Abstract
Certain H2-oxidizing rhizobacteria promote the growth of legume plants nodulated with rhizobia devoid of an uptake hydrogenase system (Hup−). We demonstrated and assessed the plant growth-promoting ability of H2-oxidizing rhizobacteria naturally associating with lentil roots nodulated by rhizobia possessing an uptake hydrogenase system (Hup+ lentil) in semiarid Canada. The ten H2-oxidizing rhizobacteria isolated were strains of Variovorax paradoxus, Variovorax sp., Rhodococcus sp., Mycobacterium sp., Acinetobacter sp., Acinetobacter calcoaceticus, and Curtobacterium sp. Several of these strains increased Hup+ lentil shoot and root biomasses, and root nodule number in the absence or presence of drought stress. Inoculation with H2-oxidizing rhizobacteria enhanced the growth of Hup+ lentil infected by the fungal root pathogens Fusarium avenaceum, Rhizoctonia solani, and Pythium ultimum. Fusarium avenaceum growth was markedly suppressed by all H2-oxidizing rhizobacteria in vitro, and seven isolates also suppressed the growth of both R. solani and P. ultimum. Siderophore production was detected in nine isolates and one isolate could solubilize phosphate. Indole-3-acetic acid production was found in four isolates, and 1-aminocyclopropane-1-carboxylate deaminase activity in six isolates. Most H2-oxidizing rhizobacterial isolates exhibited multiple plant growth-promoting attributes and all isolates exhibited at least one. Our results suggest that the H2-oxidizing rhizobacteria naturally associating with lentil roots in semiarid Canada are beneficial in an Hup+ environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rhizobacteria benefiting plant growth are called plant growth-promoting rhizobacteria (PGPR) (Nelson 2004; Glick and others 2007; Belimov and others 2009). The beneficial effects of PGPR have been attributed to a variety of mechanisms. Some PGPR can solubilize phosphate from organic or inorganic compounds, thereby facilitating phosphate uptake and promoting plant growth (Vassilev and others 2006). Bacterial siderophores in the soil enhance iron uptake by plants and improve plant growth in iron-depleted soils (Vansuyt and others 2007). Bacterial siderophores can also act as inducers of plant disease resistance and thus have biocontrol potential (Glick and others 2007; Gan and others 2011). Some bacteria, called phytostimulators, produce substances such as the hormone indole-3-acetic acid (IAA), gibberellins, cytokinins, and the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase that stimulate the growth of plants (Nelson 2004).
Certain rhizobacteria can reduce the level of stress ethylene in plants by metabolizing its precursor with ACC deaminase, thus promoting root growth (Vanderhoef and Dute 1981; Belimov and others 2009; Laslo and others 2012). The suppression of stress ethylene can promote nodulation in legumes (Hunter 1993; Nascimento and others 2012) and protect plant productivity against drought stress (Arshad and others 2007; Belimov and others 2009). Abiotic and biotic stresses limit the growth and productivity of crops, particularly in arid and semiarid areas (Kramer and Boyer 1995). Several studies have reported that certain rhizosphere bacteria elicit tolerance to abiotic stresses such as drought and soil salinity (Zahir and others 2009; Shahzad and others 2010; Erskine and others 2011) and biotic stresses such as root rot diseases (Dworkin and Foster 1958; Figueiredo and others 2008; Gan and others 2011; Yang and others 2012; Zafar and others 2012). Recent research has shown the beneficial effects of co-inoculation with Rhizobium and PGPR on legume crop growth and nodulation (Banchio and others 2008; Iqbal and others 2012).
N2-fixing root nodules produce hydrogen (H2) gas that diffuses into soil. The exposure of soil to H2 gas from nodules was shown to stimulate H2-oxidizing rhizobacteria (Dong and Layzell 2001, 2002; Dong and others 2003; Maimaiti and others 2007) due to their ability to use H2 as an energy source (Zhang 2006; Annan and others 2012), and the growth-promoting effect of certain H2-oxidizing rhizobacteria associated with N2-fixing soybean (Glycine max) was presented as a natural mechanism offsetting the inefficiency of nitrogenase (Maimaiti and others 2007). This research highlighted the plant growth-promoting (PGP) potential of H2-oxidizing rhizobacteria (Dong and others 2003; Irvine and others 2004) in crop production.
Hydrogen evolution may benefit plants in stimulating H2-oxidizing PGPR in soil, but has a very high energetic cost to the plant. Over half the electrons allocated to nitrogenase by N2-fixing plants can be lost to H2 gas evolution (Simpson and Burris 1985). The amount of H2 released by N2-fixing nodules varies greatly as certain strains have an uptake hydrogenase system that improves their efficiency by recycling within the cell the H2 produced in the process of N2 fixation. Hydrogenase is rare in Rhizobium leguminosarum, the symbiont of lentil, but it exists, and the gain in N2 fixation efficiency provided by uptake hydrogenase explains why the strains selected by the industry of inoculants are ‘Hup+’ strains, that is, they possess an uptake hydrogenase system (Fernández and others 2005).
Recent research showed that the presence of H2 gas may be unessential for effective plant growth promotion by H2-oxidizing rhizobacteria. Inoculation with H2-oxidizing PGPR under controlled conditions produced the same level of soybean growth promotion in the presence or absence of H2 gas (Sakunpon and others 2014). This result is interesting because commercially available inoculants contain the energy-efficient Hup+ strains (Fernández and others 2005). Thus, using PGPR that are highly effective in the absence of a source of H2 gas appears to be a most interesting option for legume growth promotion. However, the plant growth-promoting ability of H2-oxidizing rhizobacteria in an Hup+ environment should first be tested.
Plant growth-promoting H2-oxidizing rhizobacteria are found in the rhizosphere of field-grown legumes, at least in Canada, suggesting their adaptation to life in the legume rhizosphere (Yang 2012). The selection of highly effective PGPR among these adapted rhizobacteria and the use of superior strains in combination with energy-efficient Hup+ rhizobial inoculants may benefit lentil production.
We isolated H2-oxidizing rhizobacteria from the rhizosphere of field-grown lentil and tested under controlled conditions the hypothesis that they promote the growth of lentil plants nodulated with commercial Hup+ rhizobial inoculants. This research is the first to examine the effect of H2-oxidizing rhizobacteria on lentil. Certain H2-oxidizing rhizobacteria were shown to promote the growth of N2-fixing soybean (Glycine max) (Dong and others 2003; Irvine and others 2004) and alfalfa (Medicago sativa L.) (Dean and others 2006), but no such research has been reported for other legumes.
Lentil is an important food legume and Canada is the world’s largest lentil producer (Canadian Agri-Food Trade Alliance 2016). Here we show the beneficial effects of H2-oxidizing rhizobacteria naturally associating with lentil roots on the productivity and nodulation of Hup+ lentil plants grown under optimal, drought stress, and biotic stress conditions. We report that all the H2-oxidizing rhizobacteria we isolated from field-grown lentil have the potential to promote lentil growth in an Hup+ environment and that most of them have multiple modes of plant growth promotion.
