Abstract
Desert plants develop different types of seed dormancy that can only be broken once they are exposed to the proper environmental signals that enhance seedling establishment. The objective of this study was to assess the role of different dormancy regulating chemicals (DRCs) and light in alleviating innate dormancy of 16 annual plants from the Arabian deserts. Seeds of the different species were stored for three months and incubated at the optimal temperature for their germination (15/25 °C) in both 12 h light/12 h darkness and in complete darkness. All species had deep innate dormancy. In four Plantago species, dormancy alleviation was high in all species by kinetin, partial by GA3 in three species, and by nitrate and thiourea in two species. In three grasses, all DRCs succeeded in breaking dormancy, but success was slight in Aristida adscensionis and partial in both Eragrostis ciliaris and Tragus racemosus. In these grasses, the dormancy break was greater in light than in dark. In three species of Brassicaceae, none of the DRCs succeeded in alleviating dormancy of Brassica tournefortii and Eremobium aegyptiacum, but all resulted in greater than 90% germination in Anastatica hierochuntica. Interestingly, two species of Resedaceae and one from Aizoaceae failed to respond to any DRCs in releasing the dormancy. DRCs partially alleviated dormancy in the other three species from different families. Possible mechanisms involved in the alleviation of deep innate dormancy of seeds from desert annuals by DRCs and their ecological adaptations to the environment are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arid desert habitats are characterized by temporal and spatial heterogeneities. Rainfall levels in deserts vary substantially among years in both amount and timing. Success of annual plants in arid/hyper arid deserts necessitates the completion of their full life cycle within a few months, sometimes weeks when soil moisture is available (Mulroy and Rundel 1977). Annuals commonly exhibit specific physiological and morphological adaptations to complete their life cycles successfully in unpredictable desert environments. As germination is a critical stage in the life cycle of desert annuals, seed dormancy is an important strategy for ensuring successful germination and seedling establishment under appropriate environmental conditions (Rezvani and others 2014). Dormancy strongly affects the fate of seedlings and consequently can also influence both population and species-level processes such as adaptation, time of colonization, and extinction (Bewley 1997; Willis and others 2014).
Germination of desert plants is usually prevented by adverse climatic conditions, such as drought and/or high temperatures or by dormancy mechanisms (Kigel 1995). Seed dormancy is a property that defines the environmental conditions in which seeds are able to germinate. Dormancy and germination are complex phenomena that are controlled by a set of genes, which are affected by both developmental and environmental factors prevailing in the maternal habitats and during seed storage (Koornneef and others 2002; Kucera and others 2005; Finch-Savage and Leubner-Metzger 2006; El-Keblawy 2013). Rainfall, light, temperature, and some soil factors such as salinity and nitrate all interact to control seed dormancy, which determines the time and level of germination. In addition, seed dormancy can be controlled by internal factors such as physiological and morphological embryo maturation, permeability of the integuments to water or oxygen, and/or the presence of inhibitors (Khan 1978; El-Keblawy and Al-Rawai 2005).
Seeds of plants growing in unpredictable environments, such as deserts, have developed different types of dormancies that can only be broken once they are exposed to the proper environmental signals that enhance seedling establishment (Finch-Savage and Leubner-Metzger 2006; Finch-Savage and Footitt 2012). Physiological dormancy is the most common dormancy type in desert annuals (Baskin and Baskin 2004). It provides seasonal cueing, ensuring that germination occurs only after specific environmental events (Finch-Savage and Leubner-Metzger 2006). Physiological dormancy is usually controlled by the balance of internal and external factors. The adjustment of physiological dormancy in seeds to their external environment is highly specific, and increased germination occurs in response to specific temperature, chemical, or light signals (Baskin and Baskin 2004). To regulate physiological dormancy and the initiation of germination, seeds have evolved highly efficient environmental sensors that respond to their prevailing environment and also to their environmental history (Huang and others 2015). Consequently, seeds detect the prevailing environmental signals to determine the time and place of seed germination and subsequent seedling emergence. Temperature, light, salinity, and soil nitrate are among the most important environmental signals influencing seed germination. For example, light is an important signal, especially for small seeded plants, in soils frequently exposed to natural disturbance, for example, mobile sand dunes (Huang and Gutterman 1998; Koutsovoulou and others 2014), or to man-made disturbances such as farms (Milberg and others 2000; Batlla and Benech-Arnold 2014). Light signals interact with internal factors to regulate dormancy release and the germination process. For example, it has been reported that light interacts with nitrate to detect gaps suitable for seedling establishment in dense vegetation (Pons 1989).
