Abstract
Abies fabri (Mast.) Craib is an endemic and dominant species in typical subalpine dark coniferous forests distributed in the eastern Tibetan Plateau. To assess how A. fabri may respond and adapt to future climate changes, we investigated the effects of drought and warming on the growth, resource allocation in biomass, membrane stability, and oxidative stress of the seedlings over two growing seasons. Drought (11.4 % average reduction in soil moisture) was created by excluding natural precipitation with a plastic roof and warming was performed by an infrared heater above the plots. Drought increased root length, the root-to-shoot ratio, N concentration, and N/P ratio in all organs, and decreased seedling height and C/N ratio in all organs. Moreover, warming (2 °C) decreased seedling height, root length, total biomass, and N concentration in stems but increased the C/N ratio. Furthermore, the combination of drought and warming decreased seedling height and biomass in all organs, which further increased the N concentration and N/P ratio in all organs. A significant decrease in the membrane stability index and an increase in malondialdehyde, superoxide radical (O2 −), and hydrogen peroxide (H2O2) were exactly matched with a dramatic decrease of total biomass under the combination of drought and warming treatment. Together these results implied that drought alone and warming alone were unfavorable for the early growth of A. fabri, and drought plus warming will intensify the opposite effect of drought alone or warming alone. Moreover, N will be a limited nutrient under extant and future climate changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Earth’s surface temperature has increased by 0.74 °C due to climate change generated by elevated atmospheric greenhouse gases (IPCC 2007). A clear warming trend on the Tibetan Plateau has also been observed over the last 50 years (Thompson and others 2000) and future warming was predicted to be “greater than the global average” (Giorgi and others 2001). Because of continuous global warming and a substantial shift in the precipitation pattern, global soil moisture will decrease. Long-term droughts have become three times more common (Sheffield and Wood 2008), and warming and drought may be concurrent under extant and future climate changes. Moreover, most general circulation models (GCMs) indicate that due to the socioeconomic scenario and greenhouse gases, the average surface temperature of the Earth will increase by 1.1–6.4 °C by end of the 21st century (IPCC 2007).
Such changes in climate are expected to influence plant growth, the regulation of biochemical reaction rates, plant physiological processes, and biomass allocation and accumulation (Sairam and others 1997; Starr and others 2000; Saxe and others 2001; Peñuelas and others 2004; Llusià and others 2006; Sardans and others 2008), and then alter the establishment, survival, and reproduction of terrestrial vegetation, resulting in dramatic effects on ecosystems (Loik and others 2004). Drought increases the rate of production of activated oxygen species (AOS) [that is, H2O2 (hydrogen peroxide) and O2 − (superoxide)] by enhancing leakage of electrons to molecular oxygen. These cytotoxic AOS can destroy normal metabolism through oxidative damage to lipids, proteins, and nucleic acids. Oxidative damage will result in negative effects on plant performance and biomass accumulation (Fazeli and others 2007). Moreover, temperature also is one of the most important factors controlling the physiological activity and growth of plants. Elevated temperature can promote plant growth and photosynthesis in all plants as long as their optimal temperature is not exceeded (Warren 1966). Drought plus warming can induce changes in plant N concentrations, C/N ratios, N use efficiency (NUE), and N relative allocation to different tissues in temperate (An and others 2005) and arctic ecosystems (Weintraub and Schimel 2005). In addition, N and P status and N/P stoichiometry in plant tissues, especially in leaves, have been studied intensively to determine how drought and warming limit plant growth (Reich and Oleksyn 2004; Zheng and Shangguan 2007). Up to now, substantial efforts have been made to study the potential impacts of single factors on terrestrial ecosystems (Peñuelas and others 2007; Sardans and others 2008), however, few studies (but see Qaderi and others 2006; Wardlaw 2002) have investigated the combined effects on plant performance and physiological traits. The interaction between drought and warming on the early growth of conifers is still not well understood. It is important to understand the possible ecophysiological responses of plants to future climate warming with low precipitation because temperate and boreal trees will likely be affected by climate change in the coming century (Matala and others 2006; Scholze and others 2006).
