Abstract
A greenhouse hydroponic experiment was performed to evaluate how peanut seedlings (Arachis hypogaea L.) responded to iron (Fe) deficiency stress in the presence of sodium nitroprusside (SNP), a nitric oxide (NO) donor. The results showed that Fe deficiency inhibited peanut plant growth, decreased chlorophyll and active Fe concentrations, and dramatically disturbed ion balance. The addition of 50, 100, 250, and 500 µM SNP, significantly promoted the absorption of Fe in the cell wall, cell organelles, and soluble fractions, increased the concentrations of active Fe and chlorophyll in peanut plants, and alleviated the excess absorption of manganese (Mn) and copper (Cu) induced by Fe deficiency. In addition, SNP also significantly increased the activities of superoxide dismutase, peroxidase, and catalase, which is beneficial to inhibit the accumulation of malondialdehyde and reactive oxygen species. Addition of 250 µM SNP had the most significant alleviating effect against Fe-deficiency stress, and after 15 days of treatment, the plants with the 250 µM SNP treatment achieved comparable NO levels with those grown under optimal nutrition conditions. However, the effects of SNP were reversed by addition of hemoglobin (Hb, a NO scavenger). These results suggest that NO released from SNP decomposition was responsible for the effect of SNP-induced alleviation on Fe deficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iron (Fe) deficiency is a widespread agricultural problem in many crops cultivated in alkaline, calcareous soils, where the availability of Fe is often insufficient to fulfill plant needs (Rombolà and others 2005). Thus, Fe deficiency is one of the major limiting factors affecting crop yields, food quality, and human nutrition (Zuo and Zhang 2011). The World Health Organization (WHO) has stated that the lack of sufficient micronutrients, such as Fe and Zn, represents a major threat to the health and development of the world population. Two billion people are anemic, mainly due to Fe deficiency (WHO 2007). Therefore, how to raise Fe density in food is important. Fe deficiency impairs chlorophyll biosynthesis and chloroplast development in both dicotyledonous and monocotyledonous species (Graziano and others 2002) and reduces the primary productivity of crops. Interveinal chlorosis is the major typical symptom in plants growing under Fe-deficiency conditions and it is caused by a reduction of the pigments and proteins in the photosynthetic apparatus with a resultant decrease in the photosynthetic rate.
In response to Fe deficiency, plants have developed a suite of responses to readjust cellular homeostasis, including induced ferric-chelate reductase (FCR) embedded in the root epidermal cell plasma membrane proton pump, which increases proton exudation. Another common biochemical change occurring when plants are subjected to Fe-deficiency stress is the accumulation of reactive oxygen species (ROS), which unbalances the cellular redox in favor of oxidized forms, thereby creating oxidative stress that can damage DNA, inactivate enzymes, and cause lipid peroxidation (Mittler 2002). To control the level of ROS, plants have evolved the antioxidant defense system comprised of enzymes such as superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (GPX), ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), glutathione reductase (GR), and non-enzymatic constituents such as ascorbate and glutathione, which are responsible for scavenging excessively accumulated ROS in plants under stress conditions (Shi and others 2007). Accordingly, the regulation of these antioxidant constituents by an exogenous substance may mediate plant tolerance to Fe-deficiency stress. Peanut (Arachis hypogaea L.) is the major oilseed crop in China, accounting for 30 % of the total oilseed production in the country. As one of the important staple crops, an increase in mineral nutrient content may have a significant effect on human nutrition (Nestel and others. 2006). However, increasing Fe concentration in peanut is complicated. Although Fe is abundant in soil, the availability of Fe is often limited because of low solubility of oxidized forms (Xiong and others 2012).
Nitric oxide (NO) is a small water-soluble and fat-soluble molecule whose role in human and animal nervous, cardiovascular, and immune systems has been studied extensively. In recent years, there has been much progress in research into NO in plants, with reports that NO is a signaling molecule regulating plant growth, development, and iron availability (Lamattina and others 2003; Zhang and others 2008; Wang and others 2013b). In relation to abiotic stresses, it was shown that the application of the SNP, a NO donor, reduced the harmful effects of heavy metals, salinity, and Fe deficiency on plants (Lopez-Carrion and others 2008; Leonor and others 2011; Wang and others 2013a). Delledonne and others (1998) reported that 0.5 mM SNP can approximately produce 2.0 µM NO. Graziano and others (2002) showed for the first time that treatment with SNP prevented the typical interveinal chlorosis in maize plants grown under Fe deficiency. Graziano and Lamattina (2005) reported that NO was associated with Fe homeostasis in plants. Sun and others (2007) also reported that NO could protect maize plants from Fe deficiency-induced oxidative stress by reacting with ROS directly or by changing the activities of ROS-scavenging enzymes. In addition, Fe deficiency induced endogenous NO accumulation in the root epidermis of tomato (Graziano and Lamattina 2007). NO can be a key signaling molecule in signal transduction pathways. Under ROS-related toxicity, NO may act as a chain breaker and thus limit the oxidative damage.
Considering that NO as a ubiquitous signal molecule participates in major physiological processes in plant growth and development, and in response to biotic and abiotic stresses (Besson-Bard and others 2008, 2009), the objective of this study was to investigate the possible role of exogenous NO in Fe-deficiency-induced changes of growth and antioxidant systems, as well as the subcellular distribution of Fe in peanut seedlings.
Materials and Methods
Plant Material and Culture Conditions
Peanut seeds were sterilized with 5 % sodium hypochlorite for 15 min and washed extensively with distilled water, then germinated on moist filter paper in the dark at 25 °C for 2 days. The germinated seeds were transferred to plastic trays filled with vermiculite and grown in a greenhouse. One-week-old seedlings were planted on perforated polystyrene plates, floating on an aerated half-strength Hoagland nutrient solution (Hoagland and Arnon 1950) for 3 days. Then, the solution was replaced with full-strength Hoagland solution. When the third leaf expanded fully, the uniform seedlings were removed of cotyledons and transferred into 2.5-L black glass containers of different nutrient solutions with four seedlings per container. The nutrient solution was renewed every 3 days.