Several studies have reported that mechanisms for releasing dormancy or inducing germination under harsh environmental conditions are influenced by several factors, including alternating temperatures, light, and dormancy regulating chemicals (DRCs) (Bouwmeester and Karssen 1993; Bewley and Black 1994; Khan and Gul 2006; El-Keblawy and others 2010; El-Keblawy 2013). Several DRCs such as plant hormones and nitrogenous compounds are extremely important for the regulation of seed dormancy and germination (Koornneef and others 2002; Finkelstein 2004; Finkelstein and others 2008). Hormones known to mediate plant development include abscisic acid (ABA), gibberellins (GAs), ethylene, brassinosteroids (BR), auxins, cytokinins, and other signaling molecules (Kucera and others 2005). Both ABA and GA are important components of seeds of most plants and play a central role in dormancy regulation (Willis and others 2014). Dormancy is controlled by a balance between ABA, as growth inhibitor, and GAs, as a growth promotor (Kucera and others 2005; Finch-Savage and Leubner-Metzger 2006).
The germination promoting effect of growth hormones in breaking seed dormancy has been reported in many species (Macchia and others 2001; Duan and others 2004; El-Keblawy and others 2010; Bahrani and Pourreza 2012). For example, exogenous application of GA, kinetin, thiourea, nitrate, fusicoccin, proline, and betaine releases dormancy in many perennial plants (Ungar 1977; Plyler and Proseus 1996; Gulzar and Khan 2001; El-Keblawy and others 2005, 2010; El-Keblawy 2013; Yarnia and Tabrizi 2012). In addition, some nitrogenous compounds such as nitric oxide, nitrate, nitrite, and thiourea are known to stimulate the germination of seeds of many perennials (Bethke and others 2004; Li and others 2005). The exogenous application of nitrate and thiourea has been used to alleviate dormancy in species such as Cicer arietinum (Aldosaro and others 1981), Lasiurus scindicus (El-Keblawy and others 2010), Centropodia forsskalii, and Sporobolus spicatus (El-Keblawy 2013). Several studies have assessed the impact of DRCs on innate dormancy of many perennial plants; however, few included annual desert species, which usually have deep innate dormancy. The aim of the present study, therefore, was to assess the impact of DRCs such as GA3, kinetin, thiourea, and nitrate in alleviating seed dormancy (that is, stimulating germination) in 16 desert annuals from the arid deserts of the United Arab Emirates (UAE). The effect of light and its interaction with the different DRCs was also assessed. We hypothesized that light, as an environmental signal, would perhaps modify the effect of these DRCs.
Materials and Methods
Seed Collection and Variation in Size and Mass
Mature seeds of 16 common annual plant species were collected during April 2013 from different localities in the Northern Emirates of the UAE. Seeds of each species were randomly collected from the population to diminish the effect of genetic variation within the population. Immediately after collection, seeds were cleaned from the surrounding structures and debris and stored in brown paper bags at room temperatures (20 ± 2 °C) until the germination experiment was started in July 2013.
For each species, the average seed mass was determined by weighing three replicates, each of 100 seeds. In addition, average seed size of each species was assessed by measuring the length of 50 seeds, including any dispersal appendage if present. Seed color was assessed visually and classified as brown, dark brown, green, or black.
The climate of the Northern Emirates is generally hot and dry with a sub-tropical arid climate, which is warm in winter with hot and humid conditions in summer. The region is characterized by two distinctive seasons: a long dry season (April to November) with very high temperatures and a short season (December to March) with mild to warm temperatures and light rainfall. The mean daily temperature ranges between 12.1 °C in January and about 42 °C in June–August. Temperatures can reach up to 48 °C in summer. The average annual rainfall in the coastal area is 120 mm, but in some mountainous areas it could reach 350 mm (Böer 1997).
Germination Experiment
Seeds were germinated in four different DRCs: GA3 (3 mM), kinetin (0.25 mM), nitrate (20 mM), and thiourea (5 mM). These concentrations were commonly used in previous studies and assessed the impact of DRCs on the germination in both saline and non-saline solutions (for example, El-Keblawy and others 2005, 2010; El-Keblawy 2013; Khan and others 2002).
Germination experiments were conducted in a programmed incubator adjusted to a daily night/day temperature regime of 15/25 °C in both continuous darkness and alternating 12 h darkness/12 h light, hereafter referred as dark and light, respectively. These conditions are optimal for seed emergence in the natural habitats. A dark condition was achieved by wrapping the Petri dishes with two layers of aluminum foil. Seeds were germinated in 9-cm tight-fitting Petri dishes containing one disk of Whatman No. 1 filter paper with 10 ml of test solution. Each dish was placed in a 10-cm plastic dish as an added precaution against water loss by evaporation. Four replicate dishes, each with 25 seeds, were used for each treatment. A seed was considered to have germinated when the radicle had emerged. Germinated seedlings were counted and removed every alternate day for 20 days following seed soaking. Seeds incubated in the dark were checked only once after 20 days. Therefore, they were not exposed to any light during the incubation period.