Abies fabri, mainly distributed in mountainous regions of the eastern Qinghai–Tibetan Plateau, China, is one of the endemic and dominant species of the subalpine dark coniferous forests, which is one of the most sensitive of terrestrial ecosystems. Here we used a novel climate-change experiment with a combination of reduced rainfall and infrared heater to examine the responses of coniferous seedlings to single and combined climate changes in situ. In this study we tested the responses over two growing seasons; furthermore, we evaluated how drought and warming affect seedling growth, nutrient allocation, and biochemical reactions in alpine conifer forests in the eastern Tibetan Plateau. Considering the cool and wet climate in the region (mean annual temperature of 3.8 °C, mean annual rainfall of 1,940 mm), two questions were addressed: (1) will warming improve A. fabri growth in the cold and wet region? and (2) will warming intensify the negative effects of drought on A. fabri or alleviate it?
Materials and Methods
Study Site and Plant Material
The present study was conducted in the Alpine Ecosystem Observation and Experiment Station (3,000 m a.s.l.), situated on the eastern slope of Mount Gongga (29°20′–30°20′ N, 101°30′–102°15′ E, 7,556 m a.s.l.). Mount Gongga is located on the Quaternary sections of the eastern Tibetan Plateau and its transit zone to Sichuan province, southwest China. The climate is dominated by the southeastern Pacific monsoon. The eastern slope of Mount Gongga reaches down into the deep Dadu River valley at 1,100 m a.s.l., with a horizontal distance of less than 30 km, and the western slope blends into the eastern Tibetan Plateau at 3,000–3,500 m (Jia and others 2008). The mean annual temperature of the experimental site is 3.8 °C (−4.3 °C in January and 11.9 °C in July). The mean annual precipitation is 1,940.0 mm, 60.6 % of which occurs from June to September, and the annual air relative humidity is 90.2 %. The soil is typical mountain dark brown soil with high sand content and strong permeability. The vegetation consists mainly of a dark coniferous forest dominated by Faber’s fir [A. fabri (Mast.) Craib].
Three-year-old healthy A. fabri seedlings of uniform size from a tree nursery on Mt. Emei (29°36′ N, 103°29′ E, 3,099 m a.s.l.) were selected based on plant height, basal stem diameter, and root length. The averages of plant height, basal stem diameter, and root length were 14.8 ± 1.1 cm, 3.06 ± 0.16 mm, and 10.6 ± 1.3 cm, respectively. Seedlings were transplanted into the experimental field between 18 and 22 October 2008.
Warming and Drought Treatments
Four treatments were performed in this study: control (C), drought alone (D), warming alone (T), and drought plus warming (D + T). The experiment was conducted in a semiopen field with 12 plots (area of 2 × 2 m2 per plot). Samples were taken only from an internal area of 1.5 × 1.5 m2 to avoid the effect of edge disturbance. The tests were started on 9 May 2009 and three replicates per treatment were performed.
The plots were treated with warming during nighttime by an infrared heater since May 2009. One 165 × 15 cm infrared heater (Kalglo Electronics Inc., Bethlehem, PA, USA), with a radiation output of about 100 W m−2, was hung 1.5 m above each warmed plot. Meanwhile, a “dummy” heater with the same shape and size as the infrared heater was used in the control plot to simulate the shading effects of the heater. The distance between the control and the warmed plots was approximately 6 m to avoid having the infrared heater warm the control plot.
To reduce the rainfall input in the growing season (May to September), the drought treatment was performed by covering each plot with a metal scaffold carrying a transparent polyethylene plastic roof approximately 2.5–3.0 m above the ground. A rain sensor (RainBird Sprinkler Mfg. Co. Inc., Canada) was connected to the plastic roof to enable the roof to cover the plots when it rains and to remove the roof when the rain stops. The ambient precipitation plots were equipped with the same scaffolding as the drought treatment plots but without a functioning roof; this was done to simulate the shading effects of the scaffolding. The shielding roof had no effect on light interception in this experiment (Yang and others 2012).
Air temperature (20 cm above ground) and soil moisture (−20 cm) were measured in the four plots (C, D, T, D + T) at 10 min intervals by EC-TM moisture and temperature sensors (Decagon Devices Inc., Pullman, WA). Data were recorded by EM50 ECH2O data loggers (Decagon Devices Inc.).
Dry Mass Determination
Nine seedlings were sampled randomly from each treatment. Needles, shoots, and roots were separated. Shoot height, stem diameter, and root length were measured. For dry mass (DM) determination, samples were oven-dried (65 °C for 72 h) to constant mass and then weighed. The root/shoot ratio was then calculated.
Sample Preparation for Chemical Measurements
Current-year needles from each treatment were collected and immediately weighed after the water in the needles was removed by drying, and then frozen in liquid nitrogen. Next, the samples of roots, stems, and leaves were dried at 65 °C for 72 h and then were ground and passed through a 20 mesh screen followed by measurements.