The experimental design consisted of a control (full-strength Hoagland solution; indicated by CK) and six treatments (Hoagland solution omitted FeNaEDTA, as indicated by –Fe; the “–Fe” treatment amended with 50, 100, 250, or 500 µM SNP, designated as SNP1, SNP2, SNP3, SNP4, respectively; and the SNP4 treatment amended with 3 % NO scavengers (bovine hemoglobin) as designated as SNP + Hb). Each treatment had three replicates, giving a total of 21 containers. All the containers were arranged following a randomized block design. The culture experiment was carried out under controlled conditions in a growth chamber, with 25 ± 1 °C/20 ± 1 °C (day/night) temperatures, and a 16 h photoperiod at a light intensity of 300 ± 10 μEm−2s−1 provided by reflector sunlight dysprosium lamps. Relative humidity ranged from 65 to 70 %. After 20 days of treatment, the plants were harvested and the roots and shoots were separated and washed with 5 mM CaCl2 first and then repeatedly washed with deionized distilled water. For the estimation of plant dry matter and concentrations of Fe and other mineral nutrients in plant, the plant samples were oven-dried at 80 °C to constant weight. For measurement of enzyme, fresh plant samples were frozen in liquid nitrogen and stored at −70 °C prior to analysis.
Determination of NO Levels
NO levels were determined according to the method described by Chen and others (2010). Fresh samples were cut into approximately 5-mm segments and immediately incubated with 100 U CAT and 100 U SOD for 5 min to remove endogenous ROS before addition of oxyhemoglobin (5 mM). After 3 min of incubation, NO was quantified by spectrophotometric measurement of the conversion of oxyhemoglobin to methemoglobin.
Determination of Plant Growth and Concentrations of Fe and Other Minerals in Plants
After harvest, the plants were separated into roots and shoots. Shoot height, root length, and their fresh weights were measured. The root volume was determined by the water displacement method. The plant samples were oven-dried for 30 min at 105 °C and then at 80 °C until constant weight. The dried plant tissues were weighed to obtain dry biomass yield and ground to <1 mm powder, and the concentrations of Fe and other minerals in the plant were determined by atomic absorption spectrophotometer (SHIMADZU AA-6300, Kyoto, Japan) after digestion with mixed acid [HNO3 + HClO4 (3:1 v/v)] (Graziano and others 2002).
Determination of Root Activity
Root activity was determined by the triphenyl tetrazolium chloride (TTC) method. Roots were first washed with deionized water and cut into small pieces, and then 1–2 g of fresh root was weighed. Roots were then placed into test tubes and mixed with 5 mL 0.4 % (w/v) TTC and 5 mL 66 mM Na2HPO4–NaH2PO4 (pH 7.0). The mixture was incubated at 37 °C for 3 h and then 2 mL of 1 M H2SO4 was added. Six mL ethyl acetate was added and the root was ground with mortar and pestle, and the resultant suspension was then filtered with a Whatman #41 filter paper. Four mL ethyl acetate was added to the filtrate and the solution was shaken on a mechanical shaker for 1 min. The color intensity of the solution was then measured using a spectrophotometer (Shimadzm UV-2210) at wavelength of 485 nm. Root activity was expressed as absorbance per gram of fresh root (Kong and others 2014).
Determination of Chlorophyll (chl) Concentrations
The chlorophyll concentrations were determined according to the method of Knudson and others (1977). Fresh leaf tissue (0.5 g) was extracted with 2 mL 95 % (v/v) ethanol for 24 h in the dark, and the extract was analyzed for the concentrations of chlorophyll a, b and carotenoid using a spectrophotometer (SHIMADZU UV-2450, Kyoto, Japan), by taking the absorbance readings at 665, 649, and 470 nm, respectively. The leaf chlorophyll concentration is expressed as mg per gram-fresh weight (mg g−1 FW).
Determination of Nutrient Solution pH
The pH of the nutrient solution was measured with a MV-pH meter (DMP-2), and each treatment was replicated three times. After 4 days of treatment, the pH of the nutrient solution was measured from 8 a.m. to 6 p.m. every 2 h on the first day, and measured at 2 p.m. on the second and third day. During the measurement period, the nutrient solution was not replaced, and only distilled water was added to replenish that lost by evaporation.
Isolation of Plasma Membrane and the Measurement of H+-ATPase Activity
A membrane fraction enriched in plasma membrane vesicles was prepared as described by Briskin and others (1987) with minor changes. Excised roots were homogenized (1/2, w/v) with a mortar and pestle in a cold grinding medium containing 25 mM HEPES-Tris (pH 7.2), 250 mM mannitol, 5 mM EDTA, 5 mM EGTA, 1 mM DTT, and 1.5 % (w/v) PVP. The entire isolation process was carried out at 4 °C. The homogenate was filtered through a four-layer cheesecloth filter and centrifuged at 560×g for 12 min, and then the supernatant was centrifuged at 10,000×g for 15 min, and then at 60,000×g for 30 min to yield a crude membrane fraction. The resulting pellet was resuspended in 1 mL of gradient buffer containing 20 mM HEPES-Tris (pH 7.5), 5 mM EDTA, and 0.5 mM EGTA. The supernatant was layered on top of a step gradient consisting of 1 mL 45, 33, and 15 % (w/w) sucrose, respectively, and then centrifuged for 2 h at 70,000×g.
ATP hydrolysis assays were performed as described by Briskin and others (1987) in 0.5 mL of the reaction medium containing 36 mM Tris-Mes (pH 6.5), 30 mM ATP-Na2, 3 mM MgSO4, 1 mM NaN3, 50 mM KNO3, 1 mM Na2MoO4, and 0.02 % (w/v) Triton A-100, in the presence or absence of 2.5 mM Na3VO4. The reaction was triggered by adding 50 µL PM vesicles. After 30 min incubation at 37 °C, the reaction was quenched by adding 50 µL 55 % (w/v) TCA. The H+-ATPase activity was determined by the release of Pi (Ohinishi and others 1975).
Soluble protein content was measured by the Coomassie Brilliant Blue G-250 method (Bradford 1976). Soluble protein content was expressed as mg g−1 FW.
