Abstract
The aim of this study was to evaluate and quantify the phytotoxic effects of hexane and ethyl acetate fractions obtained from a leaf extract of Sapindus saponaria on the germination and seedling growth of Euphorbia heterophylla (wild poinsettia) and Echinochloa crus-galli (barnyardgrass) and to isolate and identify the major bioactive compounds. A crude ethanol extract was prepared from 100 g of dry plant material in 500 mL of ethanol. The extract was fractionated by liquid–liquid extraction, and the hexane and ethyl acetate fractions were solubilized at concentrations of 0.625, 1.25, 2.5, and 5.0 mg mL−1. The effect of these fractions was compared with the oxyfluorfen herbicide in bioassays. The hexane and ethyl acetate fractions inhibited germination, induced abnormalities, and reduced seedling growth of E. crus-galli and E. heterophylla with concentration-dependent effects. The phytotoxicity of the fractions ranged according to the receptor species, and the ethyl acetate fraction showed a greater inhibitory effect than the hexane fraction on seedling development. For both species, the oxyfluorfen herbicide inhibited mainly shoot growth, whereas the plant extracts inhibited seedling root growth. The S. saponaria fractions caused a reduction of more than 50 % in the size of metaxylem cells of E. heterophylla roots. The ethyl acetate fraction obtained from S. saponaria leaves was subjected to fractionation, and the substance isolated was identified as 3-(1,2-dimethyl-5-oxabicyclo [2.1.1] hexan-2-yl) but-2-enoic acid. This compound also inhibited the germination and seedling growth of E. crus-galli and E. heterophylla, but the ethyl acetate fraction was more potent in reducing seedling growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bioactive phytochemicals released into the environment can influence plant growth and development (Sodaeizadeh and others 2009). There is an increasing evidence that these plant chemicals can suppress the germination and growth of different weed species (Tawaha and Turk 2003; Sampietro and Vattuone 2006). Echinochloa crus-galli (L.) P. Beauv. (Poaceae, barnyardgrass) and Euphorbia heterophylla L. (Euphorbiaceae, wild poinsettia) are weeds with a very common occurrence in Brazil and cause large impacts on agricultural systems (Aliotta and others 2006; Vidal and others 2007). The main control method for these species is the application of synthetic herbicides, with consequent environmental impacts and selection of biotypes with herbicide resistance (Batish and others 2007; Grisi and others 2013). Indeed, chemical control of weeds is the method most widely used due to its high efficiency and practicality (Panozzo and others 2014). However, the intense application of pesticides has resulted in environmental damage, acting on the balance of microorganisms and causing changes in the physico-chemical properties of the soil, resulting in decreased crop productivity (Chou 1999).
The incorporation of substances with allelopathic activity in agriculture can reduce environmental pollution and increase agricultural production for sustainable weed management (Parvez and others 2003; He and others 2004; Sodaeizadeh and others 2009). Plants are able to develop defense mechanisms based on the synthesis of certain secondary metabolites, which when released into the environment will interfere at some stage of the life cycle of other plants (Marco and others 2012). The accumulation of substances with allelopathic effects has been found in all plant organs, with a tendency for accumulation in the leaves (Dorning and Cipollini 2006; Khan and others 2011). However, allelochemicals vary in composition, concentration, and plant location (Hong and others 2004). Their phytotoxic effects are mediated by chemical substances belonging to different classes of compounds, such as phenols, terpenes, alkaloids, polyacetylenes, fatty acids, peptides, glycosides, and saponins (Wink 2003).
Species from the Sapindaceae family are known for their traditional medicinal uses as a diuretic, stimulant, expectorant, natural surfactant, sedative, anthelmintic, and agent against stomachache and dermatitis in many parts of the world (Tsuzuki and others 2007). Chemical investigations of this family have led to the isolation of saponins, diterpenes, and flavonoids, among other secondary metabolites (Pelegrini and others 2008). Sapindus saponaria L. (soapberry, Sapindaceae) is a medium size tree distributed all over the tropics and usually occurs in rain and semi-deciduous forests (Murgu and Rodrigues-Filho 2006; Tsuzuki and others 2007). The main bioactive substances found in its fruits are saponins and acyclic sesquiterpene oligoglycosides (Murgu and Rodrigues-Filho 2006). Despite the potential importance of Sapindus plants as a good source of bioactive saponins, there is a lack of detailed studies on the chemical composition of S. saponaria (Murgu and Rodrigues-Filho 2006). In our previous report, we found that aqueous extracts of S. saponaria leaves exhibited inhibitory effects on the germination and seedling growth of E. crus-galli and Ipomoea grandifolia (Dammer) O’Donell (Grisi and others 2012). Based on this information, S. saponaria leaves may produce biologically active compound(s) and possess potential sources of natural herbicides.
Thus, the aims of this study were (1) to evaluate and quantify the phytotoxic effects of hexane and ethyl acetate fractions from an ethanolic extract of S. saponaria leaves on diaspore germination and seedling growth of E. heterophylla and E. crus-galli; (2) to isolate and identify bioactive compounds; and (3) to examine the phytotoxicity of the isolated compound compared with its derivative mixture.