Lipid Peroxidation and Membrane Stability
Lipid peroxidation was determined by estimating malondialdehyde (MDA) content with a slight modification (Heath and Packer 1968) using the thiobarbituric acid (TBA) reaction. Current-year needles (1 g) were homogenized with 5 cm3 of 20 % trichloroacetic acid (TCA) and the homogenate was centrifuged at 3,500×g for 20 min. Two cubic centimeters of 20 % TCA containing 0.5 % TBA and 100 mm3 of 4 % butylated hydroxytoluene in ethanol was added to 2 cm3 of the supernatant. The mixture was heated at 95 °C for 30 min and then quickly cooled on ice. The resulting mixture were centrifuged at 10,000 ×g for 15 min and the absorbance was measured at 532 nm. The content of MDA was calculated from the absorbance at 532 nm (corrected by using the extinction coefficient of 155 mM−1 cm−1).
Membrane stability was estimated by measuring the electrolyte leakage (conductivity) using the method of Shanahan and others (1990). Needles (1 g) were put in a glass vial containing 10 ml double-distilled water and kept at 10 °C for 24 h with shaking. The initial conductivity (C1) was recorded by a conductivity meter at 25 °C. Next, the samples were autoclaved at 0.1 MPa for 10 min and then cooled to 25 °C; the final conductivity (C2) and distilled water conductivity (C0) were recorded. The membrane stability index (MSI) was calculated as: MSI = [1−(C1/C2)] × 100.
Soluble Sugar, Starch, and Free Proline Content
Soluble sugars were determined by the anthrone method (Li 2000) modified by Wu and Xia (2006). Current-year needles (0.3 g) were cut into pieces and placed in a 25 ml cuvette with 10 ml distilled water. The cuvette was incubated at 100 °C for 60 min and the contents were filtered into 25 ml volumetric flasks. The reaction mixture of 7.5 ml contained 0.5 ml extract, 0.5 ml distilled water, 0.5 ml mixed reagent (1 g anthrone + 50 ml ethyl acetate), 5 ml H2SO4 (98 %), and 1.5 ml distilled water. The mixture was heated at 100 °C for 1 min and the absorbance was read at 630 nm. The remainder of measured soluble sugar was transferred to a 25 ml cuvette containing 10 ml distilled water and 1.0 ml HClO4 (9.2 mol L−1). The cuvette was placed in a boiling water bath for 30 min, cooled to 30 °C, and filtered into 25 ml volumetric flasks. The concentration of starch was determined colorimetrically as described above.
Free proline was extracted and its content determined according to the procedure used by Bates and others (1973). Current-year needles (0.3 g) were homogenized in 5 ml of 3 % sulfosalicylic acid and then centrifuged at 3,000×g for 20 min. The supernatant was treated with ninhydrin dissolved in acetic acid and then boiled for 1 h. The absorbance at 520 nm was recorded with a calibration curve. Free proline content was expressed as mg g−1 FW.
O2 − (Superoxide Radical) and H2O2 (Hydrogen Peroxide)
Superoxide anion radical (O2 −) was measured with slight modification to the method (Ke and others 2002) by monitoring nitrite formation from hydroxylamine in the presence of O2 −. Fresh leaves (0.3 g) were homogenized with 6 ml of 50 mM potassium phosphate (pH 7.8) and centrifuged at 5,000×g for 10 min. The incubation mixture contained 0.5 ml of 50 mM phosphate buffer (pH 7.8), 1 ml of 1 mM hydroxylamine hydrochloride, and 0.1 ml of the supernatant and was incubated at 25 °C for 1 h; then 1 ml of 17 mM sulfanilamide and 1 ml of 7 mM α-naphthylamine were added to the incubation mixture. After keeping the reaction at 25 °C for 20 min, the absorbance of the aqueous solution was measured at 530 nm. A standard curve with NO2 − was used to calculate the production rate of O2 − from the chemical reaction of O2 − and hydroxylamine.
Hydrogen peroxide (H2O2) content was also determined (Prochazkova and others 2001). Fresh leaves (0.3 g) were ground with 5 ml of cooled acetone and centrifuged at 10,000×g for 10 min. The mixture contained 1 ml of the supernatant, 2 ml titanium reagent, and 5 ml ammonium solution to precipitate the titanium–hydrogen peroxide complex. The reaction mixture was centrifuged at 10,000×g for 10 min. The precipitate was dissolved in 3 ml of 1 mM H2SO4 and then recentrifuged. The absorbance of the supernatant was read at 415 nm.