Materials and Methods
Isolation, Selection, and Identification of H2-Oxidizing Rhizobacteria
A total of 14 lentil cultivars were grown in field plots in Swift Current, Saskatchewan (50°8170N, 107°8410W; elevation 825 m), in the middle of the Canadian lentil-producing area. Rhizosphere soil, defined as the soil adhering to roots, was collected by careful brushing from nodulated lentil plants. These lentil plants were inoculated at seeding with a commercial strain of R. leguminosarum (Nitragin-C powder, Novozymes, Bagsvaerd, Denmark). The methylene blue reduction assay (Lambert and others 1985) confirmed the presence of an uptake hydrogenase system in that strain, thus its Hup+ status.
All soil samples were pooled together, mixed well to yield one soil sample, and kept in a sealed plastic bag at −20 °C for further analysis.
The isolation procedure used in this study followed the protocol of Maimaiti and others (2007). The rhizosphere soil sample was incubated for a month in air containing 0.3% H2 gas, and then the soil was serially diluted (10−3 to 10−10) with sterile distilled water. Then, 100-µL aliquots from the dilutions were pipetted and spread onto mineral salt agar (MSA) in Petri plates (Schlegel and Meyer 1985; Maimaiti and others 2007) and incubated under H2-enriched air (0.3% H2) for 3 weeks.
All bacterial isolates were recovered from the plates and tested for hydrogen-oxidizing ability. The 24 isolates that grew on the MSA were transferred onto 5-mL MSA slants in sealed tubes exposed to the same H2-enriched air as described above. A similar but uninoculated MSA slant was used as a negative control. The isolates and negative control were prepared in triplicate. After three weeks of growth, all tubes were flushed with H2-enriched air and sealed with gas-tight caps to create a closed environment. After 2 days, a 100-µL gas sample was taken from each test tube for H2 concentration determination (Qubit Flow Gas Exchange System, Kingston, ON, Canada).
The rhizobacterial isolates oxidizing H2 were further characterized. They were identified by comparison of their 16S rRNA gene sequence with known sequences in GenBank (Maimaiti and others 2007). DNA was extracted from individual bacterial colonies from each Petri plate using the DNeasy Plant Mini Kit (Qiagen, Toronto, ON, Canada) as per the manufacturer’s protocol. The extracted DNA was diluted tenfold and subjected to polymerase chain reaction (PCR) using primers 968f/1401br (Watanabe and others 2001). Platinum PCR Super Mix (Cat. No. 11306-016; Invitrogen life technologies, Carlsbad, CA, USA) was used in the PCR reaction mixture. Thermal cycling was conducted in a Veriti 96-Well Fast Thermal Cycler (Applied Biosystems, Carlsbad, CA, USA) under the following conditions: 4 min of initial denaturation at 94 °C; 30 cycles of 45 s of denaturation at 94 °C, 45 s of annealing at 56 °C, and 1 min of elongation at 72 °C; and 15 min of final elongation at 72 °C.
All PCR products were purified with AMPure XP (Beckman Coulter, Brea, CA, USA). The concentration of the purified PCR products was measured with a Qubit 2.0 fluorometer (Invitrogen life technologies, Carlsbad CA, USA). The DNA concentration of each sample was adjusted to 10 ng µL−1 and sent for Sanger sequencing at the Plant Biotechnology Institute (Saskatoon, SK, Canada). The sequences obtained were queried for similarities with known sequences in GenBank using the BLAST search tool at NCBI (http://www.ncbi.nlm.nih.gov/). Identification was based on 97–99% sequence similarity (Kim and others 2002; Xiang and others 2005; Satola and others 2012).
Assessment of Plant Growth-Promoting Effects
The plant growth-promoting effects of the H2-oxidizing rhizobacteria were tested in the greenhouse facility at Agriculture and Agri-Food Canada’s Semiarid Prairie Agricultural Research Centre, in Swift Current, Saskatchewan, Canada. The experiment had 11 treatments, namely the ten bacterial isolates and the sterile mineral salt solution as the control, in a randomized complete block design with four blocks.
Pre-germinated seeds of the lentil (Lens culinaris) cultivar CDC Maxim were inoculated with bacterial treatments (Belimov and others 2001; Maimaiti and others 2007) as follows: The bacterial colonies were grown in 30% nutrient broth (Becton, Dickinson, USA) at 28 °C, suspended in 50% sterile MSA medium, and grown overnight on an orbital shaker at 100 rpm. The concentration of the bacterial solution was adjusted to 5 × 107 cells mL−1 (Maimaiti and others 2007). The seeds were surface-sterilized with a mixture of 70% ethanol and 30% hydrogen peroxide for 2 min and rinsed several times with sterile distilled water. The seeds were pre-germinated on a moist filter paper in a Petri plate overnight at 24 °C in the dark. Seeds at the same germination stage were transferred into Petri plates lined with sterile filter paper. Five Petri plates with ten seeds each were prepared for each treatment. Then, 6 mL of bacterial suspension was added to each Petri plate. All dishes were covered and incubated at 24 °C in the dark for 2 days. Inoculated seeds at the same stage of germination were selected and transferred to 1-L pots filled with 900 mL packed pasteurized field soil. The soil had a silt loam texture, a pH of 6.5, an electrical conductivity of 1.41 mS, and contained 2.8 mg kg−1 of available N and 15.67 mg kg−1 of available P. Seeds pre-germinated in sterile MS solution were used as the control. About 0.05 g of Nodulator self-adhering peat-based inoculant for pea and lentil (Becker Underwood, Saskatoon, SK, Canada), containing a minimum of 1 × 109 viable bacterial cells of R. leguminosarum per gram, was applied as a root dip to the seeds when they were transplanted into each pot. The Hup+ status of the R. leguminosarum strain was confirmed by the methylene blue reduction assay (Lambert and others 1985). Three seedlings were placed in holes prepared in soil-filled pots and covered with 3 cm of soil. After emergence, the plants were thinned to one per pot.
The pots were kept in the greenhouse under optimal conditions, that is, a day/night temperature regime of 22/15 °C, a light/dark photoperiod of 15/9 h, and a relative humidity of 75%. The plants were observed daily and watered as needed. The pots were re-randomized within blocks weekly to ensure that every plant had an equal chance to be in any location in its block, thus minimizing any border effect that may have existed otherwise. After six weeks of growth, plant shoots and roots were harvested and dried separately in open paper bags at 40 °C in a forced-air dryer and the dry biomasses were recorded.
