Abstract
Cadmium (Cd) is a biologically non-essential and phytotoxic heavy metal pollutant. In this study, we estimated the Cd accumulation potential of Indian mustard and identified factors responsible for Cd uptake, tolerance, and detoxification. Eight transcriptomic libraries were sequenced and ≈ 230 million good quality reads were generated. The alignment rate against B. juncea reference genome V1.5 varied in the range of 85.03–90.06%. Comparative expression analysis using DESeq2 revealed 11,294 genes to be significantly differentially expressed under Cd treatment. The agriGO singular enrichment analysis revealed genes related to response to chemical, oxidative stress, transport, and secondary metabolic process were upregulated, whereas multicellular organismal development, developmental process, and photosynthesis were downregulated by Cd treatment. Furthermore, 616 membrane transport proteins were found to be significantly differentially expressed. Cd-related transporters such as metal transporter (Nramp1), metal tolerance protein (MTPC2, MTP11), cadmium-transporting ATPase, and plant cadmium resistance protein (PCR2, PCR6) were upregulated whereas cadmium/zinc-transporting ATPase (HMA2, HMA3, HMA4), high-affinity calcium antiporter (CAX1), and iron transport protein (IRT1) were downregulated by Cd treatment. A total of 332 different gene-networks affected by Cd stress were identified using KAAS analysis. Various plant hormones signaling cascades were modulated suggesting their role in Cd stress tolerance. The regulation overview using MapMan analysis also revealed gene expression related to plant hormones, calcium regulation, and MAP kinases were altered under Cd stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a toxic heavy metal (HM) and a widespread environmental pollutant. The concentration of Cd in soil is increasing over the years, for example, it is reported to increase by 0.2% per year in Scandinavia (Jarup 2003). Although the sources of Cd are both natural and anthropogenic, the contribution of the latter far exceeds the former. The anthropogenic factors for Cd pollution are atmospheric deposition, metal smelting, fuel combustion, application of phosphate fertilizers, incineration and dumping of Cd-polluted waste, and sewage and sludge disposal. Cd can immobilize in soil by binding to organic matter. The agricultural sites polluted with Cd are of major concern as being highly soluble Cd is readily absorbed by plants and enters the food chain (Sarwar et al. 2010). The dietary consumption of such Cd-containing crops can cause chronic toxicity in human. Besides being carcinogenic, Cd intoxication can damage the kidney, liver, bones, and lungs (Rahimzadeh et al. 2017). Cd is a non-essential element for plant growth and can cause stunting, leaf rolls, chlorosis, oxidative stress, genotoxicity and inhibition of the photosynthesis, respiration and nitrogen metabolism in plants (Andresen and Küpper 2013).
Remediation of environmental pollutants is a financial and technical challenge. The physicochemical methods like excavation, adsorption, biosorption, ion-exchange, precipitation, and so on, are neither cost-effective nor suitable for widespread pollutants like cadmium. Plants that accumulate HMs in their aboveground tissue have the potential to be used for HM remediation. The technology of removing pollutants from the environment with the help of plants is termed as phytoremediation (Cunningham and Berti 1993). The ideal plant for phytoremediation purposes should be able to (1) tolerate the pollutant, (2) accumulate the pollutant in an easily harvestable part, (3) adapted to grow locally, (4) and accumulate high biomass. The idea of phytoremediation is not new, hitherto the true potential of phytoremediation remains to be achieved. For its successful implementation, selection of suitable plant species plays an important role. Members of the family Brassicaceae are known to accumulate various metal (loid) s in the aboveground tissue. Previous studies have suggested Indian mustard (Brassica juncea) as a potential species for phytoremediation. Indian mustard is reported to accumulate various metal(loid)s, for example, Cd, As, Pb and Cu in its aboveground part (Goswami and Das 2015).
Cd toxicity can generate oxidative stress in plants although it is not a redox-active element. The accumulated Cd ions disturb the redox balance resulting in the generation of reactive oxygen species (ROS) which further leads to the destruction of cellular and metabolic function. The level of enzymatic and non-enzymatic antioxidants is reported to increase in response to Cd exposure to alleviate the oxidative damage (Ahmad et al. 2011; Choudhury and Kumar 2004). Stress perception is important in providing tolerance towards a particular stress. The Cd stress is reported to activate various kinases of the MAPK signaling cascade which further phosphorylate numerous substrates including transcription factors (DalCorso et al. 2010; Liu et al. 2010). The MAPK signaling cascade in plants is involved in the perception of environmental and developmental stimuli (Jonak et al. 2002). Calcium ions (Ca2+) are also shown to be involved in signal cascades of environmental stimuli. Studies have shown the protective role of Ca against Cd toxicity (Wang and Song 2009). Ca modulates the activity of target proteins and protein kinases via direct binding or through calmodulin (CALM) (Roberts and Harmon 1992). Another consequence of Cd toxicity is the modulation of plant hormone signaling. The level of abscisic acid (ABA) is shown to be increased under Cd stress in oilseed rape (Brassica napus L.) (Yan et al. 2016). Transcriptomic studies have identified transcription factors belonging to several families, for example, WRKY, ethylene-responsive factor (ERF) and myeloblastosis protein (MYB) to control gene expression under Cd stress. Previous studies were limited to biochemical analysis, determination of Cd bio-accumulation or characterization of a limited number of genes for Cd tolerance and accumulation (Mobin and Khan 2007; Qadir et al. 2004; Zhu et al. 1999). The underlying mechanism and the gene networks involved in Cd tolerance and accumulation in Indian mustard remain to be identified. With the advances in high-throughput technology, it has become possible to study the whole stress transcriptome for non-modal crops. In the present study, we investigated Indian mustard cultivar RLM514 for understanding the molecular mechanism of Cd tolerance and identification of modulated gene networks using RNA-sEq. The cultivar RLM514 has been tested to accumulate arsenic (As) in a separate study conducted in our laboratory. Thus, the cultivar RLM514 constitutes a fascinating plant material to study uptake, translocation, and detoxification of metal(loid)s and can contribute significantly to phytoremediation of sites contaminated with multiple metal(loid)s.