Materials and Methods
Fraction Preparation
Mature leaves (dark green color and papyraceous texture) of S. saponaria were collected in São Carlos-SP, (22°02′S and 47°52′W), Brazil, in May 2011. The region is characterized by Aw climate (Köppen 1948), with dry winters (April to September) and wet summers (October to March). The collected leaves were dried at 40 °C for 72 h and ground using an industrial mill.
The ethanolic extract was prepared in the proportion of 100 g of dry plant material in 500 mL of ethanol. The plant material was exhaustively extracted with ethanol under dark and cold conditions. After 72 h, the solution was filtered and concentrated in a rotary evaporator under reduced pressure, resulting in a crude ethanol extract. The resulting crude extract (60 g) was suspended in methanol (MeOH) and deionized water at a ratio of 1:3 (w/w). This solution was fractionated by liquid–liquid extraction using hexane, ethyl acetate (EtOAc), and butanol solvents. The solutions obtained were evaporated under reduced pressure producing hexanic (22.27 g), EtOAc (5.43 g), butanolic (10.92 g), and aqueous (11.34 g) fractions. Each fraction was subjected to a wheat coleoptile bioassay. The hexane and EtOAc fractions, which showed higher inhibitory activity, were solubilized in a buffer solution (10 mM 2-[N-morpholino]ethanesulfonic acid (MES) and 1 M NaOH, pH 6) and DMSO (dimethyl sulfoxide, 5 μL mL−1) at concentrations 5.0, 2.5, 1.25, and 0.625 mg mL−1 (Macías and others 2010). Germination and seedling growth bioassays included two controls: a negative control with the buffer solution and DMSO and a positive control with the oxyfluorfen herbicide (240 g.i.a. L−1) at doses of 1.0 and 2.0 L ha−1. Oxyfluorfen is a selective herbicide, and not systemic, and is used for the weed control (monocotyledonous and eudicotyledonous) in pre-emergence or early post-emergence applications (Rodrigues and Almeida 2011).
Germination and Seedling Growth Bioassay
The hexane and EtOAc fractions from the ethanol extract of S. saponaria leaves were applied on E. heterophylla and E. crus-galli diaspores. The bioassays were conducted in Petri dishes (9 cm of diameter) with two sheets of filter paper moistened with 5 mL of the fractions, herbicide, or buffer solution and DMSO (5 μL mL−1). The experimental design was completely randomized, using four replicates of 30 diaspores. The experiment was conducted in a germination chamber at 25 °C, with a photoperiod of 12 h. The germination criterion was embryo protrusion, which was evaluated every 12 h during the first 7 days of the experiment and at intervals of 24 h thereafter until the stabilization of germination. Germinability, mean germination time and rate (Maguire’s index) were calculated according to Ranal and Santana (2006).
For the analysis of E. heterophylla and E. crus-galli seedling growth, diaspores were previously germinated in distilled water. Only seedlings with 3 mm-long roots were selected and transferred to transparent plastic boxes (13 × 8 × 3 cm) containing, as a substrate, filter paper moistened with 5 mL of the fractions, herbicide, or buffer solution and DMSO (5 μL mL−1) under the same concentrations adopted for the germination test. The boxes were kept in a germination chamber at 25 °C, with a photoperiod of 12 h. The experimental design was completely randomized, with four replicates of 10 seedlings. After 7 days, the seedlings were classified as normal or abnormal (Brasil 2009), and the shoot and primary root lengths of the seedlings were measured using a caliper.
Examination of Metaxylem Cells
Euphorbia heterophylla seedlings were grown in negative control solutions and in the hexane and EtOAc fractions of S. saponaria leaves under the same conditions adopted for the growth bioassay. After 4 days, the primary root segments of the seedlings were removed and immersed in 70 % alcohol (Gatti and others 2010). The modified Fuchs staining method was used (Kraus and Arduin 1997), where the roots were immersed in alcohol (70 %) for 5 days and placed in a solution of 25 % NaOH at 60 °C for 48 h, until the material was clarified.
Then, the root segments were immersed in safranin (C20H19N4C1) and caustic soda (10 % NaOH) for 24 h at 60 °C. After staining, the material was mounted on glass slides in Apathy’s syrup (Kraus and Arduin 1997), with the roots, for observation under an optical microscope (Olympus-BX41) coupled to a camera (Sony CCD-IRIS). Four primary roots of E. heterophylla seedlings grown in different concentrations of the fractions and control solutions were used. Half of the length of each root from the central region upward was photographed. From each photograph, 15 central cells of the metaxylem were measured at 20× magnification (Image Pro Plus 5.0 program) (Gatti and others 2010).