Characterization of Plant Growth-Promoting Traits
We tested the ten H2-oxidizing rhizobacteria indirectly for phosphate-solubilizing capacity, siderophore production, IAA production, ACC deaminase activity, and antagonistic activity using the methods described by other researchers (Patten and Glick 2002; Hynes and others 2008). Strain 2-106, which solubilizes phosphate and produces siderophores, strain 5-51, which produces IAA, and strain 6-8, which produces ACC deaminase (Hynes and others 2008), were used as positive controls in the assays. These strains were kindly provided by Prof. Louise Nelson of the University of British Columbia (Kelowna, BC, Canada).
We also tested the PGP ability of the ten H2-oxidizing rhizobacteria directly, using biomass productivity, biocontrol activity, enhancement of nodulation, and protection against nodule shedding under drought conditions, as performance indicators.
All assays had complete randomized designs. Six repetitions were used except when otherwise stated.
Screening for Phosphate-Solubilizing Capacity
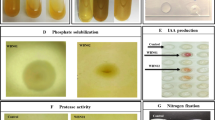
The phosphate-solubilizing ability of the ten H2-oxidizing rhizobacterial isolates was assessed visually on K2HPO4-amended glucose-yeast (GY) agar medium in Petri plates.
Two sterilized solutions, one containing 5 g of K2HPO4 in 50 mL of distilled water and the other containing 10 g of CaCl2 in 100 mL of distilled water, were added aseptically to 1 L of GY medium (10 g of glucose, 2 g of yeast extract, and 15 g of agar per liter) just before the medium was poured into Petri plates, to form an insoluble layer of calcium phosphate that made the medium opaque (Giongo and others 2013). The plates were inoculated with one of the ten H2-oxidizing PGPR isolates or with the phosphate-solubilizing strain 2-106, which was used as a positive control for comparison. The Petri plates were incubated for 5 days at 24 °C.
A zone of clearing around the bacterial colony indicated phosphate solubilization. The Petri plates were scanned, and the area (mm2) of the clear zone around the colonies was recorded using the ImageJ software (http://rsbweb.nih.gov/ij/) (Nguyen and others 1992).
Screening for Siderophore Production
We used the universal siderophore assay with chrome azurol S (CAS) and hexadecyltrimethylammonium bromide (HDTMA) as indicators (Schwyn and Neilands 1987) to assess siderophore production by the ten H2-oxidizing rhizobacterial isolates under study. Strain 2-106, a siderophore-producing strain, was used as a positive control.
First, CAS-blue agar was prepared by dissolving 60.5 mg of CAS in 50 mL of distilled water and then mixing it with 9 mL of an iron (III) solution containing 1 mM FeCl3 6H2O in 10 mM HCl. Separately, 72.9 mg of HDTMA was dissolved in 40 mL of water and then mixed with the CAS–iron (III) solution. The resulting dark blue solution was autoclaved at 121 °C for 15 min. Another solution containing 750 mL of water, 100 mL of minimal media 9 (MM9) salt solution, 30.24 g of piperazine-N,N′-bis (2-ethanesulfonic acid) (PIPES), and 15 g of agar was prepared. The pH of the solution was adjusted to 6.8 with 1 M NaOH before the addition of the agar. The solution was then autoclaved and subsequently cooled to 50 °C. Then, 30 mL of sterile casamino acid solution and 10 mL of a sterile glucose solution (20%) were added to the MM9/PIPES solution and mixed thoroughly (Schwyn and Neilands 1987).
Petri plates were filled with 30 mL of medium and, after solidification, inoculated with one of the H2-oxidizing rhizobacterial isolates. After incubation at 24 °C for 5 days, siderophore production was assessed on the basis of a change in color of the medium from blue to orange. The area of any orange zone surrounding the colony grown on CAS agar was measured (mm2) using the ImageJ software (Nguyen and others 1992).
Screening for IAA Production
The ability of the isolates to produce IAA was assayed on the basis of the method of Patten and Glick (2002) using Salkowski’s reagent. First, H2-oxidizing rhizobacterial isolates were inoculated on Luria–Bertani agar medium containing 5 mM L-tryptophan, 0.06% sodium dodecyl sulfate, and 1% glycerol. Each inoculated plate was overlaid with a sterile Whatman No. 1 filter paper and incubated for 3 days at 24 °C. After incubation, the filter papers were removed from the plates and soaked in Salkowski’s reagent (2% 0.5 M ferric chloride in 35% perchloric acid) in Petri plates at room temperature for 60 min (Patten and Glick 2002). The formation of a red halo on the paper where the colony had grown revealed the production of IAA. Strain 5-51, an IAA-producing strain, was used as a positive control.
Screening for ACC Deaminase Activity
The ACC deaminase activity of the H2-oxidizing rhizobacterial isolates was determined in Petri plates using Dworkin and Foster (DF) salt minimal medium (Dworkin and Foster 1958) containing ACC as the sole nitrogen (N) source. Strain 6-8 is able to obtain N from ACC and was used as a positive control. The ten H2-oxidizing rhizobacterial isolates plus the control were inoculated on DF salt minimal agar plates. Per liter, the DF salt minimal medium contained 4 g of KH2PO4, 6 g of Na2HPO4, 0.2 g of MgSO4, 2 g of glucose, 2 g of gluconic acid, 2 mg of citric acid, 1 mg of FeSO4 7H2O, 10 µg of H3BO3, 11.19 µg of MnSO4 H2O, 124.6 µg of ZnSO4 7H2O, 78.22 µg of CuSO4, 10 µg of MoO3, and 14 g of agar. Then, 100-µL aliquots of 3 mM ACC were aseptically pipetted and spread over the DF salt minimal medium and then allowed to dry for 10 min. The bacteria were streaked aseptically onto the agar surface; growth in that medium indicates ACC deaminase activity. Growth was observed after 2 days of incubation at 24 °C. The identity of the H2-oxidizing rhizobacterial isolates with ACC deaminase activity was recorded.
Screening for Antagonistic Activity
The ability of the isolates to antagonize in vitro the growth of select fungal pathogens was tested in an agar plate assay, as described by McSpadden and Fravel (2002), with some modifications. The fungi Fusarium avenaceum, Pythium ultimum, and Rhizoctonia solani were used as model pathogens, and the assay was conducted on potato dextrose agar (PDA).
A 0.25 cm2 piece of the mycelium of one PDA-grown phytopathogen was placed on the center of a PDA plate. One fresh colony of each bacterial isolate grown on PDA was suspended in 1 mL of phosphate-buffered peptone solution. A single streak of 10-µL aliquots of the bacterial suspension was inoculated in two opposite parallel lines, 2.5 cm from the center of the PDA plate. For controls, PDA plates inoculated only with a phytopathogenic fungus were used. The plates were inverted and placed randomly in a dark incubator at 24 °C.
Mycelial growth areas (cm2) were measured after one week using the ImageJ software. The results are reported as the means of mycelial growth area in the presence and absence of bacteria.