Determination of Active Fe Concentration in Plants
Fresh young leaves were cut into pieces, and 2.00 g was weighed and extracted with 20 mL 1 N HCl (in 1:10 tissue: extractant), shaken for 5 h, and filtered, and the subsequent Fe concentration in the extract was measured using an atomic absorption spectrophotometer (PE-2100B, Perkin Elmer Co. Ltd., MA, USA) (Gao and Shi 2007).
Assay of Ferric-Chelate Reductase (FCR)
Plants roots were immersed in saturated CaSO4 solution for 5 min, washed with deionized water, and then transferred to the nutrient solution mentioned above, which contained 0.1 mM Fe-EDTA and 0.4 mM 2, 2-bipyridyl, pH 5.0. The environmental conditions during the measurement were kept the same as for plant growth. After 2 h, the nutrient solution mentioned above which contained 0.1 mM Fe-EDTA and 0.4 mM 2, 2-bipyridyl, pH 5.0 as blank handling and Fe-reduction capacity by the roots was determined by measuring the concentration of Fe2+-dipyridyl complex formed at A523 using a spectrophotometer (Shimadzm UV-2210, Tokyo, Japan) (Zhang and others 2012).
Determination of Fe Distribution in Subcellular Structure
After harvest, roots were separated and immersed in 20 mM disodium ethylenediamine tetraacetic acid (Na2-EDTA) for 15 min and were then rinsed three times with deionized water to remove any Fe attached to the external surface of the root. Twenty-five g of fresh root and leaf samples was homogenized in 50 mL of chilled extracting buffer containing 50 mM Hepes (pH 7.5), 500 mM sucrose, 1 mM DTT, 5 mM ascorbate, and 1 % polyvinylpolypyrrolidone (PVPP). The homogenate was centrifuged at 500×g for 5 min to isolate the cell wall fraction. The supernatant was separated and then centrifuged at 20,000×g for 45 min to sediment cell organelles, and the resultant supernatant solution was referred to as the soluble fraction. All steps were performed at 4 °C. The fractions of the samples were digested in a mixture of HNO3 and HClO4 (4:1, v/v) at 120 °C for at least 3 h. The concentration of Fe was then determined using an atomic absorption spectrometer (AA-6800, Shimadzu, Tokyo, Japan) (Kong and others 2014).
Antioxidant Enzyme and Malondialdehyde (MDA) Assay
At harvest, the second fully expanded leaves of the plants were sampled for enzymatic analysis. Samples were homogenized in 0.05 mol L−1 phosphate buffer (pH 7.8) by grinding with a mortar and pestle under chilled condition with liquid nitrogen. The homogenate was filtered through a four-layer muslin cloth filter and centrifuged at 12,000×g for 10 min at 4 °C. All spectrophotometric analyses were conducted on a SHIMADZU UV-2450 spectrophotometer (Kyoto, Japan). Superoxide dismutase (SOD) activity was assayed by measuring its capacity to inhibit the photochemical reduction of nitroblue tetrazolium following the method of Stewart and Bewley (1980). Catalase (CAT) activity was measured as the decline in absorbance at 240 nm due to the decrease in the extinction of H2O2 according to the method of Patra and others (1978). Peroxidase (POD) activity was measured by the increase in absorbance at 470 nm due to guaiacol oxidation (Nickel and Cunningham 1969). The level of lipid peroxidation in fresh leaves was measured in terms of MDA concentration by the thiobarbituric acid reaction method (Heath and Packer 1968). MDA concentration was expressed as nmol g−1 FW.
Determination of the Superoxide Anion (O •−2 ) Generation Rate
For the measurement of O •−2 generation rate, 0.3 g of fresh leaves was ground in liquid N2 and extracted in 3 mL of ice-cold 50 mM sodium phosphate buffer (PBS) (pH 7.0). One milliliter supernatant of fresh root and leaf extracts was added to 0.9 mL of 65 mM phosphate buffer solution (pH 7.8) and 0.1 mL of 10 mM hydroxylammoniumchloride. One milliliter 50 mM sodium phosphate buffer (PBS) (pH 7.0) instead of one milliliter supernatant of fresh leaf extract was used as a blank. The reaction was incubated at 25 °C for 35 min. Solution (0.5 mL) from the above reaction mixture was then transferred to the container with 0.5 mL 17 mM sulfanic acid and 0.5 mL 7.8 mM α-naphthylamine solution. After 20 min of reaction, 2 mL of ether was added and then mixed well. The solution was centrifuged at 1500×g at 4 °C for 5 min. The absorbance of the pink supernatant was measured at 530 nm using the spectrophotometer. Absorbance values were calibrated to a standard curve generated with known concentrations of HNO2 (Wang and others 2013a).
Determination of Hydrogen Peroxide (H2O2) Concentration
Fresh root and leaf samples (1 g) were homogenized in 2 mL ice-cold acetone. Titanium reagent (2 % TiCl2 in conc. HCl) was added to a known volume of extracting solution to give a Ti(IV) concentration of 2 %. The Ti–H2O2 complex, together with unreacted Ti, was then precipitated by adding 0.2 mL 17 M ammonia solution for each 1 mL of extract. The precipitate was washed five times with ice acetone by resuspension and drained in 1 M H2SO4 (3 mL). The absorbance of the solution was measured at 410 nm against blanks, which had been similarly prepared but without plant tissue (Wang and others 2013a).
Statistical Analysis
Analysis of variance (ANOVA) was performed using SAS software (SAS Institute, Cary NC). Differences between treatments were separated by the least significant difference (LSD) test at a 0.05 probability level.
Results
NO Production
Compared to CK, Fe-deficiency treatment significantly decreased NO content in leaves and roots after 5 and 15 days, and addition of 100, 250, or 500 µM SNP reversed this process (Fig. 1). However, the effect of 500 µM SNP was eliminated by addition of hemoglobin. The SNP + Hb treatment resulted in similar NO levels in leaves and roots as –Fe treatment. In addition, after 15 days of treatment, with 250 µM SNP treatment, the plants achieved comparable NO levels with those grown under optimal conditions (Fig. 1).