Fractionation and Identification of Secondary Metabolites
The EtOAc fraction (3.0 g), which was selected from the results obtained by the bioassays, was suspended in MeOH and deionized water at a ratio of 1:3 (w/w). This solution was fractionated by liquid–liquid extraction using hexane, dichloromethane (CH2Cl2), and EtOAc. The solutions were evaporated under reduced pressure producing hexane (89.7 mg), CH2Cl2 (577.2 mg), EtOAc (624.4 mg), and aqueous (964.8 mg) fractions. According to observations by silica gel thin-layer chromatography (TLC), the CH2Cl2 and EtOAc fractions were pooled and subjected to silica gel (230–400 mesh) column chromatography and eluted with hexane/acetone mixtures of increasing polarity followed by methanol. This procedure yielded 41 sub-fractions, which were monitored by TLC. According to TLC observations, the sub-fractions F19 (hexane/acetone 7:3, 3.2 mg), F20 (hexane/acetone 6:4, 3.9 mg), F21 (hexane/acetone 6:4, 54.3 mg), F22 (hexane/acetone 6:4, 5.1 mg), F23 (hexane/acetone 1:1, 3.0 mg), and F24 (hexane/acetone 1:1, 3.4 mg) were pooled and subjected to analyses by 1D and 2D Nuclear Magnetic Resonance (NMR) (Bruker 600 MHz, 14.1 T) and Mass Spectrometry (LC–MS and LC–MS/MS) for identification of the purified substance.
To analyze the compound through LC–MS and LC–MS/MS, the sample was solubilized in water:acetonitrile (1:1; v/v) to a concentration of 650 µg mL−1. Afterward, this solution was diluted to 32.5 µg mL−1. Then, the sample was analyzed using an Agilent 1290 Series Liquid Chromatography apparatus (Agilent Technologies, USA). The analysis was carried out in the reversed-phase gradient mode using a stainless steel Zorbax Extend-C18 (1.8 μm, 2.1x50 mm, Agilent Technologies, USA) and a mobile phase consisted of acetonitrile (phase B) and TFA 0.1 % (v/v). The gradient method was as follows: 0–1 min 50 % B; 1–2 min 90 % B; 2–5 min 90 % B. The flow rate was 0.5 mL min−1 at 25 °C, and injected volume was 2 μL. Acquisition was carried out using an Agilent ifunnel Q-TOF 6550 LC–MS with source Dual Agilent Jet Stream ESI (Dual AJS-ESI) in the following conditions: drying gas at 280 °C, drying gas flow 14 L min−1, nebulizer at 20 psi; sheath gas at 350 °C; sheath gas flow 12 L min−1, VCap 2500; nozzle voltage 0 V; fragmentor 110 V, OCT 1 RF Vpp 750 V, and collision energy 20 V using N2. Mass spectrometer parameters acquisition ranged from 50 to 220 m/z to MS and targeted MS/MS.
The isolated compound was also subjected to germination and seedling growth bioassays with E. heterophylla and E. crus-galli under the same conditions described above. The compound was solubilized in a buffer solution and DMSO (5 μL mL−1) at concentrations 1 mM, 300, 100, 30, and 10 µM. This germination bioassay was conducted in Petri dishes (5 cm of diameter) with one sheet of filter paper moistened with 1.5 mL of the compound or control solution. The experimental design was completely randomized, using four replicates of 20 diaspores.
Statistical Analysis
The data were subjected to normality (Shapiro–Wilk) and homogeneity (Levene) tests. When these two assumptions were met, an analysis of variance (ANOVA) was applied, followed by Tukey’s test at a significance level of 0.05. Linear or quadratic regression models were adjusted when the ANOVA F was significant. The goodness of the models was evaluated by the coefficient of determination (R2). The data were subjected to a conjoint analysis because the ratio between the larger and smaller residual mean square was not greater than 7 (Pimentel-Gomes 1990).
Results
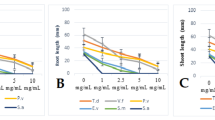
The hexane fraction of the ethanol extract of S. saponaria leaves significantly inhibited the germination process and seedling growth of E. heterophylla and E. crus-galli. For E. crus-galli, the hexane fraction linearly increased the mean germination time (1.77 h for each addition of 1 mg mL−1 of fraction); in contrast, germination (60.60 %) and the Maguire’s index (0.2602 caryopses h−1) reached minimum values at estimated concentrations of 3.46 and 3.96 mg mL−1, respectively (Fig. 1). The E. heterophylla seeds showed minimum values of germinability (45.63 %) and Maguire’s index (0.2911 seeds h−1) at estimated concentrations of 4.13 and 3.91 mg mL−1, respectively. Moreover, the mean germination time reached a maximum (55.55 h) at a concentration 3.95 mg mL−1 for the hexane fraction (Fig. 1). E. crus-galli seedlings grown in solutions containing the hexane fraction had the lowest shoot (24.73 mm) and root (10.31 mm) lengths at concentrations of 3.62 and 3.85 mg mL−1, respectively. However, the shoot (34.12 mm) and root (15.09 mm) lengths of the E. heterophylla seedlings were minimal at estimated concentrations of 3.68 and 3.87 mg mL−1, respectively (Fig. 1).
The EtOAc fraction from S. saponaria leaves also showed phytotoxic effects on the development of the studied weeds. For E. crus-galli, minimum values were observed for germinability (60.90 %) and Maguire’s index (0.2465 caryopses h−1) at estimated concentrations of 3.42 and 3.88 mg mL−1, respectively, whereas the mean germination time increased linearly by 2.90 h for each addition of 1 mg mL−1 of the fraction (Fig. 2). For E. heterophylla seeds seeded with the EtOAc fraction, there was a linear decrease and increase in the germinability and mean germination time (1.59 % and 3.27 h for each addition of 1 mg mL− 1 of fraction), respectively. At the concentration 4.24 mg mL−1, the EtOAc fraction showed the lowest Maguire’s index (0.2856 seed h−1) with the E. heterophylla seeds (Fig. 2), indicating that the allelochemicals also had the potential to reduce seed vigor. The lowest shoot lengths of the E. crus-galli (36.31 mm) and E. heterophylla (33.64 mm) seedlings grown in a solution containing the EtOAc fraction were recorded at concentrations of 4.32 and 3.75 mg mL−1, respectively (Fig. 2). The root lengths of E. crus-galli and E. heterophylla seedlings showed null values at estimated EtOAc fraction concentrations of 3.31 and 3.53 mg mL−1, respectively (Fig. 2). For both species, a greater inhibitory effect was found on the root system.