Carbon (C), Nitrogen (N), and Phosphorus (P) Concentration
The total contents of nitrogen (N) and carbon (C) were analyzed using the Kjeldahl method and the rapid dichromate oxidation technique (Nelson and Sommers 1982), respectively. The total C/N ratio was calculated as an estimate for long-term nitrogen use efficiency. P content (%) of the leaves was measured using the ammonium molybdate method after persulfate oxidation (Kuo 1996) and quantified by reference to a national standard material with known P concentration (reference code GBW08513; General Administration of Quality Supervision, People’s Republic of China).
Data Analyses
To examine the effects of drought, warming, and their combination compared with the control treatment, we used a one-way analysis of variance (ANOVA) with treatment as the fixed factor and growth parameters, N content, C/N, N/P, soluble sugar, starch, proline, MSI, MDA, O2 −, and H2O2 as separate dependent variables. Duncan’s test was used to identify whether there were significantly different responses at the level of p < 0.05. The effect of the treatments on soil moisture was tested by comparing drought, warming, and drought plus warming with the control separately by using monthly averages. Statistical analyses were conducted using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL).
Results
Soil Moisture and Temperature
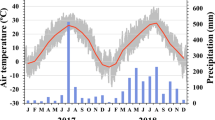
During the growing season (from May to September), the drought treatment led to a mean reduction in soil moisture of 11.4 % with respect to the control treatment. Monthly soil moisture at a depth of 20 cm was significantly decreased in D and D + T plots compared with the C plot (D, p = 0.03; D + T, p = 0.01; Fig. 1), but there was no difference between D and T plots (T, p = 0.47; Fig. 1). Monthly air temperature at 20 cm aboveground was increased by 0.1, 2.0, and 2.2 °C under drought alone, warming alone, and drought plus warming, respectively.
Growth and Biomass Effects in Abies fabri
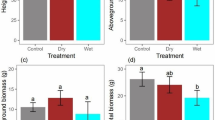
Drought, warming, and drought plus warming treatments induced a significant decrease in seedling height compared with the control treatment (Table 1, p < 0.05). An increase induced by drought alone and a decrease induced by warming alone were found in the root length. Although drought alone or warming alone induced changes of biomass partition in organs in different directions, drought plus warming decreased seedling biomass accumulation in all organs compared with control treatment. Accordingly, significant decreases in total biomass and increases in the R/S ratio were detected under drought alone, warming alone, and drought plus warming treatments relative to the control treatment. Obviously, the negative effects of warming alone and drought alone on seedling performance and growth will be intensified by the drought plus warming treatment.
Resource Accumulation and Allocation
After 2 years of treatment to the A. fabri seedlings, drought increased N concentration and changed the N/P and C/N ratios in all organs. Drought significantly increased the N concentration in roots (by 34.55 %), stems (by 22.06 %), and needles (by 126.65 %) and the N/P ratio in roots (by 71.36 %), stems (by 29.07 %), and needles (by 71.17 %) compared with the control treatment. Because of the sharp increases in N concentration and slight changes in C concentration, the C/N ratio in the drought treatment significantly decreased in roots (by 16.37 %), stems (by 21.82 %), and needles (by 57.22 %) (Fig. 2).
N concentrations (%), C/N, and N/P concentration ratios in roots, stems, and needles of Abies fabri (mean ± SE, n = 3) under control (C), drought (D), warming (T), and combined drought and warming (D + T) treatments. The measurement was finished in late September. Different letters indicate significant statistical differences between treatments (p < 0.05)
Warming increased N concentration in roots by 10.26 % and the N/P ratio by 53.64 % (Fig. 2). In contrast, warming decreased N concentration in stems by 11.48 %, and as a result it increased the C/N in stems by 9.73 %. Warming had no effect on the N/P ratio in stems. Compared with the control, warming also significantly increased N concentration and the N/P ratio and decreased the C/N ratio in needles; this was related to the decrease in C and P concentrations (Table 2). Warming also changed the N allocation in all organs and strongly affected the N/P and C/N ratios.
Compared with the control, the drought plus warming treatment significantly increased the N concentration in roots, stems, and particularly needles by 161.76 % (Fig. 2) and the N/P ratio in all organs. However, although there was a significant increase in P concentration, drought plus warming decreased the C/N ratio particularly in needles by 62.43 %, which is due to the decrease in C concentration. This decreased C concentration was matched with the decreased biomass accumulation in the drought plus warming treatment.