Data Analyses
Two-way ANOVAs were performed to assess the effects of incubation with the DRCs and light, and their interaction on final germination of each of the 16 studied species. Tukey’s HSD test was performed for multiple comparisons to determine significant differences among the treatments at P = 0.05. The germination percentages were arcsine transformed to meet the assumptions of ANOVA. The transformation improved normality of distribution of data. All statistical methods were performed using SYSTAT, version 13.0.
Results
Seed Mass, Size, and Color Variations
Seeds of the different species were very small, that is, with small sizes and lighter masses. The largest seeds were those of Aristida adscensionis (length = 1.0 cm, including the appendage), but the smallest seeds were those of Oligomeris linifolia (0.05 cm). Eight species have average seed lengths of 0.1 cm or less. Seed mass ranged between 0.034 mg in Oligomeris linifolia to 1.426 mg in Brassica tournefortii. Nine species have average seed masses less than 0.2 mg. In addition, eight species have seed lengths of less than 0.3 cm, including the appendages, if present. Most of the species have brown (10 species) or black (4 species) seed coats (Fig. 1; Table 1).
Germination Behavior
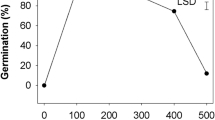
Seeds of 15 out of the studied 16 species had high levels of innate dormancy; almost no germination occurred in 3-month-stored seeds. The effects of light and growth regulators and their interaction were significant on the final germination of four Plantago species (P < 0.001), except P. ovata that showed insignificant effects for light and the interaction between light and DRCs (P > 0.05, Table 2). The highest dormancy alleviation in Plantago was achieved in seeds treated with kinetin; 60–90% of the treated seeds germinated in both light and dark in the different species, except P. ciliata, in which no germination occurred in the dark. GA3 partially alleviated dormancy in both light and dark in P. afra, and in light only in P. ovata. Nitrate partially alleviated innate dormancy in both light and dark in P. afra and P. ciliata. For thiourea, the alleviation was partial only in light in P. afra and P. ciliata (Fig. 2).
The impacts of light and DRCs and their interaction were significant on the final germination of three studied grasses (P < 0.05, Table 2). Almost no germination occurred in non-treated seeds of the three species. None of the DRCs was able to enhance germination of Aristida adscensionis seeds; few treated seeds germinated (Fig. 3a). All DRCs partially relieved innate dormancy in light, but not in the dark in Eragrostis ciliaris. Light germination ranged between 19% for seeds treated with thiourea to 28 and 33% for seeds treated with kinetin and GA3, respectively (Fig. 3b). In Tragus racemosus, all DRCs partially alleviated innate dormancy in both light and dark, but germination was significantly greater in light than in darkness for seeds treated with GA3 and kinetin (Fig. 3c).
The effect of DRCs varied greatly among three studied Brassicaceae species. In Anastatica hierochuntica, all DRCs resulted in greater than 80% germination in both light and darkness (Fig. 4a). In Eremobium aegyptiacum and B. tournefortii, the different DRCs had very limited effects in enhancing seed germination. Maximum germination was 18.8% for B. tournefortii seeds treated with GA3 in darkness and 31.3% for E. aegyptiacum seeds treated with thiourea in light (Fig. 4b, c). In E. aegyptiacum, a low level of germination occurred in seeds treated by GA3 and kinetin in the light, but not in darkness (Fig. 4c).
None of the DRCs succeeded in alleviating innate dormancy in two species of Resedaceae (O. linifolia and Reseda aucheri) (Fig. 5a, b). In Aizoon canariense (Aizoaceae), none of the DRCs succeeded in alleviating dormancy, except GA3, which resulted in 12% germination when seeds were incubated in darkness (Fig. 5c). In Senecio desfontainei (Asteraceae), all DRCs partially alleviated innate dormancy in both light and darkness. Germination ranged between about 18% for both kinetin and thiourea treatments in light as compared to 62.5% for kinetin in darkness. Germination was significantly greater in darkness than in light for seeds treated with kinetin, GA3, and thiourea (Fig. 5d). In Silene villosa (Caryophyllaceae), kinetin, nitrate, and thiourea partially alleviated dormancy in both light and darkness. Germination was significantly greater in the light than in darkness for seeds treated with kinetin (Fig. 5e). It is interesting to note that Viola cinerea (Violaceae) had the lowest innate dormancy of the studied species; 32.5% of non-treated seeds germinated in light, but none germinated in the darkness. Both GA3 and kinetin significantly increased germination in light, compared to controls (Fig. 5f).