The maximum percentage of abnormal seedlings of E. crus-galli was recorded at estimated concentrations of 3.95 (55.78 %) and 4.25 mg mL−1 (69.21 %) of the hexane and EtOAc fractions, respectively (Figs. 1, 2). The E. heterophylla seedlings were more sensitive, with a higher incidence of abnormal seedlings observed at concentrations of 3.56 (73.80 %) and 4.24 mg mL−1 (92.96 %) of the hexane and EtOAc fractions, respectively (Figs. 1, 2). For both weeds, the EtOAc fraction resulted in a higher percentage of abnormal seedlings than the hexane fraction (Table 1). The herbicide treatment was more efficient at controlling these weeds, with a higher incidence of abnormal seedlings. However, the effect of the EtOAc fraction was similar to the herbicide on the E. heterophylla seedlings (Table 1). The main anomalies observed for the abnormal E. crus-galli and E. heterophylla seedlings are provided in Table 1.
Because E. heterophylla was the most sensitive target species, the anatomical study of its roots allowed better visualization of the action mode of different fractions at the cellular level. The average size of the metaxylem cells of the seedlings grown in control was 400.25 μm (±9.76). However, the S. saponaria fractions caused a reduction of more than 50 % in the size of root metaxylem cells (Hexane: 278.27–123.25 μm, EtOAc: 182.51–80.88 μm) (Figs. 3, 4). The metaxylem cell size of E. heterophylla roots grown under the influence of the hexane and EtOAc fractions was inversely proportional to fraction concentrations (Fig. 3). For all concentrations tested, the EtOAc fraction showed greater inhibitory effect on metaxylem cell elongation than the hexane fraction, with a minimum size of 80.88 μm at a concentration of 5.0 mg mL−1 (Figs. 3, 4).
Size (µm) of the root metaxylem cells of E. heterophylla seedlings grown in different concentrations of the hexane and ethyl acetate fractions of S. saponaria leaves. Means followed by the same capital letters for fractions and small letters for concentrations do not differ significantly by Tukey’s test at 0.05 % probability. Vertical bars represent standard deviations
Comparing the effect of different fractions obtained from S. saponaria leaves with the oxyfluorfen herbicide, it was observed that inhibition (over control) of germinability of E. crus-galli caryopses was similar for all treatments (Fig. 6a). For E. heterophylla, the herbicide (28.95 %), followed by hexane fraction (18.92 %), showed a greater inhibitory effect on seed germination (Fig. 6b). For both weeds, shoot elongation was more affected by the herbicide, followed by the EtOAc fraction. The inhibitory effect of the hexane fraction and herbicide was similar on root length, whereas the EtOAc fraction showed greater inhibition on this organ (Fig. 6a, b). Thus, the results showed that the EtOAc fraction had a greater inhibitory effect than the hexane fraction on the growth and development of E. crus-galli and E. heterophylla seedlings.
Based on these results, the EtOAc fraction obtained from S. saponaria leaves was subjected to fractionation for the purification and identification of compounds. Using analytical methods such as classical chromatography, sub-fractions 19–24 showed significant purity for the structural characterization by 1D and 2D NMR. The spectroscopic data indicated the presence of a secondary metabolite named 3-(1,2-dimethyl-5-oxabicyclo [2.1.1] hexan-2-yl) but-2-enoic acid, belonging to the terpenes class (Fig. 5).
The structure of the metabolite isolated indicates a monoterpene acid with spectroscopic characteristics not known in the literature. The 1H NMR (600 MHz, CDCl3) spectrum of the compound showed three singlets at δ1.472 (C-6′, 3H), 1.274 (C-7′, 3H), and 1.786 (C-4, 3H) for methyl groups, a broad singlet at δ 5.690 (C-2, 1H) that corresponds to ethylene hydrogen alpha to an alpha–beta unsaturated carbonyl, two double doublets at δ 1.532 (C-3′; 14.0, 4.0 Hz,1H), 1.780 (C-5′; 14.0, 4.0 Hz,1H) and two double triplets at δ 1.980 (C-3′; 14.0, 2.4 Hz,1H), 2.460 (C-5′; 14.0, 2.4 Hz,1H) which corresponding to two methylenic carbons of the cyclopentane, and a multiplet regarding to carbinoic hydrogen at δ4.331 (C-4′; m,1H). The 13C NMR (150 MHz, CDCl3) spectrum of the compound indicated signals at δ182.6 (C-1), 112.0 (C- 2), 172.0 (C-3), 26.9 (C-4), 35.9 (C-1′), 86.7 (C-2′), 47.3 (C-3′), 66.8 (C-4′),45.6 (C-5′), 26.4 (C-6′), and 30.6 (C-7′). Analysis of the 1D and 2D NMR spectra are summarized in the Table 1S and Figs. 1S–5S. Considering the number of carbon, hydrogens, and oxygens proposed for the molecule, it was possible to obtain the molecular formula C11H16O3 with a molecular mass of 196 g mol−1 and an unsaturation index of 4. The ion m/z 197.1165, corresponding to ion [M + H]+, and identified by analysis of full scan, was confirmed by high-resolution mass spectrometry (Fig. 6S). The proposed structure was confirmed by analysis of sequential mass spectrometry (MS/MS, Fig. 7S). The analysis of the product ion m/z 197.1165 confirmed the ions m/z 179.1057, 161.0950, 133.1002, and 107.0850 as being derived from respective product ion (Fig. 7S).