Materials and Methods
Plant Material and Growth Condition
For the present study, we used genotype RLM514 of Indian mustard. Seeds were surface sterilized using 3% H2O2 and subsequently rinsed thoroughly with distilled H2O. Seeds were then germinated on trays containing autoclaved vermiculite and allowed to grow for 14 days in controlled conditions (14 h photoperiod; at 28 ± 2 °C).
Cadmium Treatment and Bioaccumulation Estimation
The seedlings grown as discussed above were uprooted carefully and washed thoroughly with dH2O. The seedlings were then transferred to hydroponic solutions and divided into two groups, that is, control and Cd-treated. The control group was grown without any Cd whereas the Cd-treated group was supplied with different concentrations of Cd (250 µM, 500 µM, 750 µM and 1000 µM) as CdCl2. After 72 h, seedlings were taken out of the hydroponic solutions and washed thoroughly with dH2O. The root and shoot tissues were then oven dried separately for 48 h at 60 °C. A total of 100 mg of the dried samples were digested using a microwave in 8 mL mix of concentrated HNO3 and H2O2 (3:1) and subsequently diluted with milliQ, filtered through Whatman filter paper and the amount of Cd bioaccumulated was determined using an atomic absorption spectrometer (Shimadzu Model AA-7000).
RNA Sequencing
After 72 h of treatment, the roots and shoots of control and maximum Cd-accumulating groups (750 µM) were harvested and ground using liquid nitrogen. Total RNA was isolated from the ground tissues using a NucleoSpin RNA Plant kit (Macherey–Nagel), as per the manufacturer’s instructions. The quantity of RNA was estimated on a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific) and the quality was confirmed using agarose gel electrophoresis. The RNA integrity was determined using Bio-analyzer (Agilent Technologies). The mRNA was isolated from the total RNA using oligo-dT beads using a TruSeq RNA sample preparation kit (Illumina). Subsequently, cDNA was synthesized and sequencing libraries were constructed using random fragmentation followed by adapter ligation and PCR amplification. A total of eight sequencing libraries were generated consisting of two biological replicates from the root and shoot tissues of control and Cd-treated groups. Prepared libraries were quantified using qPCR according to the Illumina qPCR Quantification protocol guide and sequenced on the HiSeq platform (Illumina Inc.) using 100 bp paired-end sequencing generating 20–25 million reads per library.
Transcriptome Assembly
The FastQC (version 0.11.4) was used to check the quality of raw reads (Andrews 2010). Afterward, the Trimmomatic tool (version 0.32) was used for trimming adapters and low quality reads (Phred Score ≤ 20) (Bolger et al. 2014). Paired-end reads having less than a 100 bp sequence length were removed from further analysis. Trimmed paired-end reads were aligned against the B. juncea reference genome V1.5 (Yang et al. 2016) using HISAT (Galaxy Version 2.0.5.1). The aligned reads were assembled to form potential transcripts using StringTie (Galaxy Version 1.3.3) (Pertea et al. 2015). CD-HIT-EST was used to make the master transcriptome (transcriptome consisting of all the 8 samples) non-redundant at 90% sequence identity cut-off (Huang et al. 2010).
Comparative Expression Analysis
The abundance of potential transcripts was determined from alignment files using HTSeq (Anders et al. 2015). For comparative expression analysis, the count tables generated using HTseq were analyzed using DESeq2 (Love et al. 2014). Comparisons were made between control and Cd-treated tissues. The transcripts with a false discovery rate (FDR) below 0.05 and log2 fold change is greater than 2 or less than − 2 were considered significantly differentially expressed.
Functional Annotations
A BLASTx homology search was performed against the TAIR10 database (https://www.arabidopsis.org) to determine the functional description of differentially expressed transcripts and against the transporter classification database (TCDB) for identification of the transporters (http://www.tcdb.org/) (e-value < 10−3). Gene ontology (GO) analysis was performed using agriGO singular enrichment analysis (SEA) with FDR < 0.05 (Du et al. 2010) and visualized using REVIGO (Supek et al. 2011). Various regulatory and metabolic pathways were determined and visualized using KAAS (KEGG Automated Annotation Server) (Moriya et al. 2007) and MapMan (Version 3.6.0)(Thimm et al. 2004).
Real-Time Quantitative PCR (RT-qPCR)
The root and shoot tissues from control and Cd-treated groups were harvested in liquid nitrogen after 72 h of Cd treatment. The harvested tissues were then utilized for total RNA isolation using the NucleoSpin RNA Plant kit (Macherey–Nagel), as per the manufacturer’s instructions. The quality and quantity of RNA were estimated using agarose gel electrophoresis and NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific) respectively. RNase-free water was used to dilute and adjust RNA concentration (100 ng/µL) in each sample. Later, cDNA synthesis was performed using a high-capacity cDNA reverse transcription kit (Applied Biosystems) as per the manufacturer’s instructions. Primer-pairs (Online Resource 1) were designed from selected transcripts using Primer3Plus (Untergasser et al. 2007). PowerUp SYBR Green Master Mix (Applied Biosystems) was used to perform RT-qPCR. The comparative gene expression was calculated using the 2−ΔΔCT method (Rao et al. 2013). A housekeeping gene (actin) was used as a reference gene for calculations.