Discussion
Seed dormancy is a common phenomenon found in many plant species in all major climatic regions. It is a kind of special adaptation that allows a species to determine the timing of germination and consequently to avoid unfavorable weather that might hinder subsequent establishment and reproduction (Finch-Savage and Leubner-Metzger 2006; El-Keblawy 2003). In our study, 15 out of 16 studied annuals of the Arabian deserts showed high levels of innate dormancy at 15/25 °C. These temperatures are favorable for seed germination and seedling establishment during the rainy seasons in arid deserts. Generally, seed dormancy at higher temperatures might prevent germination of winter annuals during summer conditions in arid deserts, where it is hard for any annual plant to survive. However, high dormancy noticed at moderate and lower temperatures (15/25 °C) in the 15 studied annuals could be a strategy for regulating germination in unpredictable desert environments. Seeds of annual desert plants might wait for special environmental cues, such as effective rainfall, specific soil nutrients, or specific light triggers to be able to germinate.
Numerous studies have demonstrated that seed dormancy in plants growing under harsh environmental conditions could be alleviated, and consequently, germination could be improved by applying different chemicals such as GA, kinetin, nitrate, thiourea and proline (for example, Gulzar and Khan 2001; Khan and Gul 2006; Finch-Savage and others 2007; El-Keblawy and others 2010; El-Keblawy 2013; Ahmed and others 2014). In our study, the dormancy of 13 annual desert plants (81.2% of the total studied species) was alleviated totally or partially by at least one of the tested DRCs (GA3, kinetin, nitrate and thiourea). This indicates that these desert annuals might have physiological dormancy. There is considerable evidence that DRCs are important positive regulators in releasing physiological dormancy (Kucera and others 2005).
Our result indicates that none of the DRCs succeeded in alleviating the innate dormancy in seeds of the two Resedaceae (O. linifolia, R. aucheri) or in A. canariense. The deep dormancy of these species and the inability of different DRC to alleviate this dormancy indicate that the seeds might have some inhibitors or that their embryos are not well developed (that is, morphological dormancy). In Ochradenus arabicus, another species of Resedaceae, low concentrations of GA3 (25–500 µM) were able to break deep innate dormancy, but higher concentrations failed to do so (Nadeem and others 2012). In addition, these authors indicated that germination started after 20 days of seed soaking. The failure of GA3 or the other DRCs to break dormancy in the two Resedaceae species in our study could be attributed to the high concentration of GA3 (3 mM) and/or the short period of time (20 days) during which we recorded the germination. The chosen concentration of GA and the length of the germination period have been considered as appropriate to achieve high germination in several germination studies (for example, El-Keblawy and others 2005, 2010; El-Keblawy 2013; Khan and others 2002). However, these concentrations were not appropriate to produce germination in the two Resedaceae species. The inconsistency in germination results of O. arabicus in our study and that of Nadeem and others (2012) suggests that different concentrations of DRCs and longer periods of time should be considered when assessing dormancy and germination. On the other hand, it might be plausible to suggest that the dormancy alleviation in O. linifolia, R. aucheri could be responsive to other environmental conditions such as light, temperature, storage period, or other soil conditions (El-Keblawy and Bhatt 2015; El-Keblawy and others 2015).
Nitrogen is one of the most important environmental nutrients needed for plants to complete their life cycle. Several reports have demonstrated a positive effect of nitrogenous compounds, such as nitric oxide, nitrate, nitrite, and thiourea, on relieving seed dormancy of many species (Bethke and others 2004; Li and others 2005). For example, nitrate was effective in breaking seed dormancy in several species, including Ocimum basalicum, Calendula officinalis, Cynara scolymus, and Physalis alkekengi (Aghilian and others 2014). In the present study, nitrate enhanced the germination of the dormant seeds of eight annual plants of the Arabian deserts. The germination was greater in light than in the dark in six species. Pons (1989) reached a similar conclusion in P. Lanceolata; nitrate significantly increased germination in bare (that is, exposed to light), compared to vegetated soil (that is, shaded or dark). Pons (1989) speculated that breaking seed dormancy by nitrate in light might function as a gap detection mechanism if nitrate concentrations in bare soil are high enough to break seed dormancy. The failure of seeds to germinate in vegetated soils, even in the presence of nitrate, indicates that the simulation effect of nitrate depends on the presence of light (Pons 1989). In other species, nitrate has been commonly used to break dormancy in seeds requiring light to germinate (Baskin and Baskin 1998). For instance, seed germination of Hypericum spp., which is affected by the absence of light, was enhanced by nitrate application (Çirak and others 2007). The positive effects of nitrate in enhancing germination was attributed to its role in influencing the permeability of membranes, which ultimately leads to activation of enzymes involved in protein synthesis and carbohydrate metabolism (Ghobadi and others 2012).