Evaluation of Biological Control Ability
The ten positive H2-oxidizing rhizobacteria under evaluation were tested in vivo for possible biocontrol activity against (i) F. avenaceum, (ii) R. solani, and (iii) P. ultimum. Red lentil cultivar CDC Maxim nodulated with Hup+ R. leguminosarum was the test plant. Assays were run on each fungal pathogen independently in the greenhouse. Each assay had a factorial design with 11 H2-oxidizing rhizobacteria treatments (ten isolates and a control) as one factor and two pathogen treatments (with and without) as the other. The 22 treatment combinations were arranged in a randomized complete block design with four blocks.
The lentil plants of all treatments were inoculated with the H2-oxidizing rhizobacteria and Hup+ R. leguminosarum, as described above. The fungal pathogens were propagated on wheat seeds; thus, infested wheat seeds were used as the carrier to infest the test plants (lentils) with fungal pathogens. The designated pots were inoculated by incorporating ten pieces of pathogen inoculum, into the soil touching the root at the three leaf seeding stage, that is, ten infested wheat seeds, in each pot.
The fungal pathogens were produced as follows: each of three steam-sterilized 150-mL conical flasks containing 30 g wheat grains and 50 mL of water were inoculated with 1 cm2 agar plugs from 2-week-old cultures of one of the three pathogens used in this study (that is, F. avenaceum, R. solani, and P. ultimum) and incubated at room temperature for 10 days (Abd El Daim and others 2014). After two week of incubation, the material was covered with the mycelium of the pathogen and ready for use as an inoculant.
Plants were grown in the greenhouse until disease symptoms appeared, which took 28 days. The shoots and roots were harvested and dried separately at 40 °C for at least 36 h in open paper bags. Root and shoot dry biomasses were recorded, and growth depression due to disease was calculated as follows:
where ML is the shoot or root mass of lentil not inoculated with a fungal pathogen and MLP is the shoot or root mass of lentil inoculated with a fungal pathogen.
Effect on Root Nodulation in the Presence and Absence of Drought Stress
In the greenhouse, an experiment was conducted in growth pouches (Mega International, St. Paul, Minnesota, USA) to test the effect of our bacterial isolates on the formation of root nodules. The growth pouch consisted of two-ply germination paper with a folded furrow for seed placement contained in a transparent polyethylene envelop. Red lentil seeds (CDC Maxim) were inoculated with one of the ten rhizobacterial isolates under evaluation or with the sterile carrier, as a control.
The ten bacterial isolates were grown overnight in 30% nutrient broth medium (Becton, Dickinson, USA) at 24 °C, and bacterial suspensions of 5 × 107 cells mL−1 were prepared in 50% sterile MS solution (Maimaiti and others 2007). The control treatment consisted of the sterile MS solution. Then, 30 surface-sterilized lentil seeds were pre-germinated for 2 days in each bacterial suspension at room temperature in the dark, as described earlier. Nodulator peat-based inoculant for pea and lentil (Becker Underwood), which contained an Hup+ strain of R. leguminosarum, was applied to the inoculated lentil seeds after 48 h of germination. Three germinated seeds with an emerging radical 1–2 cm in length were transferred to each sterile growth pouch, which contained 10 mL of sterilized Long Ashton nutrient solution (Valentine and others 2001). The seedling roots grew between the moist paper and the clear wall of the polyethylene envelop. The growth pouches were hung in enclosed metal boxes to keep the roots in the dark. These growth pouch-containing boxes were placed in the greenhouse under a day/night temperature regime of 22/15 °C, a light/dark photoperiod of 15/9 h, and a relative humidity of 75%. The growth pouches were rotated in the boxes every week to minimize border effects. The plants were observed every day and given nutrient solution to a maximum of 10 mL per week, or water as needed.
Three drought stress cycles were applied after three weeks of growth by withholding watering until wilting was observed. Plants were then watered with 5 mL of water. The application of the drought cycles was completed in 3 days, and the plants were then subjected to the initial watering regime for another 4 days. Control plants were always watered as needed. The number of nodules per plant was recorded on the last day of the experiment.
Statistical Analysis
We tested the H2 oxidation capacity of the 24 bacterial isolates by comparing their H2 consumption with the uninoculated control using Student’s t tests in R (Development Core Team R 2009. R: A language and environment for statistical computing, Austria, Vienna).
The significance of the effects of the H2-oxidizing rhizobacterial isolates on phosphate solubilization, siderophore production, IAA production, ACC production, pathogen growth, plant biomasses, and number of root nodules was assessed by ANOVA in JMP 6 software (SAS Institute, Cary, NC, USA). A random effect was attributed to blocks, when present. Treatment means were compared using Tukey’s HSD (honest significant difference) test, when significant treatment effects were found.
Results
Isolation and Identification of Rhizosphere Bacterial Isolates
In total, 24 rhizobacteria were successfully isolated from the rhizosphere of 14 lentil cultivars grown in field plots in Swift Current, Saskatchewan, Canada; of these, ten could oxidize H2 (Fig. 1).
The ten H2-oxidizing rhizobacteria were identified through similarity search as Variovorax paradoxus, Rhodococcus sp., Mycobacterium sp., Acinetobacter sp., Acinetobacter calcoaceticus, and Curtobacterium sp. (Table 1). The ability of these H2-oxidizing rhizobacteria to promote plant growth was assessed.
Plant Growth Promotion
The H2-oxidizing rhizobacterial isolates influenced the dry mass of lentil shoots and roots in an Hup+ environment (P < 0.0001) (Fig. 2). The inoculation of lentil with H2-oxidizing rhizobacterial isolates generally promoted lentil shoot biomass (Fig. 2a) and root biomass (Fig. 2b). However, the level of plant growth promotion with isolates L7, L10, and L14 was not significant (Fig. 2). Despite that, all isolates were screened for PGP attribute determination.
Characterization of Plant Growth-Promoting Traits
Only one H2-oxidizing rhizobacterial isolate was able to form a clear zone around colonies on K2HPO4-amended GY agar, an indication of calcium phosphate solubilization (Table 1). Isolate V. paradoxus L1 produced a larger clear zone than the phosphate-solubilizing control strain did. Nine H2-oxidizing rhizobacterial isolates produced siderophores, on the basis of the color change of CAS medium. The size of the orange zones surrounding the colonies was used to estimate the degree of siderophore production. Four rhizobacterial isolates produced less siderophores than the control strain did, and Variovorax sp. L14 was unable to produce siderophores (Table 1). Siderophore areas ranged from 23.5 mm2 for the colonies of Mycobacterium sp. L11 to 35.4 mm2 for the colonies of Variovorax sp. L1. Four H2-oxidizing rhizobacteria formed a red halo on the filter paper surrounding the colony in Salkowski’s reagent, indicating that they were able to synthesize IAA from tryptophan. Six H2-oxidizing rhizobacterial isolates were able to grow on a minimal medium containing ACC as the sole N2 source.