Results
Cadmium Bioaccumulation
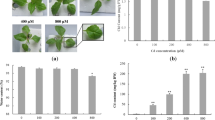
After 72 h, the active accumulation of Cd in shoots increased linearly with increase in treatment concentration until it reached a maximum value (1057.92 mg/Kg) at 750 µM of Cd (Fig. 1a) whereas in roots it was 36703.063 mg/ Kg at 500 µM of Cd (Fig. 1b). The results of the present study are in line with the study in which Cd accumulation increased with an increasing concentration of treatment in Sedum alfredii and reached a maximum of 5659.334 mg/Kg at 500 µM of Cd (Ni and Wei 2003). In another hydroponics study, Cd accumulation in roots was detected to be more than 5000 mg/Kg and 15,000 mg/Kg in poplar and willow clones, respectively (Zacchini et al. 2009).
Transcriptome Assembly and Functional Annotation
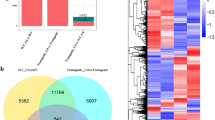
We obtained ≈ 230 million good quality reads (20–30 million paired-end reads per library) from 8 eightibraries generated from the root and shoot tissue. For reference-guided transcriptome assembly, the reads were aligned against the B. juncea reference genome (V1.5). The alignment rate varied in the range of 85.03–90.06% (Online Resource 2). The estimation of the global quality of the whole RNA-seq dataset and reproducibility of biological replicates is critical for gene-expression analysis. There are no clear standards but it is expected that in a principal component analysis (PCA) biological replicates will cluster together. The PCA performed using the alignment files clustered the biological replicates together also the different tissues, that is, root and shoot, were clustered on different quadrants (Fig. 2). The results of PCA analysis authenticate the reliability of biological replicates used in the present study (Conesa et al. 2016).
For comparative expression, comparisons were made among Cd-treated and control tissues. We found 11,294 genes to be significantly differentially expressed (log2 fold change is > 2 or < − 2 at FDR < 0.05) (Fig. 3) out of which 911 (8.06%) were mutually expressed in root and shoot, whereas 7370 (65.26%) were expressed only in root and 2101 (18.60%) were exclusive to shoots. We found 2,021 (67.09%) genes to be upregulated, whereas 992 (32.90%) were downregulated in the shoot. Similarly, 4682 (56.53%) genes were upregulated and 3599 (43.46%) were downregulated in root (Fig. 4). A total of 18,184 transcripts mapping to differentially expressed genes (DEGs) were identified by a search against the Arabidopsis thaliana TAIR10 protein database (https://www.arabidopsis.org) for functional annotation of genes.
DEGs distribution in Cd-treated root and shoot tissues. Root_UR and Root_DR represent no. of DEGs upregulated and downregulated in Cd-treated roots in comparison to control tissues. Shoot_UR and Shoot_DR represent no. of DEGs upregulated and downregulated in Cd-treated shoots in comparison to control tissues
Transporters
A total of 4670 transcripts from DEGs mapped to 616 different membrane transport proteins (Online Resource 3) Further the transport proteins were assigned to seven classes, that is, channels/pores (148), electrochemical potential-driven transporters (200), primary active transporters (131), group translocators (12), transmembrane electron carriers (13), accessory factors involved in transport (40) and incompletely characterized transport systems (65). We found Cd-related transporters such as metal transporter (Nramp1), zinc transporter (ZIP8), metal tolerance protein (MTPC2, MTP11), zinc-induced facilitator (ZIF1), cadmium-transporting ATPase, plant cadmium resistance protein (PCR2, PCR6), metal-nicotianamine transporter (YSL2) to be upregulated and cadmium/zinc-transporting ATPase (HMA2, HMA3, HMA4), high affinity calcium antiporter (CAX1), iron transport protein (IRT1), zinc transporter 1 precursor (ZRT/IRT-like protein 1) were downregulated in response to Cd treatment. Furthermore, 130 transcripts mapped to different ABC transporters among which MDR (15 transcripts) were upregulated while other ABC transporters such as ABCA12, ABCB2, ABCC5 and ABC transporter G family members were downregulated in response to Cd.
Gene Ontology (GO) and Gene-Network Analysis
Gene ontology (GO) analysis of DEGs revealed 174 and 81 significant GO terms (FDR < 0.05) were upregulated and downregulated, respectively in Cd-treated shoots. In addition, 210 and 55 significant GO terms were upregulated and downregulated in Cd-treated roots (Online Resource 4). Overall, the genes associated with response to a stimulus, jasmonic acid (JA), localization, and secondary metabolism were upregulated, whereas multi-organism process, developmental process, reproduction, and starch metabolism were downregulated in Cd-treated tissues (Fig. 5).
Statistically significant gene ontology (biological process) terms associated with DEGs in a downregulated in roots, b upregulated in roots, c downregulated in shoots, d upregulated in shoots as obtained from REVIGO at allowed similarity 0.5. Colours represent the p values (Blue and green bubbles represent more significant p values than the orange and red bubbles). (Color figure online)
We found 332 different pathways in KAAS analysis to be affected by Cd stress (Online Resource 5) which include glutathione (GSH) metabolism (87 transcripts), phenylpropanoid biosynthesis (112 transcripts), MAPK signaling pathway (125 transcripts), calcium signaling pathway (14 transcripts), and plant hormone signal transduction (184 transcripts). MapMan analysis of regulation overview also revealed the expression of various transcripts mapping to plant hormones, calcium regulation, MAP kinases, and protein degradation were altered under Cd stress (Fig. 6).