Thiourea is another nitrogenous compound that alleviated germination inhibition in several species. Thiourea was the most effective germination stimulator in dormant and non-dormant cocklebur seeds (Esashi and others 1977). In addition, exogenous application of thiourea resulted in the alleviation of innate dormancy of the perennial desert grasses Panicum turgidum (El-Keblawy and others 2010), Centropodia forsskalii, and Sporobolus spicatus (El-Keblawy 2013). Furthermore, the alleviation of innate dormancy by thiourea and nitrate was partial in Atriplex griffithii and Sporobolus arabicus, but was complete in Zygophyllum simplex (Khan and Weber 2006). It has been reported that nitrogenous compounds promote germination by acidification and softening of cell walls or by activating the pentose phosphate pathway (Esashi and others 1979). In addition, nitrogenous compounds counteract the effect of inhibitory growth regulators, such as ABA, and enhance the action of growth regulators such as GA and cytokinins in plant tissues (Kabar and Baltepe 1990). Furthermore, the stimulative effect of thiourea on seed germination was attributed to a reduction of the preventive effect of the seed coat and its cytokinin activity in overcoming inhibition (Çetinbaş and Koyuncu 2006).
Gibberellin is one of the hormones proposed to alleviate primary dormancy in many species. Our results showed that exogenous application of GA3, partially or completely, alleviated innate dormancy in P. afra, P. ciliata, P. ovata, E. ciliaris, T. racemosus, B. tournefortii, E. aegyptiacum, A. canariense, S. desfontainei, and A. hierochuntica. The alleviation was partial in all species, except A. hierochuntica. Similarly, Aghilian and others (2014) reported that GA significantly increased final germination, compared to untreated seeds of Plantago ovata, Rudbeckia hirta, and Satureja hortensis in the Iranian deserts. Other similar results have been reported in other annual plants such as Rumex dentatus (Ali and Helal 1996), Haplopappus gracilis (Galli and others 1975), and Sesamum indicum (Kyauk and others 1995). Exogenous application of GA might help balance the hormonal ratio needed for germination promotion, that is, increase the ratio of promoting hormones over inhibiting hormones such as ABA (Kucera and others 2005; Finch-Savage and Leubner-Metzger 2006). In addition, Galli and others (1975) reported that DNA synthesis was stimulated effectively in Haplopappus gracilis seeds treated with GA. More recently, Won and others (2014) suggested that GA affects the abundance of proteins involved in secondary metabolism, cell cycle, and protein degradation/synthesis in soybeans under flooding stress.
It has been reported that seed dormancy requiring germination stimulators, such as chilling and dry storage after ripening, could be overcome by applying GA (Gupta 2003). GA substitutes for light in seed germination of several species; that is, most light-requiring seeds do germinate in the dark when exogenous GAs are applied (Taylorson 1982). In addition, red light induces GA biosynthesis in photoblastic seeds. For example, in photoblastic lettuce seeds, the endogenous level of GA1 increased to a level about three times that of the dark control 6 h after a brief red light irradiation (Toyomasu and others 1993). In our study, however, there was no clear interactive effect of GA3 and light in the different desert annuals. Exogenous application of GA3 did not prompt germination in the darkness for seeds of six species (P. ovata, E. ciliaris, T. racemosus, E. aegyptiacum, S. villosa, and V. cinerea). However, GA3 resulted in better germination in darkness, compared to in light, in seeds of three species (B. tournefortii, A. canariense, and S. desfontainei).
Light has been considered as an important signal to trigger germination for seeds buried in soil. To recognize their position near the soil surface, seeds of some species, especially those with small sizes, have an initial light requirement to initiate their germination process (Baskin and Baskin 1998; Milberg and others 2000). Small seeds have limited resources that restrict seedling emergence from more than superficial depths (Bewley and Black 1994). In the present study, seeds of most studied species that were treated with at least one of DRCs showed a light requirement during their germination. This trend was very obvious in P. ciliata, E. ciliaris, and V. cinerea; their treated seeds germinated only in light. Species that have relatively larger sizes (four Plantago species, T. racemosus, A. hierochuntica, and B. tournefortii) germinated in both light and darkness. Milberg and others (2000) explained the light requirement for small seeds as a mechanism to avoid fatal germination in deeper soil that hinders seedlings from reaching the soil surface.