The metabolite 3-(1,2-dimethyl-5-oxabicyclo [2.1.1] hexan-2-yl) but-2-enoic acid inhibited the germination and seedling growth of E. crus-galli and E. heterophylla, with a concentration-dependent effect, inhibiting mainly root growth (Table 2). This compound (32 %) exerted a stronger inhibitory effect than the herbicide (16 %) and the hexane (17 %) and EtOAc (14 %) fractions on the germination of E. crus-galli caryopses. E. heterophylla germination was also affected by this compound (15 %), showing greater inhibition than the EtOAc fraction (9.8 %). The isolated compound (54 %) inhibited the root growth of E. crus-galli, with an effect similar to the herbicide (59 %) and hexane fraction (61 %). However, E. heterophylla seedlings were less affected by the compound (Fig. 6).
Inhibitory effects of the hexane and ethyl acetate fractions, herbicide and 3-(1,2-dimethyl-5-oxabicyclo [2.1.1] hexan-2-yl) but-2-enoic acid (isolated compound) from S. saponaria leaves on the germination and seedling growth of E. crus-galli (a) and E. heterophylla (b). Means followed by the same letter in the fractions are not significantly different based on Tukey’s test at 0.05 probability. * Significant difference in relation to the control
Discussion
The hexane and EtOAc fractions obtained from the ethanolic extract of S. saponaria leaves were phytotoxic and inhibited germination, reduced growth and induced the appearance of abnormalities in E. crus-galli and E. heterophylla seedlings. For all parameters, the phytotoxicity varied with the species tested, and the inhibitory effect was concentration dependent (Figs. 1, 2). Several studies show that the negative effect of chemical substances on weeds is species-dependent (Prati and Bossdorf 2004; Xuan and others 2005; Sodaeizadeh and others 2009). According to Kobayashi (2004), the susceptibility of a target species to phytotoxic substances under laboratory conditions depends on the physiological and biochemical characteristics of each species.
The effect of S. saponaria organic fractions on the germination process showed that allelochemicals may act not only in germinability but also on the mean germination time and vigor of E. crus-galli and E. heterophylla diaspores. The mean germination time is an important factor for the survival of weeds because plants that germinate more slowly may show reduced size, lower competition for resources, and less chance of establishment in the environment (Souza and others 2010; Grisi and others 2013). However, seedling development was more sensitive to the fractions than germination, and the principal reductions and morphological changes were observed for root growth. These results are in agreement with other authors who reported strong phytotoxic effects of allelochemicals on seedling growth in target species (Sodaeizadeh and others 2009; Ashrafi and others 2009; Souza and others 2010; Grisi and others 2012). Germinability, mean germination time, rate, and seedling length measurements (dependent variables) established cause-and-effect relationships with the concentration of the fractions tested (independent variable). Thus, it is possible to predict values for these characteristics at any concentration of the fractions within the studied interval (0–5.0 mg mL−1).
In the present study, shoot elongation of E. crus-galli and E. heterophylla seedlings was more affected by the synthetic herbicide. However, the hexane and EtOAc fractions showed similar and superior inhibition, respectively, to the herbicide with regard to root length. The data obtained indicate that the root growth of both species was more responsive than the shoot growth to the inhibitory phytotoxic activity induced by the fractions. According to Grisi and others (2013), the oxyfluorfen herbicide acts with more intensity in the shoot, whereas the plant extracts invest in root growth inhibition. Abnormal seedling evaluation is also an important parameter in the detection of phytotoxic effects. The main anomalies identified for these weeds were root necrosis, a twisted or curved hypocotyl, the absence of lateral roots, stunted seedlings, and gravitropic inversion (Table 1). The results of the present study corroborate the findings of Ferreira and Áquila (2000), who reported that allelochemicals can induce the appearance of abnormal seedlings, in which necrosis is the most common symptom of the phytotoxic effect with the ability to inhibit the growth of the recipient plant.