Effects of different concentrations of SNP on NO concentrations in leaves and roots of peanut plants after 5 and 15 days treatment. Values are the mean of three replicates. Bars with different letters are significantly different at P < 0.05. CK full-strength Hoagland solution, –Fe Hoagland solution omitted FeNaEDTA, SNP1 Hoagland solution omitted FeNaEDTA, supplied with 50 µM SNP, SNP2 Hoagland solution omitted FeNaEDTA, supplied with 100 µM SNP, SNP3 Hoagland solution omitted FeNaEDTA, supplied with 250 µM SNP, SNP4 Hoagland solution omitted FeNaEDTA, supplied with 500 µM SNP, SNP + Hb Hoagland solution omitted FeNaEDTA, supplied with 500 µM SNP and 3 % NO scavenger, bovine hemoglobin
Plant Growth and Root Activity
As shown in Table 1, compared with CK, Fe deficiency significantly inhibited peanut plant growth and root activity. However, the inhibition was alleviated by 50, 100, 250, or 500 µM SNP, especially the 250 µM SNP treatment. Compared with –Fe treatment, the shoot height, root length, root/shoot ratio, dry weight, and root activity in the SNP3 treatment were increased by 45.12, 115.76, 42.50, 50.28, and 63.39 %, respectively. However, the effects of SNP4 were eliminated by addition of hemoglobin. These results indicated that it is exogenous NO released from SNP decomposition that improves plant growth and root activity, and the contribution of other components from SNP may be minimal. It appears that there is an optimal range of NO concentrations for mitigating Fe-deficiency stress.
Chlorophyll Concentrations
Peanut seedlings subjected to Fe-deficiency treatment showed a significant decrease in chl a, chl b, car, and chl a/b at 10 and 20 days, compared to CK (Fig. 2). The effects of SNP on chlorophyll concentrations and chl a/b varied with concentration, with SNP3 being the most effective in increasing chl a, chl b, and car concentrations. However, SNP + Hb treatment did not reach statistically significant differences in concentrations of chl a, chl b, car, and chl a/b at 20 days, compared to –Fe treatment.
Effects of SNP at different concentrations on chlorophyll concentrations in leaves of peanut plants after 10 and 20 days of treatment. Values are the mean of three replicates. Bars with different letters are significantly different at P < 0.05. CK full-strength Hoagland solution, –Fe Hoagland solution omitted FeNaEDTA, SNP1 Hoagland solution omitted FeNaEDTA, supplied with 50 µM SNP, SNP2 Hoagland solution omitted FeNaEDTA, supplied with 100 µM SNP, SNP3 Hoagland solution omitted FeNaEDTA, supplied with 250 µM SNP, SNP4 Hoagland solution omitted FeNaEDTA, supplied with 500 µM SNP, SNP + Hb Hoagland solution omitted FeNaEDTA, supplied with 500 µM SNP and 3 % NO scavenger, bovine hemoglobin
Total Fe Concentration, Active Fe Concentration, and FCR Activity
Peanut plants subjected to Fe deficiency had a significant decrease in total Fe concentration in roots and stems (P < 0.05), and active Fe concentration in leaves, but had increased FCR activity in roots by 80 %, and minimal change in total Fe concentration in leaves, compared to CK (Table 2). The addition of SNP increased the total Fe concentration in roots and leaves, but decreased the total Fe concentration in stems. The effect varied with SNP concentration, with SNP3 (250 μM) being the most effective. The SNP3 treatment increased active Fe concentrations by 227 % compared with –Fe treatment. In addition, it also decreased FCR activity by 51.9 %, compared to the –Fe treatment. However, the SNP + Hb treatment resulted in little changes in total Fe concentrations, active Fe concentration, and FCR activity, implying that addition of Hb eliminated the effects of SNP.
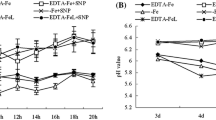
Nutrient Solution pH and H+-ATPase Activity
Compared with CK, Fe deficiency dramatically increased H+-ATPase activity in roots, but had no significant effect in leaves (Fig. 3). The amendment of SNP to the –Fe treatment, regardless of concentration, dramatically increased H+-ATPase activity in leaves and roots compared with –Fe treatment. The SNP3 treatment had the best result, increasing H+-ATPase activity by 24.4 % in roots and by 38. 8 % in leaves, compared to –Fe treatment.
Effects of SNP at different concentrations on H+-ATPase activity in leaves and roots of peanut plants after 20 days of treatment. Values are the mean of three replicates. Bars with different letters are significantly different at P < 0.05. CK full-strength Hoagland solution, –Fe Hoagland solution omitted FeNaEDTA, SNP1 Hoagland solution omitted FeNaEDTA, supplied with 50 µM SNP, SNP2 Hoagland solution omitted FeNaEDTA, supplied with 100 µM SNP, SNP3 Hoagland solution omitted FeNaEDTA, supplied with 250 µM SNP, SNP4 Hoagland solution omitted FeNaEDTA, supplied with 500 µM SNP, SNP + Hb Hoagland solution omitted FeNaEDTA, supplied with 500 µM SNP and 3 % NO scavengers bovine hemoglobin
Subcellular Distribution of Iron
To understand the mechanisms of exogenous SNP in alleviating chlorosis induced by Fe deficiency, Fe distributions in the cell wall, soluble fraction, and cell organelles of both roots and leaves were examined. Fe deficiency dramatically decreased Fe concentrations in the cell wall and cell organelles of roots, and Fe concentrations in cell organelles and the soluble fraction of leaves, but increased Fe concentrations in the cell wall of leaves and had no significant effect on the soluble fraction in roots, compared to CK (Fig. 4). The effect of SNP on the subcellular distribution of Fe in leaves varied with application rate. The SNP3 treatment decreased Fe concentrations in the cell wall by 28.5 %, but increased Fe concentrations in cell organelles and the soluble fraction by 12.6 and 43.9 %, respectively, whereas SNP1, SNP2, or SNP4 treatment decreased Fe concentrations in the cell wall and cell organelles, and increased Fe concentrations in the soluble fraction compared with –Fe treatment in roots. The SNP + Hb treatment resulted in no difference in Fe concentrations in the cell wall, cell organelles, or the soluble fraction of leaves and roots, compared to the –Fe treatment.
