):1805]. J Urol 166(6):2072–2080
Pearle MS, Lingeman JE, Leveillee R, Kuo R, Preminger GM, Nadler RB, Macaluso J, Monga M, Kumar U, Dushinski J, Albala DM, Wolf JS Jr, Assimos D, Fabrizio M, Munch LC, Nakada SY, Auge B, Honey J, Ogan K, Pattaras J, McDougall EM, Averch TD, Turk T, Pietrow P, Watkins S (2005) Prospective, randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. J Urol 173(6):2005–2009
Lee H, Ryan RT, Teichman JM, Kim J, Choi B, Arakeri NV, Welch AJ (2003) Stone retropulsion during holmium:YAG lithotripsy. J Urol 169(3):881–885
Finley DS, Petersen J, Abdelshehid C, Ahlering M, Chou D, Borin J, Eichel L, McDougall E, Clayman RV (2005) Effect of holmium:YAG laser pulse width on lithotripsy retropulsion in vitro. J Endourol 19(8):1041–1044
Lee H, Kang HW, Teichman JM, Oh J, Welch AJ (2006) Urinary calculus fragmentation during Ho: YAG and Er:YAG lithotripsy. Lasers Surg Med 38(1):39–51
Lagerveld BW, Laguna MP, Debruyne FM, De La Rosette JJ (2005) Holmium:YAG laser for treatment of strictures of vesicourethral anastomosis after radical prostatectomy. J Endourol 19(4):497–501
Futao S, Wentong Z, Yan Z, Qingyu D, Aiwu L (2006) Application of endoscopic Ho:YAG laser incision technique treating urethral strictures and urethral atresias in pediatric patients. Pediatr Surg Int 22(6):514–518
el-Nahas AR, Shoma AM, Eraky I, el-Kenawy MR, el-Kappany HA (2006) Prospective, randomized comparison of ureteroscopic endopyelotomy using holmium:YAG laser and balloon catheter: J Urol 175(2):614–618; discussion 618
Pardalidis NP, Papatsoris AG, Kosmaoglou EV (2003) Endoscopic and laparoscopic treatment of ureteropelvic junction obstruction. J Urol 168:1937–1940
Jarrett TW, Chan DY, Charambura TC et al (2002) Laparoscopic pyeloplasty: the first 100 cases. J Urol 167:1253–1256
Baldwin DD, Dunbar JA, Wells N et al (2003) Single-center comparison of laparoscopic pyeloplasty, Acucise endopyelotomy, and open pyeloplasty. J Endourol 17:155–160
Soulie M, Salomon L, Patard J et al (2001) Extraperitoneal laparoscopic pyeloplasty: a multicenter study of 55 procedures. J Urol 166:48–50
Tan BJ; Smith AD (2004) Ureteropelvic junction obstruction repair: when, how, what? Curr Opin Urol 14(2):55–59
Kuntz R, Lehrich K, Fayad A (2001) HoLEP vs open transvesical enucleation of prostates larger than 100 g: the first randomised prospective trial [Abstract]. In: Abstracts of the American Urological Association, Anaheim, 2001. J Urol 165(Suppl.):1208
El zayat EA, Elhilali MM (2006) Holmium laser enucleation of the prostate (HoLEP): the endourologic alternative to open prostatectomy. Eur Urol 49(1):87–91
El zayat E, Habib E, Elhilali M (2006) Holmium laser enucleation of the prostate in patients on anticoagulant therapy or with bleeding disorders. J Urol 175(4):1428–1432
Parr NJ, Loh CS, Desmond AD (1989) Transurethral resection of the prostate and bladder tumour without withdrawal of warfarin therapy. Br J Urol 64(6):623–625
Seki N, Mochida O, Kinukawa N, Sagiyama K, Nato S (2003) Holmium laser enucleation for prostatic adenoma: analysis of learning curve over the course of 70 consecutive cases. J Urol 170(5):1847–1850
McAllister WJ, Absalom MJ, Mir K et al (2000) Does endoscopic laser ablation of the prostate stand the test of time? Five-year results from a multicentre randomized controlled trial of endoscopic laser ablation against transurethral resection of the prostate. BJU Int 85:437–443
Anson KM, Watson GM, Shah TK, Barnes DG (1993) Laser prostatectomy: our initial experience of a technique in evolution. J Endourol 7:333–336
Martenson AC, De La Rosette JJ (1999) Interstitial laser coagulation in the treatment of benign prostatic hyperplasia using a diode laser system: results of an evolving technology. Prostate Cancer Prostatic Dis 2:148–154
Te AE, Malloy TR, Stein BS, Ulchaker JC, Nseyo UO, Hai MA, Malek RS (2004) Photoselective vaporization of the prostate for the treatment of benign prostatic hyperplasia: 12-month results from the first United States multicenter prospective trial. J Urol 172(4 Pt 1):1404–1408