The sensitivity of roots to allelochemicals is the characteristic that best indicates the allelopathic action of plant extracts. Previous studies (Souza and others 2010; Grisi and others 2012; Tanveer and others 2012) have also documented this aspect of a greater root than shoot inhibition. Possible reasons are because roots are the first to emerge and are in direct contact with extracts and thus are exposed to the peak periods and concentrations of phytotoxins (Tanveer and others 2012). Furthermore, the root is less protected by a cuticle than shoots/hypocotyls, which can result in greater penetration and concentration of these chemicals into root tissues (Yoshimura and others 2011). Root growth is characterized by high metabolic rates, and for this reason, roots are highly susceptible to environmental stress, such as allelochemicals in the substrate (Cruz-Ortega and others 1998). At the cellular level, allelochemicals induce lipid peroxidation, affect certain enzymatic activity, and rapidly depolarize the root cell membrane, resulting in a generalized increase in membrane permeability and thus blocking plant nutrient uptake (Santos and others 2008). Considering that the plant root system is its connection with the physical environment and the path of ingress of water and nutrients, poor root formation could affect the plant physiological state and thus prevent the establishment of weeds. Khanh and others (2005) reported that the highest level of allopathic suppression may occur when maximum levels of phytotoxins coincide with early stages of plant growth.
The reduction in the growth of E. heterophylla seedling roots treated with S. saponaria fractions might be associated with inhibition of metaxylem cell elongation, suggesting probable interference of allelochemicals in the hormone balance (Gatti and others 2010). Recent studies have revealed that phytohormones play an important role in controlling cell division, cell growth, and cell differentiation in distinct zones of roots (Takatsuka and Umeda 2014). Auxin is an important long- and short-distance signal and controls multiple developmental processes, including root patterning (Friml and others 2002; Petersson and others 2009), cell division, and cell elongation in roots (Ding and Friml 2010). Polar auxin transport, which is mediated by PIN-FORMED (PIN) efflux carriers, is essential for creating auxin gradients and for proper development of organs (Tanaka and others 2006).
Using phytochemical analyses, Wahab and Selim (1985) detected the presence of flavonoids, lipids, and steroids in apolar extracts from S. saponaria seeds. Lemos and others (1992) isolated a new saponin from the ethyl acetate fraction of S. saponaria fruits and reported its anti-microbial activity. Murgu and Rodrigues-Filho (2006) reported that the main glycosides in the fruits of this species were saponins (SAP) derived from triterpenes hederagenin and oleanolic acid and oligoglycosides from acyclic sesquiterpene oligoglycosides (ASOGs). These authors detected up to 30 SAPs and 63 ASOGs, which the plant produces as a complex mixture of naturally acetylated glycosides. In the present study, 3-(1,2-dimethyl-5-oxabicyclo [2.1.1] hexan-2-yl) but-2-enoic acid was identified in the EtOAc fraction obtained from S. Saponaria leaves. This metabolite is a monoterpene acid and has not been reported in the literature. Terpenes are widely found in the genus Sapindus (Pelegrini and others 2008; Huang and others 2008; Jeyabalan and Palayan 2009; Morikawa and others 2009; Eddaya and others 2013), with diverse biological activities and pharmacological properties, such as antidiabetic (Mahar and others 2011), antifungal (Tsuzuki and others 2007), and antibacterial (Lasisi and others 2012) activities.
The isolated 3-(1,2-dimethyl-5-oxabicyclo [2.1.1] hexan-2-yl) but-2-enoic acid showed phytotoxic activity on the weeds studied. Only with regard to the germination of E. crus-galli and E. heterophylla diaspores did the isolated compound have a greater inhibition than the EtOAc fraction, whereas this fraction showed the highest inhibitory activity on seedling growth. Teerarak and others (2012) also observed that the inhibitory effect of odorine (active compound) on E. crus-galli germination and seedling growth decreased in comparison with the mixture of the crude EtOAc fraction. These results indicated that the EtOAc fraction may contain many active chemical constituents that may act synergistically. This finding is in agreement with the observation of synergistic of biological compounds reported by Kilani and others (2008) and Teerarak and others (2012). This approach will require further investigation to identify other biologically active metabolites.
The EtOAc fraction obtained from the ethanolic extract of S. saponaria leaves showed an inhibitory effect on seedling development that was greater than the hexane fraction. The compound 3-(1,2-dimethyl-5-oxabicyclo [2.1.1] hexan-2-yl) but-2-enoic acid isolated and identified from the EtOAc fraction also inhibited the germination and growth of E. crus-galli and E. heterophylla, but the EtOAc fraction was more potent in inhibiting seedling growth. Thus, the compounds present in the EtOAc fraction obtained from S. saponaria leaves can be potent weed growth regulators and offer promising alternatives for the sustainable control of these species.