Effects of SNP at different concentrations on subcellular structure distribution of Fe in leaves and roots of peanut plants after 20 days of treatment. Values are the mean of three replicates. Bars with different letters are significantly different at P < 0.05. CK full-strength Hoagland solution, –Fe Hoagland solution omitted FeNaEDTA, SNP1 Hoagland solution omitted FeNaEDTA, supplied with 50 µM SNP, SNP2 Hoagland solution omitted FeNaEDTA, supplied with 100 µM SNP, SNP3 Hoagland solution omitted FeNaEDTA, supplied with 250 µM SNP SNP4 Hoagland solution omitted FeNaEDTA, supplied with 500 µM SNP, SNP + Hb Hoagland solution omitted FeNaEDTA, supplied with 500 µM SNP and 3 % NO scavenger, bovine hemoglobin
Concentration of Other Minerals
Fe deficiency decreased the concentrations of Ca, Mg, and Zn in roots, but increased the concentrations of Mn and Cu in roots (Table 3). In addition, Fe deficiency decreased Mg and Zn concentrations in leaves, but increased their concentrations in leaves. Amendment of SNP raised Ca concentrations in leaves and roots with the general trend of SNP4 > SNP3 > SNP2 > SNP1. However, addition of Hb to the SNP4 treatment eliminated the effect of SNP. Similar results were obtained with Mg concentrations in leaves. However, in roots, SNP3 treatment had the most effect on Mg concentration. SNP1, SNP2, or SNP3 treatment significantly increased Zn concentrations in roots, but decreased it in leaves. In addition, SNP1, SNP2, or SNP3 treatment decreased the concentrations of Mn and Cu in leaves and roots. The SNP + Hb treatment resulted in no difference in Mn and Cu concentrations in leaves and roots, compared to –Fe treatment.
Antioxidant Enzymes Activities
Iron deficiency enhanced SOD activity in roots and leaves, but reduced POD and CAT activities in roots and leaves (Fig. 5). Amendment of SNP to the –Fe treatment affected SOD activity but the effect varied with application rate. The SNP2 and SNP3 treatments significantly increased SOD activity in roots and leaves (P < 0.05), whereas addition of Hb eliminated this effect. The SNP1 and SNP4 had no effect on SOD activity in roots, but all of the SNP1, SNP2, and SNP3 treatments promoted the activities of POD and CAT in leaves and roots. The SNP3 application rate appeared to be optimal because it achieved the best result, increasing POD activity by 122 and 284 %, and CAT activity by 154 and 70.7 %, respectively, in leaves and roots, compared to the –Fe treatment. SNP + Hb treatment had no significant effect on POD and CAT activities in leaves and roots, likely due to the removal of NO by the added Hb.
Effects of SNP at different concentrations on the activities of SOD, POD, and CAT in leaves and roots of peanut plants after 20 days of treatment. Values are the mean of three replicates. Bars with different letters are significantly different at P < 0.05. CK full-strength Hoagland solution, –Fe Hoagland solution omitted FeNaEDTA, SNP1 Hoagland solution omitted FeNaEDTA, supplied with 50 µM SNP, SNP2 Hoagland solution omitted FeNaEDTA, supplied with 100 µM SNP, SNP3 Hoagland solution omitted FeNaEDTA, supplied with 250 µM SNP, SNP4 Hoagland solution omitted FeNaEDTA, supplied with 500 µM SNP, SNP + Hb Hoagland solution omitted FeNaEDTA, supplied with 500 µM SNP and 3 % NO scavenger, bovine hemoglobin
MDA Concentration, O •−2 Generation Rate, and H2O2 Concentration
Compared with CK, Fe deficiency significantly raised MDA concentration by 53.8 % in leaves and by 28.4 % in roots (Fig. 6a). Addition of SNP to the –Fe treatment affected MDA concentration, but the effect varied with application rate. SNP1, SNP2, and SNP3 decreased MDA concentration by 20.4, 26.3, and 31.3 %, respectively, in leaves and by 34.2, 43.2, and 64.9 %, respectively, in roots. However, the SNP4 treatment did not result in a significant difference in root MDA concentration, compared to the –Fe treatment. SNP3 treatment had the lowest MDA concentration in leaves and roots. Similar findings were obtained with the O •−2 generation rate. SNP1, SNP2, or SNP3 treatment markedly decreased O •−2 generation rate in leaves and roots, whereas SNP4 treatment had no significant effect in leaves, but significantly increased the O •−2 generation rate in roots compared to the –Fe treatment. The SNP + Hb treatment reversed the SNP effect in roots. Compared to the –Fe treatment, SNP1, SNP2, and SNP3 decreased H2O2 concentration by 5.96, 13.65, and 23.83 %, respectively, in leaves and by 9.68, 22.05, and 31.68 %, respectively, in roots, whereas the SNP + Hb treatment had no effect on H2O2 concentration, compared to the –Fe treatment.
Effects of SNP at different concentrations on the accumulation of MDA, O •−2 generation rate and H2O2 concentration in leaves and roots of peanut plants after 20 days of treatment. Values are the mean of three replicates. Bars with different letters are significantly different at P < 0.05. CK full-strength Hoagland solution, –Fe Hoagland solution omitted FeNaEDTA, SNP1 Hoagland solution omitted FeNaEDTA, supplied with 50 µM SNP, SNP2 Hoagland solution omitted FeNaEDTA, supplied with 100 µM SNP, SNP3 Hoagland solution omitted FeNaEDTA, supplied with 250 µM SNP, SNP4 Hoagland solution omitted FeNaEDTA, supplied with 500 µM SNP, SNP + Hb Hoagland solution omitted FeNaEDTA, supplied with 500 µM SNP and 3 % NO scavenger, bovine hemoglobin
Discussion
Since the function of NO as a signal in plant disease resistance was first reported in 1998 (Delledonne and others 1998), studies with different plant species have implicated NO as an important signal not only in plant disease resistance, but also in the process of seed germination, growth, development, and responses against abiotic stress (Zhang and others 2008; Leonor and others 2011; Wang and others 2013a). The objective of the present study was to prove the hypothesis that exogenous SNP supply can raise NO levels in plants, and thus influences plant growth and defense against environmental stress using peanut as an indicator plant. The results indicated that Fe deficiency decreased NO content in leaves and roots after 5 and 15 days of treatment (Fig. 1). This is consistent with the results of Sun (2006), who reported that the NO generation rate is reduced in plants under Fe deficiency, but increased in the plants with sufficient supply of Fe. However, this is opposite to the result of Graziano and Lamattina (2007), who reported that there was a 2.2-fold increase in NO production in tomato roots grown under Fe-deficiency conditions, compared to those with sufficient Fe supply after 10 days of treatment. The conflicting results indicate that the effect of Fe deficiency on NO concentration in plants is complicated and might be related to treatment time, plant parts, plant species, and genotypes. Delledonne and others (1998) proved that 500 µM SNP can produce approximately 2.0 µM NO. In the present study, different concentrations of SNP increased NO content in leaves, and the increase was greater at 15 than 5 days after treatment. However, in roots, the increase in NO concentration is lower at day 15 than day 5 of treatment. It is likely that SNP applied to growth medium first raised NO in roots, and then enhanced NO production in leaves, which is beneficial to the synthesis of chlorophyll (Sun 2006), alleviating chlorosis induced by Fe deficiency. In addition, the effects of 500 µM SNP were reversed by the addition of hemoglobin (SNP + Hb treatment). These results indicate that the mitigating effect of SNP on the chlorosis induced by Fe deficiency was ascribed to its release of NO. In the present study, we also found that 15 days of treatment with 250 µM SNP may be considered as an optimal application rate because it results in the best result in alleviating chlorosis induced by Fe deficiency.