A total of 97 transcripts mapping to the MAPK signaling pathway were upregulated and 28 were downregulated (Online Resource 6). The genes MAP kinase substrate 1 (MKS1), WRKY transcription factor 33 and 22 (WRKY33, 22), mitogen-activated protein kinase kinase 4/5 (MKK4/5), aminocyclopropane-1-carboxylate synthase (ACS), serine/threonine-protein kinase OXI1 (OXI1), mitogen-activated protein kinase kinase 9 (MKK9), calmodulin (CALM), respiratory burst oxidase (RBOH) mapping to MAPK signaling in plants were upregulated. Three genes related to calcium signaling viz., phosphatidylinositol phospholipase C, delta (PLCD), mitochondrial calcium uniporter (MCU) and voltage-dependent anion channel protein 2 (VDAC2) were upregulated in Cd-treated tissues (Online Resource 7). A total of 136 transcripts mapping to plant hormone signal transduction were upregulated and 48 transcripts were found to be downregulated by Cd treatment.
Exposure to Cd in plants is well known to induce oxidative stress although it is a non-redox metal and unable to carry out Fenton-type reactions (Laspina et al. 2005). The level of ROS is maintained carefully inside a plant cell to avoid the oxidative stress. Cd can inhibit as well as stimulate several antioxidative enzymes. To avoid the oxidative damage due to ROS, plants employ various enzymatic and non-enzymatic antioxidants. A total of 50 transcripts mapped to peroxidases out of which 28 were upregulated. The gene expression of non-enzymatic antioxidants such as coumarins and flavonoids was also modulated by Cd exposure. We found 23 differentially expressed transcripts mapping to flavonoid biosynthesis out of which ten were upregulated in Cd-treated tissue. Among the transcripts mapping to GSH metabolism, the gene expression of a total of ten important components including glutathione S-transferase (GST), glutathione synthase (GSS) and gamma-glutamyltranspeptidase (GGT) found to be modulated by cadmium (Online Resource 8). A total of 66 different transcripts encoding GST were found to be significantly upregulated.
Among the plant hormone signal transduction, four genes (auxin-responsive protein IAA (AUX/IAA), auxin response factor (ARF), auxin-responsive GH3 gene family (GH3), and SAUR family protein (SAUR)) related to the auxin signaling cascade were modulated in Cd-treated tissues (Online Resource 9). In cytokinin (CKK) signaling, three genes (CRE1 (cytokinin response 1), type-A Arabidopsis response regulators (A-ARR), type-B Arabidopsis response regulators (B-ARR)) and two genes related to gibberellin signaling (gibberellin receptor (GID1) and phytochrome-interacting factor 4 (PIF4)) were differentially expressed in Cd-treated tissues. We also detected downregulation of adenylate dimethylallyltransferase (cytokinin synthase) (IPT) and upregulation of PP2C. Similarly, we found 5,6,3 and 3 differential genes related to ethylene, brassinosteroid (BR), jasmonic acid (JA) and salicylic acid signaling. The genes mapping to ethylene receptor (ETR), ethylene-insensitive protein 3 (EIN3), ethylene-responsive transcription factor 1 (ERF1), ethylene-responsive transcription factor 1 (ERF1) were upregulated and ethylene-insensitive protein 2 (EIN2) were downregulated by Cd treatment. EIN2 and EIN3 play a central role in the ethylene signaling. The plant steroid hormones, brassinosteroids (BRs), play important roles in alleviating biotic and abiotic stress (Rajewska et al. 2016). We found two brassinosteroid-insensitive genes, BRI1and BIN2, known components of BR signaling, to be modulated under cd treatment. BRI1, essential for BR perception, was downregulated, whereas BIN2, involved in negative regulation of BR signaling, was upregulated in Cd-treated tissues (Nam and Li 2002). The gene expression of transcription factors brassinosteroid resistant 1 and 2 (BZR1and 2) was upregulated, although BIN2 is its negative regulator. The gene expression of xyloglucan:xyloglucosyl transferase (TCH4), cyclin D3 (CYCD3), the response genes of the BR signaling pathway was also upregulated in Cd-treated tissue. TCH4 encodes for cell wall protein involved in cell wall modification in response to abiotic stress, whereas CYCD3 promotes supernumerary cell division (Sun et al. 2017). The gene expression of all the three components jasmonic acid-amino synthetase (JAR1), jasmonate ZIM domain-containing protein (JAZ) and MYC2 were upregulated by Cd treatment. JAR1 conjugates JA with isoleucine (Ile) which further promotes proteasomal degradation of JAZ and hence releases transcription factors such as MYC2 involved in the induction of early JA-responsive genes. The regulatory protein (NPR1) is a critical component of SA signaling and pathogenesis-related protein 1 (PR1) is an SA response gene. NPR1 and PR1 were upregulated by Cd treatment.
Gene Expression Analysis Using Quantitative Real-Time PCR (RT-qPCR)
We performed RT-qPCR on 13 selected genes mapping to MAPK signaling, calcium signaling, plant hormone signal transduction, and GSH metabolism to validate the comparative expression analysis (Online resource 1). The RNA-seq data from comparative expression analysis and RT-qPCR data showed a positive correlation (r = 0.648) which further confirms the results of the present study (Fig. 7).