Kinetin significantly alleviated the innate dormancy in the four Plantago species, the three studied species of Brassicaceae, two grasses (E. ciliaris and T. racemosus), S. desfontainei, and V. cinerea. The positive effect of kinetin was observed in light for seven species and in the darkness for two species. Khan and Gul (2006) reviewed the impact of kinetin on the innate dormancy and reported a positive effect in enhancing the germination of some species (for example, Atriplex stocksii and Zygophyllum simplex), but no effect was observed on other species (for example, Aeluropus lagopoides, Arthrocnemum macrostachyum, and Sporobolus ioclados). In addition, kinetin induced a significant increase in the germination of chickpea (Cicer arietinum) by increasing amylase activity in the cotyledons (Kaur and others 1998). The increase in seed germination after exogenous application of GA3 and kinetin was attributed to the ability of the growth regulators to reduce the moisture requirement or to enhance water uptake during germination and early seedling growth (Sastry and Shekhawat 2001). In addition, kinetin also enhanced the biosynthesis of ethylene, which is known to promote the germination of seeds of several species when exposed to abiotic stresses (Khan and others 1993; Li and others 2005).
To conclude, desert annuals have innate dormancy. Such innate dormancy could be a viable evolutionary strategy that can help plants cope with environmental variability in the unpredictable and stressful hyper-arid deserts of the Arab Gulf region (El-Keblawy 2003). Our findings provide clear evidence that the effect of DRCs on the germination of annual plants in arid deserts is highly species specific. Different DRCs could be used for enhancing the germination of seeds of different species that would be used in restoring the degraded deserts, but nitrate is particularly suitable for field application (Saini and others 1985).
References
Aghilian S, Khajeh-Hosseini M, Anvarkhah S (2014) Evaluation of seed dormancy in forty medicinal plant species. Int J Agric Crop Sci 7:760
Ahmed MZ, Gulzar S, Khan MA (2014) Role of dormancy regulating chemicals in alleviating the seed germination of three playa halophytes. Ekoloji 23:1–8
Aldosaro JJ, Matilla A, Nicolás G (1981) Effect of ABA, fusicoccin and thiourea on germination and K+ and glucose uptake in chick-pea seeds at different temperatures. Physiol Plant 53:139–145
Ali A, Helal A (1996) Studies on germination of Rumex dentatus L. seeds. J Arid Environ 33:39–47
Bahrani A, Pourreza J (2012) Gibberlic acid and salicylic acid effects on seed germination and seedlings growth of wheat (Triticum aestivum L.) under salt stress condition. World Appl Sci J 18:633–641
Baskin CC, Baskin JM (1998) Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic Press, San Diego
Baskin CC, Baskin JM (2004) A classification system for seed dormancy. Seed Sci Res 14:1–16
Batlla D, Benech-Arnold RL (2014) Weed seed germination and the light environment: implications for weed management. Weed Biol Manag 14:77–87
Bethke PC, Gubler F, Jacobsen JV, Jones RL (2004) Dormancy of Arabidopsis seeds and barley grains can be broken by nitric oxide. Planta 219:847–855
Bewley JD (1997) Seed germination and dormancy. Plant Cell 9:1055
Bewley JD, Black M (1994) Seeds: physiology of development and germination, 2nd edn. Plenum Press, New York
Böer B (1997) An introduction to the climate of the United Arab Emirates. J Arid Environ 35:3–16
Bouwmeester HJ, Karssen CM (1993) Seasonal periodicity in germination of seeds of Chenopodium album L. Ann Bot 72:463–473
Çetinbaş M, Koyuncu F (2006) Improving germination of Prunus avium L. seeds by gibberellic acid, potassium nitrate and thiourea. Hortic Sci 33:119–123
Çırak C, Kevseroğlu K, Ayan AK (2007) Breaking of seed dormancy in a Turkish endemic Hypericum species: Hypericum aviculariifolium subsp. depilatum var. depilatum by light and some pre-soaking treatments. J Arid Environ 68:159–164
Duan DE, Liu XI, Khan MA, Gul B (2004) Effects of salt and water stress on the germination of Chenopodium glaucum L. seed. Pak J Bot 36:793–800
El-Keblawy A (2003) Effects of achene dimorphism on dormancy and progeny traits in the two ephemerals Hedypnois cretica and Crepis aspera (Asteraceae). Can J Bot 81:550–559