To prove that it is NO not other components released from SNP decomposition that is responsible for the alleviating effect on Fe deficiency, we used the 3 % NO scavenger, bovine hemoglobin, to remove NO released from SNP. The results confirmed this hypothesis. After 15 days of treatment, 500 µM SNP significantly raised NO levels in leaves, but decreased it in roots. However, this effect was eliminated by the added hemoglobin. There was no difference in NO levels in plants between the SNP + Hb and –Fe treatment (Fig. 1). Apparently, hemoglobin addition reduced NO in the plant. Moreover, the SNP + Hb treatment had no significant effect on shoot height, dry weight, and root activity (Table 1), and did not affect total Fe concentrations in leaves, stems or roots, nor active Fe concentration or FCR activity (Table 2). Therefore, it is reasonable to conclude that it is NO rather than other compositions from SNP decomposition that is responsible for the alleviation effect on Fe deficiency. This is in agreement with Sun (2006) report in maize.
Previous studies have shown that the SNP effect on different cells is either protective or toxic, depending on its concentration and the position of action (Limattina and others 2003). In the present study, the growth of peanut under Fe deficiency was significantly inhibited. However, the inhibitory effects were alleviated by SNP addition and the mitigation varied with application rate in the order of SNP3 > SNP2 > SNP1 > SNP4 (Table 1). After 15 days of treatment, plants with 250 µM SNP achieved similar NO levels to those grown under optimal conditions (Fig. 1). These results suggest that application of SNP at the optimal levels can have a direct effect on cell wall components through apoplasts including cell wall relaxation, and on the membrane lipid bilayers by enhancing membrane fluidity and promoting cell expansion and growth (Xiong and others 2009).
It has been known for years that Fe deficiency profoundly affects the formation, in the chloroplasts, of the light-harvesting pigments and electron transport chain. Also, electron microscope studies have shown that Fe deficiency reduces the number of grana and stromal lamellae in chloroplasts (Spiller and Terry 1980). Exogenous NO has a strong stimulating effect on chlorosis under Fe deficiency. In this experiment, exogenous application of SNP at low concentrations (SNP1, SNP2, and SNP3) significantly enhanced the chlorophyll concentration in peanut young leaves (Fig. 2). The results are in agreement with the report by Graziano and others (2002), Sun and others (2007), and Zhang and others (2012). The stimulating effect can be ascribed to the role of Fe in the formation of δ-aminolevulinic acid and protochlorophyllide, the precursors of the chlorophyll biosynthesis (Molassiotis and others 2005). Iron deficiency significantly decreased total Fe concentrations in roots and stems, but had no effect in leaves (Table 1). This suggested that plants under Fe-deficiency stress might show chlorosis symptoms even with Fe concentrations higher or equal to that in green leaves, although the concentration of Fe was generally lower in chlorotic leaves. Exogenous application of SNP at low concentrations increased total Fe concentrations in roots and leaves, but decreased it in stems. This suggests that exogenous NO supply improves Fe absorption and transportation. It has been reported that active Fe in leaves is a better indicator of Fe nutrition status than total Fe (Hakan and Vahap 2007). The results of Graziano and others (2002) supported a biological action of NO on the availability and/or delivery of metabolically active Fe within the plant. Exogenous application of SNP significantly increased active Fe concentration in plants (Table 1).
To understand the mechanisms of active Fe increase, Fe concentrations in subcellular structures were determined. In the roots of Fe-deficient plantlets, Fe concentrations in the cell wall and cell organelles were lower, compared to CK. However, Fe deficiency did not reduce the Fe concentration in the soluble fraction. These results suggest that Fe deficiency decreases total Fe concentrations, but does not decrease the Fe concentration in the soluble fraction in roots. This discrepancy may be attributed to the fact that Fe deficiency increased FCR activity, thus enhancing the conversion of Fe3+ into Fe2+, which is stored in the soluble fraction of cells. It is well known that reduction of FeIII to FeII by FCR is an obligatory step in Fe uptake by Strategy I plants (Molassiotis and others 2005). Therefore, Fe deficiency decreases Fe concentration in the cell wall, but not in the soluble fraction. Iron deficiency has been shown to cause increases in the concentration of organic anions (mainly citrate and malate) in roots, stems exudates, and leaves, where predominantly citrate appears to accumulate (Abadía and others 2002). In our previous study, we also found that peanut synthesized substantial amounts of organic acids, particularly malate and citrate in the roots under Fe deficiency (Zhang 2012). As a consequence of increased concentrations of organic anions and a decreased total soluble apoplastic Fe fraction in leaves, the molar ratio of citrate/Fe dramatically increases in the leaf apoplastic fluid of various plant species under Fe deficiency. When the citrate/Fe molar ratio in the leaf apoplastic fluid of Fe-deficient plants is raised above 1000, Fe uptake in chlorotic leaves would be impaired. Therefore, Fe deficiency generally decreases active Fe concentrations in young leaves.