Kuntzman RS, Malek RS, Barrett DM, Bostwick DG (1996) Potassium-titanyl-phosphate laser vaporization of the prostate: a comparative functional and pathologic study in canines. Urology 48:57
Bachmann A, Reich O, Wyler S, Ruszat R, Casella R, Gasser T, Hofstetter A, Sulser T (2004) The 80 W potassium-titanium-phosphate (KTP) laser vaporization of the prostate. Technique and 6 month follow-up after 70 procedures (German). Urologe (Ausg. A) 43(10):1262–1270
Malek RS, Kuntzman RS, Barrett DM (2000) High Power Potassium-titanyl-phosphate laser vaporization prostatectomy. J Urol 163(6):1730–1733
Bouchier-Hayes DM, Anderson P, Appledom SV et al (2005) A randomised trial comparing photo-vaporisation and trans-urethral resection of the prostate in patients with BPH (abstract). J Urol 173(Suppl 1555):42
Barber NJ, Muir GH (2004) High-power KTP laser prostatectomy: the new challenge to transurethral resection of the prostate. Curr Opin Urol 14(1):21–25
Reich O, Bachmann A, Siebels M et al (2005) High power (80 W) potassium-titanyl-phosphate laser vaporization of the prostate in 66 high risk patients. J Urol 173:158–160
Schmidlin F, Oswald M, Iselin C, Rohner S, Jichlinski P, Delacretaz G, Leisinger HJ, Graber P (1997) Vaporization of urethral stenosis using the KTP 532 laser (French). Ann Urol 31(1):38–42
Helfmann J (1992) Untersuchung der physikalischen Phanomene bei der Zertrummerung von Korperkonkrementen durch laserinduzierte Plasmen. In: Advances in laser medicine. Landsberg, Zurich: ecomed
Helfmann J, Muller G (2001) Laser lithotripsy: process overview. Med Laser Appl 16:30–37
Tischer CF, Koort H, Bazo A, Rasch R, Thiede C (2002) Clinical experiences with a new frequency-doubled double-pulse Nd:YAG Laser (FREDDY) for the treatment of urolithiasis. In: Proceedings of SPIE, vol 4609
Hochberger J, Bayer J, Tex S, Maiss J, Tschepe J, Hahn EG (1997) Frequenzverdoppelter Doppelpuls ND:YAG Laser (FREDDY) fur die Gallensteinlithotripsie—Praklinische und erste klinische Ergebnisse. Biomedizinische Technik “Laseranwendungen III” 442:330
Zorcher T, Hochberger J, Schrott KM et al (1999) In vitro study concerning the efficiency of the Frequency-doubled Double-Pulse Neodymium:YAG Laser (FREDDY) for Lithotripsy of Calculi in the Urinary tract. Lasers Surg Med 25(1):38–42
Delvecchio F, Zhu S, Weizer A, Silverstein A, Auge B, Pietrow P, Albala D, Zhong P, Preminger G (2001) In vitro fragmentation analysis of the FREDDY laser. Oral presentation at the WCE 2001, Bangkok
Bazo A, Chow WM, Coombs L, Barnes DG (2001) Freddy will crack it for you: a new device for urinary calculi lithotripsy. In: BAUS conference proceedings, section of Endourology, Sheffield, UK
Santa-Cruz RW, Leveillee RJ, Krongrad A (1998) Ex vivo comparison of four lithotripters commonly used in the ureter: what does it take to perforate? J Endourol 12(5):417–422
Harmon WJ, Sershon PD, Blute ML, Patterson DE, Segura JW (1997) Ureteroscopy: current practice and long-term complications. J Urol 157(1):28–32
Schafhauser W, Zorcher W et al (2000) Erste klinische Erfahrungen mit neuem frequenzverdoppeltem Doppelpuls Neodym:YAG Laser in der Therapie der Urolithiasis. Poster presentation at the DGU, Hamburg, Germany
Stark L, Carl P, Zauner R (2001) A new technique for Laser-Lithotripsy: FREDDY, the partially frequency-doubled double-Pulse Nd:YAG Laser. Poster presentation at the 1st int. consultation on Stone Disease, Paris
Dubosq F, Pasqui F, Girard F, Beley S, Lesaux N, Gattengno B, Thibault P, Traxer O (2006) Ednoscopic lithotripsy and the FREDDY laser: initial experience. J Endourol 20(5):296–299
Stark L, Car P (2001) First clinical experiences of laser lithotripsy using the partially frequency-doubled double-pulse neodymium:YAG laser (“FREDDY”) (abstract). J Urol 165:362A
Visuri SR, Walsh JT, Wigdor HA (1996) Erbium laser ablation of dentin hard tissue: effect of water cooling. Lasers Surg Med 18:294–300
Schlenk E, Profeta G, Nelson JS, Andrews JJ, Berns MW (1990) Laser assisted fixation of ear prostheses after stapedectomy. Lasers Surg Med 10:444–447