References
Aliotta G, Cafiero G, Otero AM (2006) Weed germination, seedling growth and their lesson for allelopathy in agruculture. In: Reigosa MJ, Pedrol N, González L (eds) Allelopathy: a physiological process with ecological implications. Springer, Dordrecht, pp 285–297
Ashrafi ZY, Sadeghi S, Mashhadi HR (2009) Inhibitive effects of barley (Hordeum vulgare) on germination and growth of seedling quack grass (Agropyrum repens). Icel Agric Sci 22:37–43
Batish DR, Arora K, Singh HP, Kohli RK (2007) Potential utilization of dried powder of Tagetes minuta as a natural herbicide for managing rice weeds. Crop Prot 26:566–571
Brasil (2009) Ministério da Agricultura e Reforma Agrária. Coordenação de Laboratório Vegetal. Regras para análise de sementes, Brasília
Chou CH (1999) Roles of allelopathy in plant biodeversity and sustainable agriculture. Crit Rev Plant Sci 18:609–630
Cruz-Ortega R, Anaya AL, Hernández-Bautista BE, Laguna-Hernández G (1998) Effects of allelochemical stress produced by Sicyos deppei on seedling root ultrastructure of Phaseolus vulgaris and Cucurbita ficifolia. J Chem Ecol 24:2039–2057
Ding Z, Friml J (2010) Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc Natl Acad Sci USA 107:12046–12051
Dorning M, Cipollini D (2006) Leaf and root extracts of the invasive shrub, Lonicera maackii, inhibit seed germination of three herbs with no autotoxic effects. Plant Ecol 184:287–296
Eddaya T, Boughdad A, Sibille E, Chaimbault P, Zaid A, Amechrouq A (2013) Biological activity of Sapindus mukorossi Gaerten (Sapindaceae) aqueous extract against Thysanoplusia orichalcea (Lepidoptera: Noctuidae). Ind Crop Prod 50:325–332
Ferreira AG, Áquila MEA (2000) Alelopatia: uma área emergente da ecofisiologia. Rev Bras Fisiol Veg 12:175–204
Friml J, Benková E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jürgens G, Palme K (2002) AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108:661–673
Gatti AB, Ferreira AG, Arduin M, Perez SCJGA (2010) Allelopathic effects of aqueous extracts of Aristolochia esperanzae O. Kuntze on development of Sesamum indicum L. seedlings. Acta Bot Bras 24:454–461
Grisi PU, Ranal MA, Gualtieri SCJ, Santana DG (2012) Allelopathic potential of Sapindus saponaria L. leaves in the control of weeds. Acta Sci Agron 34:1–9
Grisi PU, Gualtieri SCJ, Anese S, Pereira VC, Forim MR (2013) Effect of Serjania lethalis ethanolic extract on weed control. Planta Daninha 31:239–248
He HQ, Shen LH, Xiong J, Jia XL, Lin WX, Wu H (2004) Conditional genetic effect of allelopathy in rice (Oryza sativa L.) under different environmental conditions. Plant Growth Regul 44:211–218
Hong NH, Xuan TD, Tsuzuki E, Terao H, Matsuo M, Khanh TD (2004) Weed control of four higher plant species in paddy rice fields in Southeast Asia. J Agron Crop Sci 190:59–64
Huang HC, Wu MD, Tsai WJ, Liao SC, Liaw CC, Hsu LC, Wu YC, Kuo YH (2008) Triterpenoid saponins from the fruits and galls of Sapindus mukorossi. Phytochemistry 69:1609–1616
Jeyabalan S, Palayan M (2009) Antihyperglycemic and antidiabetic activity of leaves extracts of Sapindus emarginatus Vahl. Asian Biomed 3:313–318
Khan M, Hussain F, Musharaf S, Imdadullah (2011) Allelopathic effects of Rhazya stricta decne on seed germination and seedling growth of maize. Afr J Agr Res 6:6391–6396
Khanh TD, Chung MI, Xuan TD, Twata S (2005) The exploitation of crop allelopathy in sustainable agricultural production. J Agro Crop Sci 191:172–184
Kilani S, Sghaier MB, Limem I, Bouhlel I, Boubaker J, Bhouri W, Skandrani I, Neffatti A, Ammar RB, Dijoux-Franca MG, Ghedtra K, Chekir-Ghedira L (2008) In vitro evaluation of antibacterial, antioxidant, cytotoxic and apoptotic activities of the tubers infusion and extracts of Cyperus rotundus. Bioresour Technol 99:9004–9008
Kobayashi K (2004) Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol Manag 4:1–7
Köppen W (1948) Climatologia: com um estúdio de los climas de la tierra. Fondo de Cultura Econômica, México
Kraus JE, Arduin M (1997) Manual básico de métodos em morfologia vegetal. Seropedica, EDUR
Lasisi AA, Ayinde BW, Adeleye AO, Onocha PA, Oladosu IA, Idowu PA (2012) New triterpene isovanniloyl and antibacterial activity of constituents from the roots of Paullinia pinnata Linn (Sapindaceae). J Saudi Chem Soc. doi:10.1016/j.jscs.2011.12.012
Lemos TLG, Mendes AL, Sousa MP, BrazFilho R (1992) New saponin from Sapindus saponaria. Fitoterapia 63:515–517
Macías FA, Lacret R, Varela RM, Nogueiras C, Molinillo JMG (2010) Isolation and phtytotoxicity of terpenes from Tectona grandis. J Chem Ecol 36:396–404
Mahar KS, Rana TS, Ranade SA, Meena B (2011) Genetic variability and population structure in Sapindus emarginatus Vahl from India. Gene 485:32–39