In the present study, exogenous application of SNP decreased the Fe concentration in the cell wall and cell organelles of roots, but increased it in the soluble fraction. This is conducive to Fe transportation from roots to shoots. In addition, exogenous application of SNP also decreased Fe concentration in the cell wall, but increased it in cell organelles and the soluble fraction of leaves. This is conducive to the activation of Fe in leaves. It is well known that H+-ATPase in the plasma membrane plays an important role in the transport of multiple ions (Palmgren and Harper 1999), and in this experiment, SNP increased H+-ATPase activity in leaves and roots, which might make it responsible for SNP to adjust ion balance by increasing Ca, Mg, and Zn concentrations and decreasing Mn and Cu concentrations (Table 3). The protective effect of Zn has been attributed to its ability to inhibit NADPH oxidation and centered free radical generation (Cakmak 2000). SNP also increased chlorophyll concentration, and as a consequence of a high photosynthetic rate, the amount of cytosolic reducing equivalents for a plasma membrane-associated FeIII reduction may be increased. In addition, the soluble Fe concentration and photosynthesis are often synergetic, and the increased soluble Fe concentration can improve photosynthesis and the improved photosynthesis in turn raises the level of cytosolic electron donors NADPH, which can further increase the soluble Fe concentration. Therefore, SNP decreased the Fe concentration in the cell wall, but increased Fe concentrations in cell organelles and the soluble fraction in leaves of peanut seedlings.
Because Fe is an important component of the electron transport chains in chloroplasts and mitochondria, Fe deficiency impairs electron transport and probably leads to the production of ROS (Graziano and lamattina 2005). Sun and others (2007) have reported that Fe deficiency enhanced H2O2 accumulation in maize leaves. Our results also demonstrated that the concentrations of H2O2 and O •−2 increased in Fe-deficient peanut leaves and roots (Fig. 6b, c). The increased SOD activity may account for the increased accumulation of O •−2 in Fe-deficient leaves and roots (Fig. 5a, 6b). Overproduction of ROS can lead to oxidative injury such as membrane lipid peroxidation, protein oxidation, enzyme inhibition, and DNA and RNA damage (Ma and others 2008). The amounts of superoxide radicals (O •−2 ) and hydrogen peroxide (H2O2) significantly increased in leaves of plants exposed to Fe-deficiency stress and resulted in oxidative stress, which is indicated by accumulated concentrations of malondialdehyde (MDA) and electrolyte leakage.
SOD is an important enzyme involved in antioxidation processes and present in different organelles in plants, catalyzing the O •−2 conversion to H2O2. Our results indicate that the activity of SOD increased in Fe-deficient leaves and roots. CAT and POD are important H2O2 detoxifying enzymes in plants. Because they are all heme-containing enzymes, their activities are likely to be affected by Fe deficiency. Our results showed that the activities of CAT and POD were reduced under Fe deficiency (Fig. 5b, c). Recently, the function of NO in the protection of plants against oxidative stress under various adverse conditions has been increasingly reported (Sun and others 2007; Lopez-Carrion and others 2008; Wang and others 2013a). Our results showed that NO reduced the accumulation of H2O2 and O •−2 induced by Fe deficiency (Fig. 6b, c). The decreased concentration of MDA under Fe depletion coupled with NO treatment (Fig. 6a) also indicated that NO protected membrane lipids and proteins against oxidative damage.
Overall, the results from the present study suggest that NO acts as a general signal implicated in the regulation and coordination of the signaling network leading to physiological, biochemical, and morphological adaptive changes to the Fe-deficient environment.
Conclusions
The results from the present study indicate that SNP could significantly alleviate chlorosis induced by Fe deficiency and SNP + Hb reverses this effect. The mitigating mechanisms of SNP to Fe deficiency may include (1) improved absorption of Fe from the cell wall and cell organelles to the soluble fraction in roots, and from the cell wall to cell organelles and the soluble fraction in leaves; (2) increased concentration of active Fe in plant leaves, and increased chlorophyll concentrations; (3) adjusted ion balance in roots and leaves; and (4) enhanced antioxidant enzyme activities, protecting plants from oxidative stress. These findings also imply that SNP/NO has potential application for alleviating chlorosis in the production of peanut on calcareous soil.
References
Abadía J, López-Millán AF, Rombolà A, Abadía A (2002) Organic acids and Fe deficiency: a review. Plant Soil 241:75–86
Besson-Bard A, Pugin A, Wendehenne D (2008) New insights into nitric oxide signaling in plants. Annu Rev Plant Biol 59:21–39
Besson-Bard A, Gravot A, Richaud P, Auroy P, Duc C, Gaymard F, Taconnat L, Renou JP, Pugin A, Wendehenne D (2009) Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol 149:1302–1315
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Annu Rev Biochem 72:248–254
Briskin DP, Leonard RT, Hodges TK (1987) Isolation of the plasma membrane: markers and general principles. Method Enzymol 148:542–558
Cakmak I (2000) Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146:185–205
Chen F, Wang F, Sun H, Cai Y, Mao W, Zhang G, Vincze E, Wu F (2010) Genotype-dependent effect of exogenous nitric oxide on Cd-induced changes in antioxidative metabolism, ultrastructure, and photosynthetic performance in barley seedlings (Hordeum vulgare). J Plant Growth Regul 29:394–408
Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394:585–588
Gao L, Shi YX (2007) Genetic differences in resistance to iron deficiency chlorosis in peanut. J Plant Nutr 30:37–52
Graziano M, Lamattina L (2005) Nitric oxide and iron in plants: an emerging and converging story. Trends Plant Sci 10:4–8
Graziano M, Lamattina L (2007) Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J 52:949–960