El-Keblawy A (2013) Effects of seed storage on germination of two succulent desert halophytes with little dormancy and transient seed bank. Acta Ecol Sin 33:338–343
El-Keblawy A, Al-Rawai A (2005) Effects of salinity, temperature and light on germination of invasive Prosopis juliflora (SW.) D.C. J Arid Environ 61:555–565
El-Keblawy A, Bhatt A (2015) Aerial seed bank affects germination in two small-seeded halophytes in Arab Gulf desert. J Arid Environ 117:10–17
El-Keblawy A, Al-Ansari F, Al-Rawai A (2005) Effects of dormancy regulating chemicals on innate and salinity induced dormancy in the invasive multipurpose Prosopis juliflora (Sw.) DC. Shrub. Plant Growth Regul 46:161–168
El-Keblawy A, Al-Ansari F, Al-Shamsi N (2010) Impact of dormancy regulating chemicals on salinity induced dormancy in Lasiurus scindicus and Panicum turgidum: two desert glycophytic grasses. Plant Growth Regul 62:163–170
El-Keblawy A, Bhatt A, Gairola S (2015) Storage on maternal plants affects light and temperature requirements during germination in two small seeded halophytes in the Arabian deserts. Pak J Bot 47:1701–1708
Esashi Y, Katoh H, Leopold AC (1977) Dormancy and impotency of cocklebur seeds IV. Effects of gibberellic acid, benzyladenine, thiourea, and potassium nitrate on the growth of embryonic axis and cotyledon segments. Plant Physiol 59(2):117–121
Esashi Y, Ohara Y, Okazaki M, Hishinuma K (1979) Control of cocklebur seed germination by nitrogenous compounds: nitrite, nitrate, hydroxylamine, thiourea, azide, and cyanide. Plant Cell Physiol 20:349–361
Finch-Savage WE, Footitt S (2012) To germinate or not to germinate: a question of dormancy relief not germination stimulation. Seed Sci Res 22:243–248
Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytol 171:501–523
Finch-Savage WE, Cadman CS, Toorop PE, Lynn JR, Hilhorst HW (2007) Seed dormancy release in Arabidopsis Cvi by dry after-ripening, low temperature, nitrate and light shows common quantitative patterns of gene expression directed by environmentally specific sensing. Plant J 51:60–78
Finkelstein RR (2004) The role of hormones during seed development and germination. In: Davies PJ (ed) Plant hormones—biosynthesis, signal transduction, action!. Kluwer, Dordrecht, pp 513–537
Finkelstein R, Reeves W, Ariizumi T, Steber C (2008) Molecular aspects of seed dormancy. Annu Rev Plant Biol 59:387
Galli MG, Sparvoli E, Caroi M (1975) Comparative effects of fusicoccin and gibberellic acid on the promotion of germination and DNA synthesis initiation in Haplopappus gracilis. Plant Sci Lett 5:351–357
Ghobadi M, Shafei-Abnavi M, Honarmand SJ, Ghobadi ME, Mohammadi GR (2012) Effect of hormonal priming (GA3) and osmopriming on behavior of seed germination in wheat (Triticum aestivum L.). J Agric Sci 4:244–250
Gulzar S, Khan MA (2001) Seed germination of a halophytic grass Aeluropus lagopoides. Ann Bot 87:319–324
Gupta V (2003) Seed germination and dormancy breaking techniques for indigenous medicinal and aromatic plants. J Med Aromat Plants Sci 25:402–407
Huang Z, Gutterman Y (1998) Artemisia monospermaachene germination in sand: effects of sand depth, sand/water content, cyanobacterial sand crust and temperature. J Arid Environ 38:27–43
Huang Z, Ölçer-Footitt H, Footitt S, Finch-Savage WE (2015) Seed dormancy is a dynamic state: variable responses to pre-and post-shedding environmental signals in seeds of contrasting Arabidopsis ecotypes. Seed Sci Res 25:159–169
Kabar K, Baltepe S (1990) Effect of kinetin and gibberellic acid in overcoming high temperature and salinity (NaCl) stresses on the germination of barley and lettuce seeds. Phyton 30:65–74
Kaur S, Gupta AK, Kaur N (1998) Gibberellic acid and kinetin partially reverse the effect of water stress on germination and seedling growth in chickpea. Plant Growth Regul 25:29–33
Khan AA (1978) Incorporation of bioactive chemicals into seeds to alleviate environmental stress. Acta Hortic 83:2255–2264
Khan MA, Gul B (2006) Halophyte seed germination. In: Khan MA, Weber DJ (eds) Ecophysiology of high salinity tolerant plants. Springer, Dordrecht, pp 11–30
Khan MA, Weber DJ (2006) Ecophysiology of high salinity tolerant plants. Springer, Dordrecht