Discussion
Cadmium Uptake and Sequestration is Facilitated Through Various Transporters
Metal transporters play important roles in plant nutrition and metal homeostasis (Nelson 1999). Furthermore, these transporters mediate the uptake of metal ions from the surroundings and detoxification via vacuolar compartmentalization. Toxic metals like Cd can enter the plant cell via the transporters of essential elements like Ca, Zn and iron (Fe). The metal transporter encoding the gene Nramps in Arabidopsis can transport both Fe and Cd (Thomine et al. 2000). The ZIP gene family can transport a variety of metal ions including Cd, Zn, Mn and Fe (Guerinot 2000). The comparative expression analysis suggests that Nramp1 and ZIP8 could be the major Cd influx transporters present in the plasma membrane of Indian mustard. Further, the Cd2+ ions from the cytoplasm are then sequestered into vacuoles with the help of vacuolar transporters (Fu et al. 2017). Among the Zn transporters present in the vacuolar membrane, ZIF1 and MTPC2 could be involved in Cd sequestration in the present study. Besides the upregulation of transcripts encoding Cd-influx transporters, the upregulation of efflux transporters such as PCR2, PCR6, YSL2, and probable cadmium-transporting ATPase could also play an important role in improving Cd tolerance by pumping the toxic Cd ions out of the cell. The dual localization of AtPCR2 in the epidermis and vascular tissue attributes to its contrasting roles, that is, as Zn-efflux transporter and Zn-translocator (Song et al. 2010). Similarly, PCR2 can also aid in the translocation of Cd from root to shoot. YSL2 is known to transport nicotinamine (NA)-metal complexes laterally in the vasculature (DiDonato et al. 2004). The upregulation of transcripts encoding YSL2 is consistent with its role in imparting Cd tolerance. It is plausible that as a detoxification strategy, the gene expression of a few transporters such as HMA, IRT, ZRT/IRT-like protein1 and CAX1 was downregulated in the present study. The HMAs are type 1B P-type ATPases and their role in maintaining metal homeostasis is well established (Miyadate et al. 2011; Williams and Mills 2005). Sequence analysis of these P-type ATPases revealed that HMA2, HMA3, and HMA4 in Arabidopsis are closely related and essential for Zn homeostasis (Cobbett et al. 2003). Several studies reported AtHMA2, AtHMA4 and OsHMA3 to be involved in xylem loading and translocating Cd from root to shoot (Hussain et al. 2004; Miyadate et al. 2011). IRT1 and ZRT/IRT-like protein1 are important metal homeostasis proteins known to transport metals including Cd, Fe and Zn (Vert et al. 2002). CAXs are antiporters shown to transport Cd into the vacuole and export H+ (Hirschi et al. 2000; Koren’kov et al. 2007).
Cadmium-Induced Oxidative Stress Management
The HM Cd is capable of producing oxidative stress in plants. Plant species that are tolerant to Cd tend to manage the oxidative stress via upregulating gene expression of various enzymatic and non-enzymatic antioxidants. The results of the present study are in line with the results of several studies exhibiting the upregulation of antioxidants in response to Cd exposure. Among the enzymatic antioxidants CAT, DHAR, APX, and GR play important roles in alleviating oxidative stress and transduction of stress stimuli. Several studies have shown the importance of non-enzymatic antioxidants like flavonoids and coumarins in oxidative stress management (Agati et al. 2012; Gacche and Jadhav 2012; Kostova et al. 2011; Pietta 2000). The upregulation of these non-enzymatic antioxidants in the present study indicates their role in Cd-induced oxidative stress management. GSH is central in controlling the differential expression of several anti-oxidants including glutathione reductase, superoxide dismutase, phenylalanine ammonia lyase, and chalcone synthase. Several studies have reported a positive relation between Cd treatment, GSH content and GST activity (Nocito et al. 2011; Yang et al. 2018b; Zhand et al. 2013; Zhang and Ying 2008). Inside a plant cell, Cd-ions can bind to GSH and phytochelatins with high affinity leading to the formation of Cd-GSH/PC complexes which are then transported to vacuoles or apoplasts for detoxification. Glutathione S-transferase (GST) contributes to detoxification by catalyzing complexation of GSH with Cd and also helps in reducing the Cd-induced oxidative stress.
Cadmium Stress Perception and Signaling
Exposure to toxic substances leads to changes in the gene expression pattern to mitigate the stress stimuli. A number of signaling pathways lead to differential gene regulation. The mitogen-activated protein kinase (MAPK) pathway and calcium (Ca) signaling pathways are the important pathways involved in transduction of external stress stimuli to the nucleus for a pertinent response (Huang et al. 2012; Price et al. 1994). The MAPK signaling cascade modulates homeostasis of ROS, cell death, defense response to pathogens, stress adaptation and tolerance in plants. Cd-induced ROS is detected by OXI1 (oxidative signal-inducible 1) which further stimulates various serine/threonine kinases of MAPK signaling (Rentel et al. 2004). These proteins can stimulate the rapid signal transduction of a downstream mitogen-activated protein kinase (MAPK) cascade. In the present study, the MAPK signaling cascade activated a wide range of kinases and transcription factors such as WRKY 22/29 and 33, MYC2 and ERF1 which further can induce a wide array of stress and growth responses. Previous studies have also reported the activation of the MAPK signaling by cadmium (Lin and Aarts 2012; Yang et al. 2018a; Yeh et al. 2007). The concentration of Ca2+ and Ca-binding proteins (calmodulin; CALM) are known to be altered under abiotic stress (Yang and Poovaiah 2003). MCU increases cellular concentrations of Ca2+ and CALM binds to Ca2+ which brings about conformational changes in it. CALM and Ca are hypothesized to play roles in Cd signaling (Suzuki et al. 2001). The gene expression of CALM was upregulated in Cd-treated tissues which can regulate the plant’s response to Cd via a variety of mechanisms including regulation of expression, ion transport, stress tolerance, and metabolism.