Physiological Parameter Changes
Neither drought nor warming treatment significantly changed MSI, MDA, O2 −, or H2O2 compared with the control (Table 3). However, the combination of drought and warming significantly decreased membrane stability and increased MDA, hydrogen peroxide content, and the superoxide radical production rate (Table 3, p < 0.05). Obviously, drought plus warming treatment resulted in cell oxidative damage in seedlings.
Drought did not affect the osmotic regulator in A. fabri seedlings. Free proline and soluble sugar contents in needles increased in warming alone, but a significant decrease in starch content was detected only in the drought plus warming treatment (Fig. 3).
The contents of free proline, soluble sugar, and starch in Abies fabri needles under control (C), drought (D), warming (T), and combined drought and warming (D + T) treatments. Data are presented as mean ± SE (n = 3) and were acquired from the lab in early September. Different letters indicate significant statistical differences between treatments (p < 0.05)
Discussion
Performance Traits and N Accumulation in Biomass
Drought changed seedling performance and biomass accumulation, indicated by decreased height and total biomass and increased root length and root biomass. Similar results have been reported in other woody plants such as Quercus suber L. seedlings (Puértolas and others 2008), Picea asperata (Yang and others 2008), and Populus cathayana (Lu and others 2009). Accordingly, the root/shoot ratio (R/S) also increased with drought as a result of reducing aboveground biomass and promoting root growth. This kind of morphological response to drought was a plant avoidance mechanism to hostile environments, mainly improving water use efficiency by increasing the proportion of water-absorbing root biomass relative to aboveground biomass (Lei and others 2006). Additionally, the present results are similar to those previously reported for A. fabri after a 1 year drought treatment (Guo and others 2010), except for seedling height change, suggesting that drought over two growing seasons limited seedling height much more. On the other hand, consistent with similar studies on Empetrum hermaphroditum (Bokhorst and others 2009) and Picea glauca (Wilmking and others 2004), our results showed that compared with the control, artificial warming decreased seedling growth and biomass accumulation, indicating a decrease of plant growth. However, our results disagree with the studies of Picea glauca (Danby and Hik 2007) and Pinus halepensis (Prieto and others 2009) which showed that warming increased plant growth and biomass accumulation. Decreased growth with warming might be due to the physiological limitation on water transport at the study sites (Barber and others 2004; Lloyd and Fastie 2002). Although there were negative effects on seedling growth with drought alone and warming alone, drought plus warming further intensified the adverse effects, such as sharply decreased seedling height and total biomass accumulation compared with the control treatment. Obvious increases in MSI, MDA, O2 −, and H2O2 were detected under drought plus warming treatment, also suggesting that the negative effects of drought on deoxidization capacity were intensified by experimental warming.
Significant increases in N concentration were observed in all organs induced by drought alone. Comparable results have also been reported by Sinclair and others (2000) and Lu and others (2009), whereas Sardans and others (2008) reported that drought decreased leaf N content in Globularia alypum and Erica multiflora. A possible reason for the increase in N concentration in the present study may be an accumulation of soluble protein during drought, perhaps providing a reservoir for later use during the recovery period after the drought has ended (Millard 1988).
This study observed increases in leaf N concentration in warming compared to control plots, which is consistent with observations from previous studies (Nijs and others 1996; Soussana and others 1996; Luomala and others 2003). However, other warming studies showed that elevated temperature decreased leaf N concentration (Weih and Karlsson 2001; Link and others 2003). This is due mainly to the fact that N mineralization was depressed in the long-term warming experiment resulting in larger decreases in leaf N concentration over time (An and others 2005). In our study, warming substantially increased plant physiological activities such as increased soluble sugar and starch contents (Fig. 2).
The C/N ratio could be used to estimate the long-term nitrogen use efficiency (NUE) and might be an important signal for controlling gene expression in plants. Our results showed that drought plus warming significantly decreased the NUE of A. fabri seedlings (Fig. 2). Maranville and Madhavan (2002) summarized the NUE of the published literature into three cases wherein NUE increased: (1) maintaining constant biomass while reducing plant or leaf N, (2) increasing biomass at a constant level of plant or leaf N, and (3) reducing levels of plant or leaf N per unit of increase in biomass. In our study, a decrease in biomass production and an increase in leaf N concentration under drought plus warming led to decreased NUE of A. fabri.