Marco CA, Teixeira E, Simplício A, Oliveira C, Costa J, Feitosa J (2012) Chemical composition and allelopathyc activity of essential oil of Lippia sidoides Cham. Chilean J Agric Res 72:157–160
Morikawa T, Xie Y, Asao Y, Okamoto M, Yamashita C, Muraoka O, Matsuda H, Pongpiriyadacha Y, Yuan D, Yoshikawa M (2009) Oleanane-type triterpene oligoglycosides with pancreatic lipase inhibitory activity from the pericarps of Sapindus rarak. Phytochemistry 70:1166–1172
Murgu M, Rodrigues-Filho E (2006) Dereplication of Glycosides from Sapindus saponaria using Liquid Chromatography-Mass Spectrometry. J Brazil Chem Soc 17:1281–1290
Panozzo LE, Agostinetto D, Moraes PVD, Magano DA, Harter A, Pinto LB (2014) Control of Echinochloa sp. in the Irrigated Rice Crop. Int J Agron 2014:1–6
Parvez SS, Parvez MM, Nishihara E, Gemma H, Fujii Y (2003) Tamarindus indica L. leaf is a source of allelopathic substance. Plant Growth Regul 40:107–115
Pelegrini DD, Tsuzuki JK, Amado CAB, Cortez DAG, Ferreira ICP (2008) Biological activity and isolated compounds in Sapindus saponaria L. and other plants of the genus Sapindus. Lat Am J Pharm 27:922–927
Petersson SV, Johansson AI, Kowalczyk M, Makoveychuk A, Wang JY, Moritz T, Grebe M, Benfey PN, Sandberg G, Ljung K (2009) An auxin gradient and maximum in the Arabidopsis root apex shown by highresolution cell-specific analysis of IAA distribution and synthesis. Plant Cell 21:1659–1668
Pimentel-Gomes FP (1990) Curso de estatística experimental, 13th edn. Nobel, Piracicaba
Prati D, Bossdorf O (2004) Allelopathic inhibition of germination by Alliaria petiolata (Brassicaceae). Am J Bot 91:285–288
Ranal MA, Santana DG (2006) How and why to measure the germination process? Rev Bras Bot 29:1–11
Rodrigues BN, Almeida FS (2011) Guia de herbicidas, 6th edn. Edição dos Autores, Londrina
Sampietro DA, Vattuone MA (2006) Sugarcane straw and its phytochemicals as growth regulators of weed and crop plants. Plant Growth Regul 48:21–27
Santos WD, Ferrarese ML, Nakamura CV, Mourão KS, Mangolin CA, Ferrarese-Filho O (2008) Soybean (Glycine max) root lignification induced by ferulic acid. The possible mode of action. J Chem Ecol 34:1230–1241
Sodaeizadeh H, Rafieiolhossaini M, Havlik J, Damme PV (2009) Allelopathic activity of different plant parts of Peganum harmala L. and identification of their growth inhibitors substances. Plant Growth Regul 59:227–236
Souza FM, Gandolfi S, Perez SCJGA, Rodrigues RR (2010) Allelopathic potential of bark and leaves of Esenbeckia leiocarpa Engl. (Rutaceae). Acta Bot Bras 24:169–174
Takatsuka H, Umeda M (2014) Hormonal control of cell division and elongation along differentiation trajectories in roots. J Exp Bot 65:2633–2643
Tanaka H, Dhonukshe P, Brewer PB, Friml J (2006) Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell Mol Life Sci 63:2738–2754
Tanveer A, Jabbar MK, Kahliq A, Matloob A, Abbas RN, Javaid MM (2012) Allelopathic effects of aqueous and organic fractions of Euphorbia dracunculoides Lam. on germination and seedling growth of chickpea and wheat. Chil J Agric Res 72:495–501
Tawaha AM, Turk MA (2003) Allelopathic effects of Black Mustard (Brassica nigra) on germination and growth of Wild Barley (Hordeum spontaneum). J Agron Crop Sci 189:298–303
Teerarak M, Charoenying P, Laosinwattana C (2012) Physiological and cellular mechanisms of natural herbicide resource from Aglaia odorata Lour. on bioassay plants. Acta Physiol Plant 34:1277–1285
Tsuzuki JK, Svidzinski TI, Shinobu CS, Silva LF, Rodrigues-Filho E, Cortez DA, Ferreira IC (2007) Antifungal activity of the extracts and saponins from Sapindus saponaria L. An Acad Bras Cienc 79:577–583
Vidal RA, Trezzi MM, Prado R, Ruiz-Santaella JP, Vila-Aiub M (2007) Glyphosate resistant biotypes of wild poinsettia (Euphorbia heterophylla L.) and its risk analysis on glyphosate-tolerant soybeans. J Food Agric Environ 5:265–269
Wahab SMA, Selim MA (1985) Lipids and flavonoids of Sapindus saponaria. Fitoterapia 56:167–168
Wink M (2003) Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 64:3–19
Xuan TD, Shinkichi T, Khanh TD, Chung IM (2005) Biological control of weeds and plant pathogens in paddy rice by exploiting plant allelopathy: an overview. Crop Prot 24:197–206
Yoshimura H, Sawa Y, Tamotsu S, Sakai A (2011) 1,8-cineole inhibits both proliferation and elongation of by-2 cultured tobacco cells. J Chem Ecol 37:320–328
Acknowledgments
The authors acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2011/11.860-5) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Grisi, P.U., Forim, M.R., Costa, E.S. et al. Phytotoxicity and Identification of Secondary Metabolites of Sapindus saponaria L. Leaf Extract. J Plant Growth Regul 34, 339–349 (2015). https://doi.org/10.1007/s00344-014-9469-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-014-9469-2