Pratisto H, Frenz M, Ith M, Romano V, Felix D, Grossenbacher R, Altermitt HJ, Weber HP (1996) Temperature and pressure effects during erbium stapedotomy. Lasers Surg Med 18:100–108
Dietlein TS, Jacobi PC, Krieglstein GK (1998) Erbium:YAG laser trabecular ablation (LTA) in the surgical treatment of glaucoma. Lasers Surg Med 23:104–110
Chan KF, Vargas G, Parker PJ, Teichman JMH, Glickman RD, McGuff HS, Welch AJ (2000) In vitro erbium:YAG laser lithotripsy. In: Proceedings of SPIE—the international society for optical engineering, vol 3914, pp 198–206
Chan KF, Hammer DX, Choi B, Teichman JMH, McGuff HS, Pratisto H, Jansen ED, Welch AJ (2000) Free electron laser lithotripsy: threshold radiant exposures. J Endourol 14:161–167
Chan KF, Lee H, Teichman JMH, Kamerer A, McGuff HS, Welch AJ (2002) Erbium:YAG laser lithotripsy. J Urol 168:436–441
Teichman JMH, Chan KF, Cecconi PP, Corbin NS, Kamerer AD, Glickman RD, Welch AJ (2001) Erbium:Yag versus Holmium:Yag. J Urol 165:876–879
Kang HK, Lee H, Teichman JH, Welch AJ (2005) Comparison of urinary calculus fragmentation during Ho:YAG and Er:YAG lithotripsy. In: Proceedings of SPIE, vol 5686, pp 159–170
Jovanovic S, Schonfeld U, Prapavat V et al (1997) Effects of pulsed laser systems on stapes footplate. Lasers Surg Med 21:341
Jelinkova H, Kohler O, Nemec M, Koranda P, Siulc J, Drlik P, Miyagi M, Shi YW, Matsuura Y, Kokta MR (2004) Hrabal Interaction of mid-infrared laser radiation with soft ureter tissue. In: Proceedings of SPIE, vol 5610, pp 18–23
Varkarakis IM, Inagaki T, Allafa ME, Chan TY, Rogers CG, Wright EJ, Fried NM (2005) Erbium vs. Holmium laser incision of the urethra and bladder neck. In: Proceedings of SPIE, vol 5686, pp 171–175
Chaney CA, Yang Y, Fried NM (2004) Assembly and testing of Germanium/Silica Optical fibers for flexible endoscopic delivery of Erbium:YAG laser radiation. In: Proceedings of SPIE, vol 5317, pp 1–8
Iwai K, Shi Y, Matsuura Y, Miyagi M (2002) Rugged hollow fiber for the infrared and its use in laser lithotripsy. In: Proceedings of SPIE, vol 4916, pp 115–119
Kay SL, Oz MC, Haber M, Blitzer A, Treat MR, Trokel SL (1992) Soft tissue effects of the THC:YAG laser on canine vocal cords. Otolaryngol Head Neck Surg 107(3):438–443
Silkiss RZ, Axelrod RN, Iwach AG, Vassiliadis A, Hennings DR (1992) Transcanalicular THC:YAG dacryocystorhinostomy. Ophthalmic Surg 23(5):351–353
Silkiss RZ (1993) THC:YAG nasolacrimal duct recanalization. Ophthalmic Surg 24(11):772–774
Devos D, Creac’h C, Laureau E, Bourriez JL, Guieu JD (2000) Thulium laser evoked potentials. Normative values for the arms and legs (French). Neurophysiol Clin 30(5):313–322
Theisen D, Ott V, Bern HW, Danick V, Keller R, Brinkmann R (2003) Cw high power IR-laser at 2microns for minimally invasive surgery. In: Proceedings of SPIE, vol 5142, pp 96–100
El-Sherif AF, King TA (2003) Soft and hard tissue ablation with short-pulse high peak power and continuous thulium-silica fiber lasers. Lasers Med Sci 18(3):139–147
Rink K, Delacretaz G, Salathe RP (1995) Fragmentation process of current laser lithotriptors. Lasers Surg Med 16(2):134–146
Fried NM (2005) High-power laser vaporization of the canine prostate using a 110 W Thulium fiber laser at 1.91 microns. Lasers Surg Med 36(1):52–56
Fried NM, Murray KE (2005) High-power thulium fiber laser ablation of urinary tissues at 1.94 microm. J Endourol 19(1):25–31
Wollin TA, Denstedt JD (1998) The holmium laser in urology. J Clin Laser Med Surg 16(1):13–20
Fried NM (2005) Thulium fiber laser lithotripsy: an in vitro analysis of stone fragmentation using a modulated 110-watt Thulium fiber laser at 1.94 microns. Lasers Surg Med 37(1):53–58
Xia SJ, Zhang YN, Lu J, Sun XW, Zhang J, Zhu YY, Li WG (2005) Thulium laser resection of prostate-tangerine technique in treatment of benign prostate hyperplasia (Chinese). Zhonghua Yi Xue Za Zhi 30;85(45):3225–3228
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marks, A.J., Teichman, J.M.H. Lasers in clinical urology: state of the art and new horizons. World J Urol 25, 227–233 (2007). https://doi.org/10.1007/s00345-007-0163-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-007-0163-x