The H2-oxidizing rhizobacterial treatments influenced the growth of all three phytopathogens (P < 0.0001). Five H2-oxidizing rhizobacterial isolates, namely L1, L11, L20, L21, and L22, were able to suppress the growth of all phytopathogens. Isolates L10, L14, and L17 did not inhibit R. solani, and isolates L4, L7, L10, and L14 did not inhibit P. ultimum (Table 1). The H2-oxidizing rhizobacterial isolate L21 showed the highest level of antagonism in reducing the growth of F. avenaceum to 36% of that of the control (Fig. 3a, d). Isolate L20 had the strongest antagonistic effect on R. solani, reducing its growth to 43% of that of the control (Fig. 3b, e). Isolate L22 had the strongest antagonistic effect on P. ultimum, reducing its growth to 46% of that of the control (Fig. 3c, f). The isolates that could antagonize all the fungal pathogens tested were members of the genera Acinetobacter, Curtobacterium, and Variovorax.
Examples of pathogen growth inhibition by H2-oxidizing rhizobacteria: growth of a Fusarium avenaceum, b Rhizoctonia solani, and c Pythium ultimum in the absence of rhizobacterial isolates, growth inhibition of d F. avenaceum in the presence of isolate L21, e Rhizoctonia solani in the presence of isolate L20, and f Pythium ultimum in the presence of isolate L22. g Absence of growth inhibition of Pythium ultimum in the presence of isolate L4
Biocontrol Ability
Some of the bacteria reduced (P < 0.0001) the negative impact of the common pathogens F. avenaceum, P. ultimum, and R. solani on lentil shoot and root dry biomasses in the greenhouse (Fig. 4). Twenty-eight days after inoculation with the pathogens, the lentil plants inoculated with a pathogen had poor growth and pale color and were losing leaves in the absence of an H2-oxidizing rhizobacterium. Inoculation with a pathogen caused 40–55% depression in shoot and root biomasses in the absence of H2-oxidizing rhizobacteria (Fig. 4). The seven H2-oxidizing rhizobacterial isolates that showed antagonistic activity in vitro (Table 1) had at least some ability to mitigate the negative effect of pathogens on lentil shoot and root growth in the prevalent Hup+ environment (Fig. 4). In particular, L20, L21, and L22 had superior abilities and could mitigate the impact of all pathogens on the production of both lentil shoot and root biomasses in the greenhouse.
Lentil shoot (a, c, e) and root (b, d, f) dry mass reduction caused by the common root pathogens Fusarium avenaceum (a, b), Rhizoctonia solani (c, d), and Pythium ultimum (e, f) as influenced by pre-inoculation of the plants with H2-oxidizing rhizobacterial isolates, in comparison with controls. Plants were grown for six weeks in pots in the greenhouse. Bars with different letters are significantly different at α = 0.05 (Tukey’s HSD, n = 4)
Effect on Nodulation
Rhizobacterial inoculation influenced the nodulation of lentil roots (P < 0.0001). Seven H2-oxidizing rhizobacterial isolates (L1, L10, L11, L17, L20, L21, and L22) increased the number of root nodules in the absence of drought stress (Fig. 5). Isolates L4, L7, and L14 had no significant effect (Fig. 4). Most of the isolates capable of promoting lentil plant biomass and root nodulation were members of the genera Mycobacterium, Acinetobacter, Curtobacterium, and Variovorax.
Effect of the H2-oxidizing rhizobacterial isolates on the resilience of root nodules to drought stress in lentil inoculated with Rhizobium leguminosarum. The control (Rh) was only inoculated with R. leguminosarum. Bars with different letters are significantly different at α = 0.05 (Tukey’s HSD, n = 4)
Drought stress caused the H2-oxidizing rhizobacteria to influence root nodule retention in lentil differently (P < 0.0001). Drought stress decreased the number of nodules per plant in lentil inoculated with L1 and L14, but did not influence nodule number in other treatments (Fig. 5). The stimulating effect of L1 on nodulation completely disappeared with the application of the three drought cycles (Fig. 5).
Discussion
The results of this research verified our hypothesis. The H2-oxidizing rhizobacteria naturally living in the rhizosphere of field-grown lentil can promote the growth of their host plants, despite the use of a commercial Hup+ rhizobial inoculant. Because H2-oxidizing PGPR can promote plant growth in combination with commercial Hup+ rhizobia, they have potential for use in crop production.
Early research suggested that H2-oxidizing PGPR may function best in the rhizosphere of legumes nodulated by Hup− rhizobial strains as root nodules, then produce H2 gas (Dong and Layzell 2002). H2-oxidizing bacteria may have different metabolic processes and function both as lithotrophs and organotrophs (Fernández and others 2005), depending on environmental conditions (John and Whatley 1977). Hydrogenase is inhibited by oxygen and the microaerophilic conditions it requires to function (Yagi and Higuchi 2012) may not always be present in the rhizosphere of crop plants. H2-oxidizing PGPR living in the vicinity of H2 producing nodules may have a competitive advantage in using H2 gas in microaerophilic soil conditions. However, they can also promote plant growth while relying on their organotrophic metabolism. Soil oxygen is consumed by roots and microbial activity in the rhizosphere. However, agricultural soils are generally well aerated, especially in the semiarid areas where lentil is grown, and the importance of the competitive advantage provided by hydrogenase to H2-oxidizing PGPR is unclear at this time. It is possible that the capacity to use H2 enlarges the niche of the H2-oxidizing PGP rhizobacteria to some extent, but we know that this metabolism is not necessary for H2-oxidizing rhizobacteria to promote lentil growth.
The H2-oxidizing rhizobacteria found in the lentil rhizosphere belong to the Actinobacteria and Proteobacteria, phyla known to dominate the rhizosphere (Bulgarelli and others 2013). Among the ten H2-oxidizing rhizobacterial isolates assessed in the present study, we detected six different mechanisms for plant growth promotion: P solubilization, siderophore production, IAA production, ACC deaminase production, antagonism of phytopathogens, and enhancement of nodulation. The modes of action of H2-oxidizing rhizobacteria had rarely been reported except for the capacity of Variovorax and Flavobacterium to metabolize ACC (Maimaiti and others 2007) and Burkholderia to produce the rhizobitoxine (Zhang 2006).
Plant growth promotion was often attributed to the ability of the PGPR strains to produce phytohormones and enzymes (Belimov and others 2001; Laslo and others 2012). Among the ten H2-oxidizing rhizobacterial isolates assessed in the present study, L10, L11, L17, L20, L21, and L22 showed ACC deaminase activity (Table 1). The enzyme ACC deaminase hydrolyzes the precursor of ethylene, ACC, to α-ketobutyrate and ammonia, reducing ethylene production in plants and thus stimulating plant growth (Glick and others 1998; Arshad and others 2007; Kazan and Manners 2009; Gan and others 2011).