Khan AA, Andreoli C, Kuo CG (1993) Role of ethylene biosynthesis in seed germination and stand establishment under stress. In: Proceedings of an international symposium on adaptation of food crops to temperature and water stress, Taiwan, pp 81–90
Khan MA, Gul B, Weber DJ (2002) Improving seed germination of Salicornia rubra (Chenopodiaceae) under saline conditions using germination-regulating chemicals. Western N Am Nat 62:101–105
Kigel J (1995) Seed germination in arid and semiarid regions. In: Kigel J, Galili G (eds) Seed development and germination. Marcel Dekker, New York, pp 645–699
Koornneef M, Bentsink L, Hilhorst H (2002) Seed dormancy and germination. Curr Opin Plant Biol 5:33–36
Koutsovoulou K, Daws MI, Thanos CA (2014) Campanulaceae: a family with small seeds that require light for germination. Ann Bot 113:135–143
Kucera B, Cohn MA, Leubner-Metzger G (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15:281–307
Kyauk H, Hopper NW, Brigham RD (1995) Effects of temperature and presoaking on germination, root length and shoot length of sesame (Sesamum indicum L.). Environ Exp Bot 35:345–351
Li W, Liu X, Khan MA, Yamaguchi S (2005) The effect of plant growth regulators, nitric oxide, nitrate, nitrite and light on the germination of dimorphic seeds of Suaeda salsa under saline conditions. J Plant Res 118:207–214
Macchia M, Angelini LG, Ceccarini L (2001) Methods to overcome seed dormancy in Echinacea angustifolia DC. Sci Hortic 89:317–324
Milberg P, Andersson L, Thompson K (2000) Large-seeded spices are less dependent on light for germination than small-seeded ones. Seed Sci Res 10:99–104
Mulroy TW, Rundel PW (1977) Annual plants: adaptations to desert environments. BioScience 27:09–114
Nadeem M, Al-Qurainy F, Khan S, Tarroum M, Ashraf M (2012) Effect of some chemical treatments on seed germination and dormancy breaking in an important medicinal plant Ochradenus arabicus Chaudhary, Hill C. & AG Mill. Pak J Bot 44:1037–1040
Plyler DB, Proseus TE (1996) A comparison of the seed dormancy characteristics of Spartina patens and Spartina alterniflora (Poaceae). Am J Bot 83:11–14
Pons TL (1989) Breaking of seed dormancy by nitrate as a gap detection mechanism. Ann Bot 63:139–143
Rezvani M, Zaefarian F, Amini V (2014) Effects of chemical treatments and environmental factors on seed dormancy and germination of shepherd’s purse (Capsella bursa-pastoris (L.) Medic.). Acta Bot Bras 28:495–501
Saini HS, Bassi PK, Spencer MS (1985) Seed germination in Chenopodium album L: relationships between nitrate and the effects of plant hormones. Plant Physiol 77:940–943
Sastry EV, Shekhawat KS (2001) Alleviatory effect of GA3 on the effects of salinity at seedling stage in wheat (Triticum aestivum). Indian J Agric Res 35:226–231
Taylorson RB (1982) Interaction of phytochrome and other factors in seed germination. In: Khan AA (ed) The physiology and biochemistry of seed development, dormancy and germination. Elsevier Biomedical Press, Amsterdam, pp 323–346
Toyomasu T, Tsuji H, Yamane H, Nakayama M, Yamaguchi I, Murofushi N, Takahashi N, Inoue Y (1993) Light effects on endogenous levels of gibberellins in photoblastic lettuce seeds. J Plant Growth Regul 12:85–90
Ungar IA (1977) Salinity, temperature, and growth regulator effects on seed germination of Salicornia europaea L. Aquat Bot 3:329–335
Willis CG, Baskin CC, Baskin JM, Auld JR, Venable DL, Cavender-Bares J, Rubio de Casas R et al (2014) The evolution of seed dormancy: environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytol 203:300–309
Won OM, Nanjo Y, Komatsu S (2014) Analysis of soybean root proteins affected by gibberellic acid treatment under flooding stress. Protein Pept Lett 21:911–947
Yarnia M, Tabrizi EFM (2012) Effect of seed priming with different concentration of GA3, IAA and kinetin on Azarshahr onion germination and seedling growth. J Basic Appl Sci Res 2:2657–2661
Acknowledgements
This work was supported by a grant from the Qatar National Research Fund, QNRF (Grant # 5-260-1-053). We are grateful to Prof. Yougasphree Naidoo of UKZN, South Africa for linguistic revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
El-Keblawy, A., Gairola, S. Dormancy Regulating Chemicals Alleviate Innate Seed Dormancy and Promote Germination of Desert Annuals. J Plant Growth Regul 36, 300–311 (2017). https://doi.org/10.1007/s00344-016-9640-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9640-z