Plant Hormones Signaling in Response to Cadmium
Plant hormones are small signaling molecules known to regulate varied physiological processes including responses to biotic and abiotic stresses (Davies 2010). Several studies have exhibited their role in tolerance towards Cd stress in plants (Guo et al. 2007, Hsu and Kao 2003, 2005). Modulation of gene expression of phytohormone signaling components indicates their involvement in managing Cd stress in the present study. Results indicate that the plant hormones (IAA, ABA, JA, ethylene, BR, CK, and GA signaling) were active in response to Cd stress in Indian mustard. In rice under Cd stress, the phytohormones including CKs, IAA, ABA, JA, and GA were detected, suggesting their role in Cd stress response (Cai et al. 2015). IAA signaling was activated and the gene expression of an auxin-responsive gene belonging to the family GH3 and SAUR were upregulated which could be producing proteins involved in imparting Cd tolerance (Minglin et al. 2005). IAA was shown to alleviate Cd toxicity in eggplant by reducing Cd uptake and enhancing antioxidant activity/production (Singh and Prasad 2015).
The expression of transcripts mapping to components of ABA signaling was regulated under Cd stress. PP2C is a key repressor of ABA signaling and regulates its downstream responses. Out of the transcripts mapping to PP2C, 17 were upregulated and 2 downregulated which could be a possible mechanism to maintain homeostasis and regulating the ABA amount in response to Cd stress. ABA was shown to impart Cd tolerance via reducing root to shoot translocation of cadmium (Hsu and Kao 2003). JAs are known to play roles in Cd stress response (Singh and Shah 2014). JA was shown to alleviate Cd-induced oxidative stress via activation of antioxidant machinery and accumulation of phytochelatins (Maksymiec et al. 2007; Yan et al. 2013). MYC2 is a master regulator of JA signaling and involved in crosstalk between plant hormones such as ABA, SA, GA, and IAA. The upregulation of EIN3 and its target ERF1 in Cd-treated tissues exhibited that ethylene signaling is active under Cd stress. Activation of transcription factors like ERF1 then regulate the expression of downstream ethylene-responsive genes (Abozeid et al. 2017; Kendrick and Chang 2008). Ethylene is suggested to enhance the Cd stress response via controlling GSH content and the oxidative stress profile in A. thaliana (Schellingen et al. 2015). BRs are polyhydroxy steroidal plant hormones known to improve various abiotic stresses including Cd tolerance via increasing osmolytes such as proline content and increasing activities of antioxidative enzymes in B.juncea and Phaseolus vulgaris (Hayat et al. 2007; Rady 2011). Cd treatment leads to downregulation of an enzyme involved in CKK synthesis (cytokinin synthase) and upregulation of an enzyme that catalyzes the degradation of cytokinins (cytokinin dehydrogenase; CKX). The decrease in cytokinin (CKK) contents aid in reducing transpiration driven Cd translocation and hence providing tolerance towards Cd toxicity (Veselov et al. 2003).
Conclusion
Indian mustard exhibited the potential for Cd remediation as evident from bioaccumulated of a large amount of Cd in shoots. Huge reprogramming at the transcriptomic level was exhibited by Cd treatment. Various transporters involved in Cd uptake and efflux were identified. GSH is involved in detoxification of Cd ions via sequestration into vacuoles. MAPK signaling and Ca signaling were found to play important roles in Cd stress perception. Furthermore, plant hormone signaling was found to be affected by Cd which contributes to Cd tolerance through varied ways such as enhancing osmolyte content, antioxidant enzyme activity or reduction in root to shoot Cd translocation. The present study has provided a thorough understanding of the molecular mechanisms involved in Cd uptake, tolerance, and detoxification in Indian mustard.
Data Availability
The raw sequence data of the libraries are available from the NCBI SRA under the study with Accession Nos.: SRP152398 and SRP126203.
References
Abozeid A, Ying Z, Lin Y, Liu J, Zhang Z, Tang Z (2017) Ethylene improves root system development under cadmium stress by modulating superoxide anion concentration in Arabidopsis thaliana. Front Plant Sci 8:253
Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76
Ahmad P, Nabi G, Ashraf M (2011) Cadmium-induced oxidative damage in mustard Brassica juncea (L.) Czern. & Coss. plants can be alleviated by salicylic acid South African. J Bot 77:36–44
Anders S, Pyl PT, Huber W (2015) HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169
Andresen E, Küpper H (2013) Cadmium toxicity in plants. In: Cadmium: from toxicity to essentiality. Springer, New York, pp 395–413
Andrews S (2010) FastQC: a quality control tool for high throughput sequence data. Babraham Institute, Cambridge
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Cai B-D, Yin J, Hao Y-H, Li Y-N, Yuan B-F, Feng Y-Q (2015) Profiling of phytohormones in rice under elevated cadmium concentration levels by magnetic solid-phase extraction coupled with liquid chromatography tandem mass spectrometry. J Chromatogr A 1406:78–86
Choudhury S, Kumar S (2004) Role of salicylic acid in regulating cadmium induced oxidative stress in Oryza sativa L. roots. Bulg J Plant Physiol 30:95–110
Cobbett CS, Hussain D, Haydon MJ (2003) Structural and functional relationships between type 1B heavy metal-transporting P-type ATPases Arabidopsis. New Phytol 159:315–321
Conesa A et al (2016) A survey of best practices for RNA-seq data analysis. Genome Biol 17:13
Cunningham SD, Berti WR (1993) Remediation of contaminated soils with green plants: an overview. In Vitro Cell Dev Biol Plant 29:207–212