Graziano M, Beligni MV, Lamattina L (2002) Nitric oxide improves internal iron availability in plants. Plant Physiol 130:1852–1859
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ 347:1–32
Knudson LL, Tibbitts TW, Edwards GE (1977) Measurement of ozone injury by determination of leaf chlorophyll concentration. Plant Physiol 60:606–608
Kong J, Dong YJ, Xu LL, Liu S, Bai XY (2014) Effects of exogenous salicylic acid on alleviating chlorosis induced by iron deficiency in peanut seedlings (Arachis hypogaea L.). J Plant Growth Regul 33:715–729
Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54:109–136
Leonor R, Marcela S, Irene M, Eduardo Z, Lorenzo L (2011) Nitric oxide, nitrosyl iron complexes, ferritin and frataxin: a well equipped team to preserve plant iron homeostasis. Plant Sci 181:582–592
Lopez-Carrion AI, Castellano R, Rosales MA, Ruiz JM, Romero L (2008) Role of nitric oxide under saline stress: implications on proline metabolism. Plant Biol 52:587–591
Ma YH, Ma FW, Zhang JK, Li MY, Wang YH, Liang D (2008) Effects of high temperature on activities and gene expression of enzymes involved in ascorbate–glutathione cycle in apple leaves. Plant Sci 175:761–766
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Molassiotis AN, Diamantidis GC, Therios IN, Tsirakoglou V, Dimassi KN (2005) Oxidative stress, antioxidant activity and Fe(III)-chelate reductase activity of five Prunus rootstocks explants in response to Fe deficiency. Plant Growth Regul 46:69–78
Nestel P, Bouis HE, Meenakshi JV, Pfeiffer W (2006) Symposium: food fortification in developing countries. J Nutr 136:1064–1067
Nickel RS, Cunningham BA (1969) Improved peroxidase assay method using Ieuco 2,3,6-trichlcroindophenol and application to comparative measurements of peroxidase catalysis. Anal Biochem 27:292–299
Ohinishi T, Gall RS, Mayer ML (1975) An improved assay of inorganic phosphate in the presence of extralabile phosphate compounds: application to the ATPase assay in the presence of phosphocreatine. Anal Biochem 69:261–267
Palmgren MG, Harper JF (1999) Pumping with plant P-type ATPases. J Exp Bot 50:883–893
Patra HL, Kar M, Mishre D (1978) Catalase activity in leaves and cotyledons during plant development and senescence. Biochem Pharmacol 172:385–390
Rombolà AD, Gogorcena Y, Larbi A, Morales F, Baldi E, Marangoni B, Tagliavini M, Abadía J (2005) Iron deficiency-induced changes in carbon fixation and leaf elemental composition of sugar beet (Beta vulgaris) plants. Plant Soil 271:39–45
Shi QH, Ding F, Wang XF, Wei M (2007) Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant Physiol Biochem 45:542–550
Spiller S, Terry N (1980) Limiting factors in photosynthesis: ll. Iron stress diminishes photochemical capacity by reducing the number of photosynthetic units. Plant Physiol 65:121–125
Stewart RC, Bewley JD (1980) Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol 65:245–248
Sun BT (2006) Nitric oxide protect maize seedlings against iron deficiency stress [D]. [Master Dissertation]. Beijing, China: CNKI (China National Knowledge Infrastructure). Available from: http://cdmd.cnki.com.cn/Article/CDMD-10730-2007115573.htm
Sun BT, Jing Y, Chen KM, Song LL, Chen FJ, Zhang LX (2007) Protective effects of nitric oxide on iron deficiency-induced oxidative stress in maize (Zea mays). J Plant Physiol 164:536–543
Wang QH, Liang X, Dong YJ, Xu LL, Zhang XW, Hou J, Fan ZY (2013a) Effects of exogenous nitric oxide on cadmium toxicity, element contents and antioxidative system in perennial ryegrass. Plant Growth Regul 69:11–20
Wang QH, Liang X, Dong YJ, Xu LL, Zhang XW, Kong J, Liu S (2013b) Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of perennial ryegrass under cadmium stress. J Plant Growth Regul 32:721–731
WHO (2007) Micronutrient deficiency: iron deficiency anaemia. Geneva: WHO. Available from http://www.who.int/nutrition/topics/ida/>
Xiong J, An LY, Lu H, Zhu C (2009) Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230:755–765
Xiong HC, Kobayashi T, Kakei Y, Senoura T, Nakazono M, Takahashi H, Nakanishi H, Shen HY, Duan PG, Guo XT, Nishizawa NK, Zuo YM (2012) AhNRAMP1 iron transporter is involved in iron acquisition in peanut. J Exp Bot 63:4437–4446
Zhang XW (2012) Study on the effect and mechanism of exogenous nitric oxide regulates iron deficiency chlorosis of peanut. [master dissertation]. Beijing, China: CNKI(China National Knowledge Infrastructure); [cited 2012 Aug 27]. Available from: http://cdmd.cnki.com.cn/Article/CDMD-10434-1012487322.htm
Zhang YM, Yang JF, Lu SY, Cai JL, Guo ZF (2008) Overexpressing SgNCED1 in tobacco increases ABA level, antioxidant enzyme activities, and stress tolerance. J Plant Growth Regul 27:151–158
Zhang XW, Dong YJ, Qiu XK, Hu GQ, Wang YH, Wang QH (2012) Exogenous nitric oxide alleviates iron-deficiency chlorosis in peanut growing on Calcareous soil. Plant Soil Environ 58:111–120
Zuo YM, Zhang FS (2011) Soil and crop management strategies to prevent iron deficiency in crops. Plant Soil 339:83–95
Acknowledgments
The authors would like to thank English Lecturer Mr. Stuart Craig MA (England, Taishan University, China), Dr Chengliang Li (RWTH Aachen University, Germany), and lecturer Xiujuan Wang (College of Foreign Languages, Shandong Agricultural University) for their critical reading and revision of the manuscript. The authors extend their appreciation to Pingping Yang (College of Animal Science and Technology, Shandong Agricultural University, China) for her assistance in sample analysis. Special acknowledgments are also given to the editors and reviewers. This research was funded by Shandong Provincial Natural Science Foundation of China (ZR2013CM003), the Chinese National Basic Research Program (2015CB150400), and a grant from the Higher Educational Science and Technology Bureau of Shandong Province (J14LF08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kong, J., Dong, Y., Song, Y. et al. Role of Exogenous Nitric Oxide in Alleviating Iron Deficiency Stress of Peanut Seedlings (Arachis hypogaea L.). J Plant Growth Regul 35, 31–43 (2016). https://doi.org/10.1007/s00344-015-9504-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-015-9504-y