The isolates possessing ACC deaminase were the ones that stimulated nodule formation in lentil roots (Fig. 5), suggesting that ACC deaminase activity may increase nodulation in legume plants. This possibility is supported by previous studies reporting more nodules and higher shoot mass in plants co-inoculated with Rhizobium and an ACC deaminase-producing bacterium (Bai and others 2003; Belimov and others 2009). It appears that bacteria possessing ACC deaminase are particularly beneficial to legumes, because those bacteria promote nodulation by adjusting ethylene levels (Shaharoona and others 2006; Mirza and others 2007; Gan and others 2011; Chen and others 2013).
The ACC deaminase-producing PGPR help plants overcome abiotic stresses (Shahzad and others 2010). Drought stress is associated with an elevated release of endogenous ethylene by plants (Mayak and others 2004), and ethylene metabolism is involved in the response of N2 fixation to drought stress in legumes (Larrainzar and others 2014). Our isolates L1 and L14 did not mitigate the negative impact of drought stress on nodulation like most of the other isolates did, maybe because these two isolates do not possess ACC deaminase activity.
Our isolate V. paradoxus L1 was devoid of ACC deaminase activity but was the only bacterium able to solubilize phosphate (Table 1). Phosphorus is a major macronutrient for plants, and a large proportion of the phosphorus fertilizers applied to cultivated soil becomes unavailable through rapid conversion into calcium or magnesium phosphate (Richardson 2001; Goldstein 2007; Jorquera and others 2008; Richardson and others 2009). Phosphate-solubilizing bacteria are common in soil (Richardson 2001), but their numbers can be low in cultivated soil (Findenegg and Nelemans 1993; Rodríguez and Fraga 1999).
Many PGPR produce the plant hormone IAA (Huddedar and others 2002; Belimov and others ; Jiang and others 2012), which plays an important role in the regulation of plant development (Gupta and others 1998 2009; Spaepen and others 2007). Four H2-oxidizing rhizobacterial isolates in our study also produced IAA, which might have led to increased plant biomass and nutrient uptake. These isolates belong to the genera Variovorax, Curtobacterium, Mycobacterium, and Acinetobacter (Table 1). Recent literature supports a positive effect of Acinetobacter on plant growth and development not only through the production of IAA (Kazan and Manners 2009; Kang and others 2012), but also through siderophore synthesis (Sarode and others 2009).
Siderophore production by PGPR improves the availability of iron to plants as iron–siderophore complexes (Antoun and others 1998; Vansuyt and others 2007). The production of iron-chelating siderophores was the most common PGP trait observed among our H2-oxidizing rhizobacterial isolates. Nine of the ten H2-oxidizing rhizobacteria tested positive for siderophore production (Table 1). The frequency of siderophore production among the H2-oxidizing rhizobacterial associates of lentil in this study concurs with the report of Hynes and others (2008) who found that 76% of their Canadian prairie native PGPR isolates were siderophore producers.
The chelation of soluble iron by microbial siderophores can lead to the inhibition of phytopathogen growth, in addition to increasing the level of plant-available iron in soil (Bano and Musarrat 2003). Siderophores can sequester large amounts of iron (III) in the rhizosphere, effectively preventing the proliferation of fungal pathogens through iron starvation (Kloepper and others 1980). Iron deficiency in pathogens inhibits their growth and sporulation (Mathiyazhagan and others 2004). Our H2-oxidizing rhizobacteria enhanced plant growth by mitigating the negative impact of the fungal pathogens (Fig. 4). This mitigation was possibly due to the production of siderophores or phytohormones by the rhizobacteria or may have been due to the production of antifungal metabolites (McSpadden G and Fravel 2002; Haas and Defago 2005; Banchio and others 2008). Several of the H2-oxidizing rhizobacteria showed antifungal activity against fungal pathogens (Fig. 3). Isolates Curtobacterium sp. L22, Acinetobacter sp. L20, and A. calcoaceticus L21 promoted plant growth in the presence of all phytopathogens (Fig. 4) and also showed the highest antifungal properties (Fig. 3, Table 1). Studies to isolate and identify the molecules with antifungal properties produced by our bacteria are in progress.
We found that H2-oxidizing rhizobacteria grown in semiarid environments possess several PGP traits. Isolates V. paradoxus L1 and L17, Mycobacterium sp. L11, Acinetobacter sp. L20, A. calcoaceticus L21, and Curtobacterium sp. L22 possess several modes of plant growth promotion, including the reduction of stress ethylene levels with ACC deaminase production and increased nodulation. All the isolates tested produced siderophores or possessed other PGP traits. Variovorax paradoxus L1 is an IAA producer with phosphorus-solubilizing ability. Variovorax paradoxus L17 and Mycobacterium sp. L11 are IAA producers with ACC deaminase-producing ability. Acinetobacter sp. L20, A. calcoaceticus L21, and Curtobacterium sp. L22 also possess ACC deaminase activity and express a high level of antagonism against common root pathogens.
The present study showed the plant growth-promoting potential of H2-oxidizing rhizobacteria originally isolated from the rhizosphere of field-grown lentil. The PGP effect of the H2-oxidizing rhizobacteria in Hup+ environments can be attributed to multiple known modes of action. Using these H2-oxidizing rhizobacteria in the Hup+ environment created by commercial inoculants could contribute to the success of lentil production in the Canadian prairie. This remains to be demonstrated under field conditions.