N versus P limitation
The P concentration in A. fabri needles increased in drought and drought plus warming conditions without increasing biomass. Warming did not change the P concentration similarly but decreased biomass. These results indicated that P was probably not a limiting factor in this ecosystem dominated by A. fabri, whereas other nutrients such as N were possibly more limiting than P. Drought alone, warming alone, and their combination increased the N/P ratio in needles, indicating a greater accumulation of N than P in needles. A N/P ratio below 14 generally indicates N constraint, and a N/P ratio above 16 suggests P constraint. A N/P ratio between 14 and 16 indicates either or both nutrients constrain plant growth (Koerselman and Meuleman 1996). In the present study, the N/P ratio in needles was always below 14 in control, drought, warming, and their combination conditions. These results confirmed that N was probably the limiting nutrient in the ecosystem of the study region. Our results matched well with those of the study by Perakis and Hedin (2002) in that a relative lack of symbiotic N fixers resulted in significant N losses in dissolved organic N. Another factor might be that weathering in the warm and moist climate of the study region rapidly releases P into biologically available forms, and its availability to plants increases at least as fast as plant demand for these elements (Walker and Syers 1976). In contrast, the young soil of A. fabri forests (Zhong and others 1997) generally contains little or no nitrogen, leading to N limitation.
In conclusion, we demonstrated that in contrast to the response to drought alone and warming alone, biomass dramatically decreased under drought plus warming treatment. N was a limiting nutrient, although there was a sharp increase in N concentration and N:P ratio under drought plus warming. In addition, instantaneous membrane damage was observed under the drought plus warming treatment, with sharply decreased biomass of A. fabri seedlings. Thus, the present study suggests that warming intensifies the negative effects of drought on A. fabri in the eastern Tibetan Plateau. However, the mechanism behind the unfavorable reactions that A. fabri developed remains unknown. Thus, the effects of drought and warming in the longer term need further elucidation.
References
An Y, Wan SQ, Zhou X, Subedar AA, Wallace LL, Luo Y (2005) Plant nitrogen concentration, use efficiency, and contents in a tallgrass prairie ecosystem under experimental warming. Glob Change Biol 11:1733–1744
Barber VA, Juday GP, Finney BP, Wilmking M (2004) Reconstruction of summer temperatures in interior Alaska from tree-tring proxies: evidence for changing synoptic climate regimes. Clim Change 63:91–120
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bokhorst SF, Bjerke JW, Tømmervik H, Callaghan TV, Phoenix GK (2009) Winter warming event damage sub-Artic vegetation: consistent evidence from an experimental manipulation and a natural event. J Ecol 97(6):1408–1415
Danby RK, Hik DS (2007) Responses of white spruce (Picea glauca) to experimental warming at a subarctic alpine treeline. Glob Change Biol 13:437–451
Fazeli F, Ghorbanli M, Niknam V (2007) Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol Plant 51:98–103
Giorgi F, Hewitson B, Christensen J, Hulme M, Von Storch H, Whetton P, Jones R, Mearns L, Fu C (2001) Climate change 2001: regional climate information—evaluation and projections. In: Houghton JT, Ding Y, Griggs DJ, Noguer M, Linden PJV, Dai X, Maskell K, Johnson CA (eds) Climate change 2001: the scientific basis. Contribution of Working Group I to the third assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, p 583–636
Guo JY, Yang Y, Wang GX, Yang LD, Sun XY (2010) Ecophysiological responses of Abies fabri seedlings to drought stress and nitrogen supply. Physiol Plant 139:335–347
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
IPCC (2007) Climate change 2007: the physical science basis. In: Solomon S, Qin D, Manning M et al (eds) Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Jia GD, Wei K, Chen FJ, Peng PA (2008) Soil n-alkane δD vs. altitude gradients along Mount Gongga, Chinaz. Geochim Cosmochim Acta 72:5165–5174
Ke D, Wang A, Sun G, Dong L (2002) The effect of active oxygen on the activity of ACC synthase induced by exogenous IAA. Acta Bot Sin 44:551–556