DalCorso G, Farinati S, Furini A (2010) Regulatory networks of cadmium stress in plants. Plant Signal Behav 5:663–667
Davies PJ (2010) The plant hormones: their nature, occurrence, and functions. In: Plant hormones. Springer, New York, pp 1–15
DiDonato RJ, Roberts LA, Sanderson T, Eisley RB, Walker EL (2004) Arabidopsis Yellow Stripe-Like2 (YSL2): a metal-regulated gene encoding a plasma membrane transporter of nicotianamine–metal complexes. Plant J 39:403–414
Du Z, Zhou X, Ling Y, Zhang Z, Su Z (2010) agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38:W64–W70
Fu X et al (2017) Genome-wide identification of sweet orange (Citrus sinensis) metal tolerance proteins and analysis of their expression patterns under zinc, manganese, copper, and cadmium toxicity. Gene 629:1–8
Gacche RN, Jadhav SG (2012) Antioxidant activities and cytotoxicity of selected coumarin derivatives: preliminary results of a structure–activity relationship study using computational tools. J Exp Clin Med 4:165–169
Goswami S, Das S (2015) A study on cadmium phytoremediation potential of Indian mustard, Brassica juncea. Int J Phytorem 17:583–588
Guerinot M (2000) The ZIP family of metal transporters. Biochimica et biophysica acta 1465:190
Guo B, Liang Y, Zhu Y, Zhao F (2007) Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ Pollut 147:743–749
Hayat S, Ali B, Hasan SA, Ahmad A (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60:33–41
Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ (2000) Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation increased manganese tolerance. Plant Physiol 124:125–134
Hsu Y, Kao C (2003) Role of abscisic acid in cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Cell Environ 26:867–874
Hsu YT, Kao CH (2005) Abscisic acid accumulation and cadmium tolerance in rice seedlings. Physiol Plant 124:71–80
Huang Y, Niu B, Gao Y, Fu L, Li W (2010) CD-HIT Suite: a web server for clustering and comparing biological sequences. Bioinformatics 26:680–682
Huang T-L, Nguyen QTT, Fu S-F, Lin C-Y, Chen Y-C, Huang H-J (2012) Transcriptomic changes and signalling pathways induced by arsenic stress in rice roots. Plant Mol Biol 80:587–608
Hussain D et al (2004) P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell 16:1327–1339
Jarup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Jonak C, Ökrész L, Bögre L, Hirt H (2002) Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol 5:415–424
Kendrick MD, Chang C (2008) Ethylene signaling: new levels of complexity and regulation. Curr Opin Plant Biol 11:479–485
Koren’kov V et al (2007) Enhanced Cd2+-selective root-tonoplast-transport in tobaccos expressing Arabidopsis cation exchangers. Planta 225:403–411
Kostova I, Bhatia S, Grigorov P, Balkansky S, Parmar S, Prasad VK, Saso A L (2011) Coumarins as antioxidants. Curr Med Chem 18:3929–3951
Laspina N, Groppa M, Tomaro M, Benavides M (2005) Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci 2:323–330
Lin Y, Aarts M (2012) The molecular mechanism of zinc and cadmium stress response in plants Cellular and molecular life sciences. CMLS 69:3187–3206
Liu X-M et al (2010) Cadmium activates Arabidopsis MPK3 and MPK6 via accumulation of reactive. oxygen species. Phytochemistry 71:614–618
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550
Maksymiec W, Wojcik M, Krupa Z (2007) Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and ascorbate. Chemosphere 66:421–427
Minglin L, Yuxiu Z, Tuanyao C (2005) Identification of genes up-regulated in response to Cd exposure in Brassica juncea. L. Gene 363:151–158
Miyadate H et al (2011) OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol 189:190–199
Mobin M, Khan N (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164:601–610
Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M (2007) KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res 35:W182–W185
Nam KH, Li J (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110:203–212
Nelson N (1999) Metal ion transporters and homeostasis. EMBO J 18:4361–4371
Ni T-H, Wei Y-Z (2003) Subcellular distribution of cadmium in mining ecotype Sedum alfredii. Acta Botanica Sinica 45(8):925–928
Nocito FF, Lancilli C, Dendena B, Lucchini G, Sacchi GA (2011) Cadmium retention in rice roots is influenced by cadmium availability, chelation and translocation. Plant Cell Environ 34:994–1008
Pertea M, Pertea GM, Antonescu CM, Chang T-C, Mendell JT, Salzberg SL (2015) StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33:290
Pietta P-G (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Price A, Taylor A, Ripley S, Griffiths A, Trewavas A, Knight M (1994) Oxidative signals in tobacco increase cytosolic calcium. Plant Cell (USA) 6:1301–1310
Qadir S, Qureshi M, Javed S, Abdin M (2004) Genotypic variation in phytoremediation potential of Brassica juncea cultivars exposed to Cd stress. Plant Sci 167:1171–1181
Rady MM (2011) Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci Hortic 129:232–237
Rahimzadeh MR, Rahimzadeh MR, Kazemi S, Moghadamnia A-a (2017) Cadmium toxicity and treatment: an update. Casp J Intern Med 8:135
Rajewska I, Talarek M, Bajguz A (2016) Brassinosteroids and response of plants to heavy metals action. Front Plant Sci 7:629
Rao X, Huang X, Zhou Z, Lin X (2013) An improvement of the 2ˆ (–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinform Biomath 3:71
Rentel MC et al (2004) OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 427:858
Roberts DM, Harmon AC (1992) Calcium-modulated proteins: targets of intracellular calcium signals in higher plants. Ann Rev Plant Biol 43:375–414