References
Abd El Daim IA, Häggblom P, Karlsson M, Stenström E, Timmusk S (2014) Paenibacillus polymyxa A26 Sfp-type PPTase inactivation limits bacterial antagonism against Fusarium graminearum but not of F. culmorum in kernel assay. Front Plant Sci. 6:368. doi:10.3389/fpls.2015.00368
Annan H, Golding A-L, Zhao Y, Dong Z (2012) Choice of hydrogen uptake (Hup) status in legume-rhizobia symbioses. Ecol Evol 2:2285–2290
Antoun H, Beauchamp C, Goussard N, Chabot R, Lalande R (1998) Potential of Rhizobium and Bradyrhizobium species as plant growth promoting rhizobacteria on non-legumes: effect on radishes (Raphanus sativus L.). Plant Soil 204:57–67
Arshad M, Saleem M, Hussain S (2007) Perspectives of bacterial ACC deaminase in phytoremediation. Trends Biotechnol 25:356–362
Bai Y, Zhou X, Smith DL (2003) Enhanced soybean plant growth resulting from coinoculation of Bacillus strains with Bradyrhizobium japonicum. Crop Sci 43:1774–1781
Banchio E, Bogino PC, Zygadlo J, Giordano W (2008) Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Bioch Syst Ecol 36:766–771
Bano N, Musarrat J (2003) Characterization of a new Pseudomonas aeruginosa Strain NJ-15 as a potential biocontrol agent. Curr Microbiol 46:0324–0328
Belimov A, Safronova V, Sergeyeva T, Egorova T, Matveyeva V, Tsyganov V, Borisov A, Tikhonovich I, Kluge C, Preisfeld A, Dietz K, Stepanok V (2001) Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol 47:642–652
Belimov AA, Dodd IC, Hontzeas N, Theobald JC, Safronova VI, Davies WJ (2009) Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol 181:413–423
Bulgarelli D, Schlaeppi K, Spaepen S, Van Themaaf EVL, Schulze-Lefert P (2013) Structure and function of the bacterial microbiota of plants. Ann Rev Plant Biol 64:807–838
Canadian Agri-Food Trade Alliance (2016) Pulses. http://cafta.org/pages/agri-food-exports/pulses/
Chen L, Dodd IC, Theobald JC, Belimov AA, Davies WJ (2013) The rhizobacterium Variovorax paradoxus 5C-2, containing ACC deaminase, promotes growth and development of Arabidopsis thaliana via an ethylene-dependent pathway. J Bot 64:1565–1573
Dean CA, Sun W, Dong Z, Caldwell CD (2006) Soybean nodule hydrogen metabolism affects soil hydrogen uptake and growth of rotation crops. Can J of Plant Sci 86:1355–1359
Dong Z, Layzell DB (2001) H2 oxidation, O2 uptake and CO2 fixation in hydrogen treated soils. Plant Soil 229:1–12
Dong Z, Layzell DB (2002) Why do legume nodules evolve hydrogen gas? In: Finan T, O’Brian M, Layzell D, Vessey K, Newton W (eds) Nitrogen fixation, global perspectives. CABI, New York, pp 331–335
Dong Z, Wu L, Kettlewell B, Caldwell CD, Layzell DB (2003) Hydrogen fertilization of soils—is this a benefit of legumes in rotation? Plant Cell Environ 26:1875–1879
Dworkin M, Foster JW (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–603
Erskine W, Sarker A, Kumar S (2011) Investing in lentil improvement toward a food secure world. Food Secur 3(2):127–139
Fernández D, Toffanin A, Palacios JM, Ruiz-Argüeso T, Imperial J (2005) Hydrogenase genes are uncommon and highly conserved in Rhizobium leguminosarum bv. viciae. FEMS Microbiol Lett 253:83–88
Figueiredo MVB, Burity HA, Martínez CR, Chanway CP (2008) Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl Soil Ecol 40:182–188
Findenegg G, Nelemans J (1993) The effect of phytase on the availability of P from myo-inositol hexaphosphate (phytate) for maize roots. Plant Soil 154:189–196
Gan Y, Liang C, Hamel C, Cutforth H, Wang H (2011) Strategies for reducing the carbon footprint of field crops for semiarid areas. A review. Agron Sustain Dev 31:643–656
Giongo A, Beneduzi A, Gano K, Vargas LK, Utz L, Passaglia LMP (2013) Characterization of plant growth-promoting bacteria inhabiting Vriesea gigantea Gaud. and Tillandsia aeranthos (Loiseleur) L.B. Smith (Bromeliaceae). Biota Neotrop 13:80–85
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. Theor Biol 190:63–68
Glick BR, Todorovic B, Czarny J, Cheng Z, Duan J, McConkey B (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26:227–242
Goldstein A (2007) Future trends in research on microbial phosphate solubilization: one hundred years of insolubility. In: Velázquez E, Rodríguez-Barrueco C (eds). First International Meeting on Microbial Phosphate Solubilization. Vol 102 of the series Developments in Plant and Soil Sciences. Springer, Netherlands, pp 91–96
Gupta A, Saxena AK, Gopal M, Tilak KVBR (1998) Effect of plant growth promoting rhizobacteria on competitive ability of introduced Bradyrhizobium sp. (Vigna) for nodulation. Microbiol Res 153:113–117
Haas D, Defago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307
Huddedar S, Shete A, Tilekar J, Gore S, Dhavale D, Chopade B (2002) Isolation, characterization, and plasmid pUPI126-mediated indole-3-acetic acid production in Acinetobacter strains from rhizosphere of wheat. Appl Biochem Biotech 102–103:21–39
Hunter WJ (1993) Ethylene production by root nodules and effect of ethylene on nodulation in Glycine max. Appl Environ Microbiol 59:1947–1950
Hynes RK, Leung GCY, Hirkala DLM, Nelson LM (2008) Isolation, selection, and characterization of beneficial rhizobacteria from pea, lentil, and chickpea grown in western Canada. Can J Microbiol 54:248–258
Iqbal MA, Khalid M, Shahzad SM, Ahmad M, Soleman N, Akhtar N (2012) Integrated use of Rhizobium leguminosarum, plant growth promoting rhizobacteria and enriched compost for improving growth, nodulation and yield of lentil (Lens culinaris Medik.). Chil J Agric Res 72(1):104
Irvine P, Smith M, Dong Z (2004) Bacteria or fungi? Acta Hort 631:239–242
Jiang F, Chen L, Belimov AA, Shaposhnikov AI, Gong F, Meng X, Hartung W, Jeschke DW, Davies WJ, Dodd IC (2012) Multiple impacts of the plant growth-promoting rhizobacterium Variovorax paradoxus 5C-2 on nutrient and ABA relations of Pisum sativum. J Exp Bot 63:6421–6430
John P, Whatley FR (1977) The bioenergetics of Paracoccus denitrificans. BBA Rev Bioenerg 463:129–153
Jorquera M, Hernandez M, Rengel Z, Marschner P, Mora M (2008) Isolation of culturable phosphobacteria with both phytate-mineralization and phosphate-solubilization activity from the rhizosphere of plants grown in a volcanic soil. Biol Fertil Soils 44:1025–1034
Kang S-M, Khan A, Hamayun M, Shinwari ZK, Kim Y-H, Joo G, Lee I-J (2012) Acinetobacter calcoaceticus ameliorated plant growth and influenced gibberellins and functional biochemicals. Pak J Bot 44:365–372
Kazan K, Manners JM (2009) Linking development to defense: auxin in plant–pathogen interactions. Trends Plant Sci 14:373–382
Kim D, Kim Y-S, Kim S-K, Kim SW, Zylstra GJ, Kim YM, Kim E (2002) Monocyclic aromatic hydrocarbon degradation by Rhodococcus sp. Strain DK17. Appl Environ Microbiol 68:3270–3278