Koerselman W, Meuleman AFM (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33(6):1441–1450
Kuo S (1996) Phosphorus. In: Sparks DL, Page AL, Loeppert PA, Soltanpour PN, Tabatabai MA, Johnston CT et al (eds) Methods of soil analysis Part 3: chemical methods. Soil Science Society of America and American Society of Agronomy, Madison, pp 869–920
Lei Yanbao, Yin Chunying, Li Chunyang (2006) Differences in some morphological, physiological, and biochemical responses to drought stress in two contrasting populations of Populus przewalskii. Physiologia Plantarum 127:182–191
Li HS (2000) Principles and techniques of plant physiological biochemical experiment. Higher Education Press, Beijing
Link SO, Smith JL, Halvorson JJ, Bolton H Jr (2003) A reciprocal transplant experiment within a climatic gradient in a semiarid shrub-steppe ecosystem: effects on bunchgrass growth and reproduction, soil carbon, and soil nitrogen. Glob Change Biol 9:1097–1105
Lloyd AH, Fastie CL (2002) Spatial and temporal variability in the growth and climate response of treeline trees in Alaska. Clim Change 52:481–509
Llusià J, Peñuelas J, Alessio GA, Estiarte M (2006) Seasonal contrasting changes of foliar concentrations of terpenes and other volatile organic compound in four dominant species of a Mediterranean shrubland submitted to a field experimental drought and warming. Physiol Plant 127:632–649
Loik ME, Still CJ, Huxman TE, Harte J (2004) In situ photosynthetic freezing tolerance for plants exposed to a global warming manipulation in the Rocky Mountains, Colorado, USA. New Phytol 162:331–341
Lu YW, Duan BL, Zhang XL, Korpelainen H, Berninger F, Li CY (2009) Intraspecific variation in drought response of Populus cathayana grown under ambient and enhanced UV-B radiation. Ann For Sci 66:613
Luomala EM, Laitinen K, Kellomäki S, Vapaavouri E (2003) Variable photosynthetic acclimation in consecutive cohorts of Scots pine needles during 3 years of growth at elevated CO2 and elevated temperature. Plant Cell Environ 26:645–660
Maranville JW, Madhavan S (2002) Physiological adaptations for nitrogen use efficiency in sorghum. Plant Soil 245:25–34
Matala J, Ojansuu R, Peltola H, Raitio H, Kellomäki S (2006) Modelling the response of tree growth to temperature and CO2 elevation as related to the fertility and current temperature sum of a site. Ecol Model 199:39–52
Millard P (1988) The accumulation and storage of nitrogen by herbaceous plants. Plant Cell Environ 11:1–8
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Part 2. American Society of Agronomy and Soil Science, Madison, pp 539–579
Nijs IA, Teughels HA, Blum H, Hendrey G, Impens I (1996) Simulation of climate change with infrared heaters reduces the productivity of Lolium perenne L. in summer. Environ Exp Bot 36:271–280
Peñuelas J, Gordon C, Llorens L, Nielsen T, Tietema A, Beier C, Bruna P, Emmett B, Estiarte M, Gorissen A (2004) Nonintrusive field experiments show different plant responses to warming and drought among sites, seasons, and species in a North-South European gradient. Ecosystems 7:598–612
Peñuelas J, Prieto P, Beier C, Cesaraccio C, De Angelis P, De Dato G, Emmett BA, Estiarte M, Garadnai J, Gorissen A, Láng EK, Kröeldulay G, Llorens L, Pellizzaro G, Riis-nielsen T, Schmidt IK, Sirca C, Sowerby A, Spano D, Tietema A (2007) Response of plant species richness and primary productivity in shrublands along a north-south gradient in Europe to seven years of experimental warming and drought. Reductions in primary productivity in the heat and drought year of 2003. Glob Change Biol 13:2563–2581
Perakis SS, Hedin LO (2002) Nitrogen loss from unpolluted South American forests by dissolved organic compounds. Nature 415:416–419
Prieto P, Peñuelas J, Llusià J, Asensio D, Estiarte M (2009) Effects of experimental warming and drought on biomass accumulation in a Mediterranean shrubland. Plant Ecol 205(2):179–191
Prochazkova D, Sairam RK, Srivastava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci 161:765–771
Puértolas J, Pardos M, Jiménez MD, Aranda I, Oardos JA (2008) Interactive responses of Quercus suber L. seedlings to light and mild water stress: effects on morphology and gas exchange traits. Ann For Sci 65:611–621