Sarwar N, Malhi SS, Zia MH, Naeem A, Bibi S, Farid G (2010) Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agric 90:925–937
Schellingen K, Van Der Straeten D, Remans T, Vangronsveld J, Keunen E, Cuypers A (2015) Ethylene signalling is mediating the early cadmium-induced oxidative challenge in Arabidopsis thaliana. Plant Sci 239:137–146
Singh S, Prasad SM (2015) IAA alleviates Cd toxicity on growth, photosynthesis and oxidative damages in eggplant seedlings. Plant Growth Regul 1:87–98
Singh I, Shah K (2014) Evidences for structural basis of altered ascorbate peroxidase activity in cadmium-stressed rice plants exposed to jasmonate. Biometals 27:247–263
Song W-Y et al (2010) Arabidopsis PCR2 is a zinc exporter involved in both zinc extrusion and long-distance zinc transport. Plant Cell 22:2237–2252
Sun J, Wang P, Zhou T, Rong J, Jia H, Liu Z (2017) Transcriptome analysis of the effects of shell removal and exogenous gibberellin on germination of Zanthoxylum seeds. Sci Rep (Nature Publisher Group) 7:1
Supek F, Bošnjak M, Škunca N, Šmuc T (2011) REVIGO summarizes and visualizes long lists of gene ontology terms. PloS one 6:e21800
Suzuki N, Koizumi N, Sano H (2001) Screening of cadmium-responsive genes in Arabidopsis thaliana. Plant Cell Environ 24:1177–1188
Thimm O et al (2004) MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37:914–939
Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI (2000) Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci USA 97:4991–4996
Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA (2007) Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 35:W71-W74
Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat J-F, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14:1223–1233
Veselov D, Kudoyarova G, Symonyan M, Veselov S (2003) Effect of cadmium on ion uptake, transpiration and cytokinin content in wheat seedlings. Bulg J Plant Physiol 29:353–359
Wang CQ, Song H (2009) Calcium protects Trifolium repens L. seedlings against cadmium stress. Plant Cell Rep 28:1341–1349
Williams LE, Mills RF (2005) P1B-ATPases–an ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci 10:491–502
Yan Z, Chen J, Li X (2013) Methyl jasmonate as modulator of Cd toxicity in Capsicum frutescens var. fasciculatum seedlings. Ecotoxicol Environ Saf 98:203–209
Yan H, Filardo F, Hu X, Zhao X, Fu D (2016) Cadmium stress alters the redox reaction and hormone balance in oilseed rape (Brassica napus L.) leaves. Environ Sci Pollut Res 23:3758–3769
Yang T, Poovaiah B (2003) Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci 8:505–512
Yang J et al (2016) The genome sequence of allopolyploid Brassica juncea and analysis of differential homoeolog gene expression influencing selection. Nat Genet 48:1225
Yang H et al (2018a) Transcriptome assembly and expression profiling of the molecular responses to cadmium toxicity in cerebral ganglia of wolf spider Pardosa pseudoannulata (Araneae: Lycosidae). Ecotoxicology 27:198–208
Yang H et al (2018b) Metatranscriptome analysis of the intestinal microorganisms in Pardosa pseudoannulata in response to cadmium stress. Ecotoxicol Environ Saf 159:1–9
Yeh C-M, Chien P-S, Huang H-J (2007) Distinct signalling pathways for induction of MAP kinase activities by cadmium and copper in rice roots J Exp Bot 58: 659–671
Zacchini M, Pietrini F, Mugnozza GS, Iori V, Pietrosanti L, Massacci A (2009) Metal tolerance, accumulation and translocation in poplar and willow clones treated with cadmium in hydroponics. Water Air Soil Pollut 197:23–34
Zhand C-h, Wu Z-y, Ting J, Ying G (2013) Purification and identification of glutathione S-transferase in rice root under cadmium stress. Rice Sci 20:173–178
Zhang C-H, Ying G (2008) Response of glutathione and glutathione S-transferase in rice seedlings exposed to cadmium stress. Rice Sci 15:73–76
Zhu YL, Pilon-Smits EA, Tarun AS, Weber SU, Jouanin L, Terry N (1999) Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing γ-glutamylcysteine synthetase. Plant Physiol 121:1169–1177
Acknowledgements
This work was supported by the Council for Scientific and Industrial Research, India under Grant No. 38(1403)/15/EMR-II. ST and SC acknowledge the fellowship received from ICMR towards Ph.D.
Author information
Authors and Affiliations
Contributions
PB designed and conceived of the study. ST analyzed and interpreted data, drafted the manuscript. SC helped in the acquisition of data and data analysis. PB and SC coordinated further in improving the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 3
: Details of BLASTx analysis against the transporter classification database. (XLSX 510 KB)
Online Resource 5
: KAAS summary and details of metabolic and regulatory pathways. (XLSX 22 KB)
Online Resource 6
: MAPK signaling pathway (The boxes in green represent transcripts with significant differential expression under Cd stress) (PNG 37 KB)
Online Resource 7
: Calcium signaling pathway (The boxes in green represent transcripts with significant differential expression under Cd stress) (PNG 27 KB)
Online Resource 8
: Glutathione metabolism pathway (The boxes in green represent transcripts with significant differential expression under Cd stress) (PNG 30 KB)
Online Resource 9
: Plant hormone signal transduction pathway (The boxes in green represent transcripts with significant differential expression under Cd stress) (PNG 32 KB)
Rights and permissions
About this article
Cite this article
Thakur, S., Choudhary, S. & Bhardwaj, P. Comparative Transcriptome Profiling Under Cadmium Stress Reveals the Uptake and Tolerance Mechanism in Brassica juncea. J Plant Growth Regul 38, 1141–1152 (2019). https://doi.org/10.1007/s00344-019-09919-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-09919-8