Kloepper JW, Leong J, Teintze M, Schroth MN (1980) Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286:885–886
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. Academic Press, New York
Lambert GR, Hanus FJ, Sterling RA, Evans HJ (1985) Determination of the hydrogenase status of individual legume nodules by a methylene blue reduction assay. Appl Environ Microbiol 50:537–539
Larrainzar E, Molenaar JA, Wienkoop S, Gil-Quintana E, Alibert B, Limami AM, Arrese-Igor C, González EM (2014) Drought stress provokes the down-regulation of methionine and ethylene biosynthesis pathways in Medicago truncatula roots and nodules. Plant Cell Environ 37:2051–2063
Laslo É, György É, Mara G, Tamás É, Ábrahám B, Lányi S (2012) Screening of plant growth promoting rhizobacteria as potential microbial inoculants. Crop Prot 40:43–48
Maimaiti J, Zhang Y, Yang J, Cen YP, Layzell D, Peoples M, Dong Z (2007) Isolation and characterization of hydrogen-oxidizing bacteria induced following exposure of soil to hydrogen gas and their impact on plant growth. Environ Microbiol 9:435–444
Mathiyazhagan S, Kavitha K, Nakkeeran S, Chandrasekar G, Manian K, Renukadevi P, Krishnamoorthy AS, Fernando WGD (2004) PGPR mediated management of stem blight of Phyllanthus amarus (Schum and Thonn) caused by Corynespora cassiicola (Berk and Curt) Wei. Arch Phytopathol Plant Prot 37:183–199
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci 166:525–530
McSpadden GB, Fravel D (2002) Biological control of plant pathogens: research, commercialization, and application in the USA. Plant Health Prog. doi:10.1094/PHP-2002-0510-01-RV
Mirza BS, Mirza MS, Bano A, Malik KA (2007) Coinoculation of chickpea with Rhizobium isolates from roots and nodules and phytohormone-producing Enterobacter strains. Aust J Exp Agric 47:1008–1015
Nascimento F, Brígido C, Alho L, Glick BR, Oliveira S (2012) Enhanced chickpea growth-promotion ability of a Mesorhizobium strain expressing an exogenous ACC deaminase gene. Plant Soil 353:221–230
Nelson LM (2004) Plant growth promoting rhizobacteria (PGPR): prospects for new inoculants. Crop Manag. doi:10.1094/CM-2004-0301-05-RV
Nguyen C, Yan W, Le Tacon F, Lapeyrie F (1992) Genetic variability of phosphate solubilizing activity by monocaryotic and dicaryotic mycelia of the ectomycorrhizal fungus Laccaria bicolor (Maire) P.D. Orton. Plant Soil 143:193–199
Patten CL, Glick BR (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801
Richardson A (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust J Plant Physiol 28:897–906
Richardson A, Barea J, McNeill A, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339
Rodríguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Sakunpon N, Boonkerd N, Teaumroong N, Okazaki S, Tittabutr P (2014) Influence of H2 and plant growth promoting rhizobacteria (PGPR) containing uptake hydrogenase on soybean growth promotion. Int Proc Chem Biol Environ Eng 70:147
Sarode PD, Rane MR, Chaudhari BL, Chincholkar SB (2009) Siderophoregenic Acinetobacter calcoaceticus isolated from wheat rhizosphere with strong PGPR activity. J Microbiol 5:6–12
Satola B, Wübbeler JH, Steinbüchel A (2012) Metabolic characteristics of the species Variovorax paradoxus. Appl Microbiol Biotechnol 97:541–560
Schlegel H, Meyer M (1985) Isolation of hydrogenase regulatory mutants of hydrogen-oxidizing bacteria by a colony-screening method. Arch Microbiol 141:377–383
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Shaharoona B, Arshad M, Zahir ZA (2006) Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.). Lett Appl Microbiol 42:155–159
Shahzad S, Khalid A, Arshad M, Kalil-ur R (2010) Screening rhizobacteria containing ACC-deaminase for growth promotion of chickpea seedlings under axenic conditions. Soil Environ 29:38–46
Simpson FB, Burris RH (1985) A nitrogen pressure of 50 atmospheres does not prevent evolution of hydrogen by nitrogenase. Science 224:1095–1097
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Valentine AJ, Osborne BA, Mitchell DT (2001) Interactions between phosphorus supply and total nutrient availability on mycorrhizal colonization, growth and photosynthesis of cucumber. Sci Hortic 88:177–189
Vanderhoef LN, Dute RR (1981) Auxin regulated wall loosening and sustained growth in elongation. Plant Physiol 67:146–149
Vansuyt G, Robin A, Briat J-F, Curie C, Lemanceau P (2007) Iron acquisition from Fe-pyoverdine by Arabidopsis thaliana. Mol Plant Microb Interact 20:441–447
Vassilev N, Vassileva M, Nikolaeva I (2006) Simultaneous P-solubilizing and biocontrol activity of microorganisms: potentials and future trends. Appl Microbiol Biotechnol 71:137–144
Watanabe K, Kodama Y, Harayama S (2001) Design and evaluation of PCR primers to amplify bacterial 16S ribosomal DNA fragments used for community fingerprinting. J Microbiol Methods 44:253–262
Xiang S, Yao T, An L, Xu B, Wang J (2005) 16S rRNA sequences and differences in bacteria isolated from the Muztag Ata glacier at increasing depths. Appl Environ Microbiol 71:4619–4627
Yagi T, Higuchi Y (2012) Studies on hydrogenase. Proc Jpn Acad Ser B 89:16–32
Yang C (2012) Response of rhizobacterial community to agronomic practices in chickpea field, and its effects on pulse-cereal rotation system. PhD Dissertation, University of Saskatchewan. http://hdl.handle.net/10388/ETD-2012-03-367
Yang C, Hamel C, Gan Y, Vujanovic V (2012) Bacterial endophytes mediate positive feedback effects of early legume termination times on the yield of subsequent durum wheat crops. Can J Microbiol 58:1368–1377
Zafar M, Abbasi MK, Khan MA, Khaliq A, Sultan T, Aslam M (2012) Effect of plant growth-promoting rhizobacteria on growth, nodulation and nutrient accumulation of lentil under controlled conditions. Pedosphere 22:848–859
Zahir Z, Ghani U, Naveed M, Nadeem S, Asghar H (2009) Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch Microbiol 191:415–424
Zhang Y (2006) Mechanisms of isolated hydrogen-oxidizing bacteria in plant growth promotion and effects of hydrogen metabolism on rhizobacterial community structure. Master’s Thesis, Saint Mary’s University, Halifax
Acknowledgements
This research was supported by Saskatchewan’s Agriculture Development Fund and Saskatchewan Pulse Growers.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Abdellatif, L., Ben-Mahmoud, O.M., Yang, C. et al. The H2-oxidizing Rhizobacteria Associated with Field-Grown Lentil Promote the Growth of Lentil Inoculated with Hup+ Rhizobium Through Multiple Modes of Action. J Plant Growth Regul 36, 348–361 (2017). https://doi.org/10.1007/s00344-016-9645-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9645-7