Qaderi MM, Kurepin LV, Reid DM (2006) Growth and physiological responses of canola (Brassica napus) to three components of global climate change: temperature, carbon dioxide and drought. Physiol Plant 128:710–721
Reich PB, Oleksyn J (2004) Global patterns of plant foliar N and P in relation to temperature and latitude. Proc Natl Acad Sci USA 101:11001–11006
Sairam RK, Deshmukh PS, Shukla DS (1997) Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agron Crop Sci 178:171–178
Sardans J, Peñuelas J, Estiarte M, Prieto P (2008) Warming and drought alter C and N concentration, allocation and accumulation in a Mediterranean shrubland. Glob Change Biol 14:1–13
Saxe H, Cannell MGR, Johnsen Ø, Ryan MG, Vourlitis G (2001) Tree and forest functioning in response to global warming. New Phytol 149:369–400
Scholze M, Knorr W, Arnell NW, Prentice IC (2006) A climate-change risk analysis for world ecosystems. Proc Natl Acad Sci USA 103:13116–13120
Shanahan JF, Edwards IB, Quick JS, Fenwick JR (1990) Membrane thermostability and heat tolerance of spring wheat. Crop Sci 30:247–251
Sheffield J, Wood EF (2008) Projected changes in drought occurrence under future global warming from multi-model, multi-scenario, IPCC AR4 simulations. Clim Dyn 31:79–105
Sinclair TR, Pinter PJ, Kimball BA, Adamsen FJ, LaMorte RL, Wall GW, Hunsaker DJ, Adam N, Brooks TJ, Garcia RL, Thompson T, Leavitt S, Matthias A (2000) Leaf nitrogen concentration of wheat subjected to elevated [CO2] and either water or N deficits. Agric Ecosyst Environ 79:53–60
Soussana JF, Casella E, Loiseau P (1996) Long term effects of CO2 enrichment and temperature increase on a temperate grassland sward. II. Plant nitrogen budgets and root fraction. Plant Soil 182:110–114
Starr G, Oberbauer SF, Pop EW (2000) Effects of lengthened growing season and soil warming on the phenology and physiology of Polygonum bistorta. Glob Change Biol 6:357–369
Thompson LG, Yao T, Mosley-Thompson E, Davis ME, Henderson KA, Lin PN (2000) A high-resolution millennial record of the South Asian monsoon from Himalayan ice cores. Science 289:1916–1919
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–9
Wardlaw IF (2002) Interaction between drought and chronic high temperature during kernel filling in wheat in a controlled environment. Ann Bot 90:469–476
Warren WJ (1966) Effect of temperature on the net assimilation rate. Ann Bot 30:753–761
Weih M, Karlsson PS (2001) Growth response of Mountain birch to air and soil temperature: is increasing leaf-nitrogen content an acclimation to lower air temperature? New Phytol 150:147–155
Weintraub MN, Schimel JP (2005) Nitrogen cycling and the spread of shrubs control changes in the carbon balance of arctic tundra ecosystems. Bioscience 55:408–415
Wilmking M, Juday GP, Barber VA, Zald HSJ (2004) Recent climate warming forces contrasting growth responses of white spruce at treeline in Alaska through temperature thresholds. Glob Change Biol 10:1724–1736
Wu QS, Xia RX (2006) Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J Plant Physiol 163:417–425
Yang Y, Guo JY, Wang GX, Yang LD, Yang Y (2012) Effects of drought and nitrogen addition on photosynthetic characteristics and resource allocation of Abies fabri seedlings in eastern Tibetan Plateau. New For 43:505–518
Yang Y, Han C, Liu Q, Lin B, Wang JW (2008) Effect of drought and low light on growth and enzymatic antioxidant system of Picea asperata seedlings. Acta Physiol Plant 30:433–440
Zheng SX, Shangguan ZP (2007) Spatial patterns of leaf nutrient traits of the plants in the Loess Plateau of China. Trees 21:357–370
Zhong XH, Wu N, Luo J, Yin KP, Tang Y, Pan ZF (1997) Researches of the forest ecosystems on Gongga Mountain. Chengdu University of Science and Technology Press, Chengdu, pp 10–14
Acknowledgments
This research was supported by the Knowledge Innovation Project (KZCX2-EW-309-2, KZCX2-YW-331-2) and the “West Light” Talent Plan Project (Y0R2100100) of the Chinese Academy of Sciences. We thank Quan Lan for providing valuable field help. We are also grateful for all workers in the Alpine Ecosystem Observation and Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Y., Wang, G., Yang, L. et al. Effects of Drought and Warming on Biomass, Nutrient Allocation, and Oxidative Stress in Abies fabri in Eastern Tibetan Plateau. J Plant Growth Regul 32, 298–306 (2013). https://doi.org/10.1007/s00344-012-9298-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-012-9298-0