Abstract
Supplying a sufficient amount of available iron (Fe) for plant growth in hydroponic nutrient solutions is a great challenge. The chelators commonly used to supply Fe in nutrient solutions have several disadvantages and may negatively affect plant growth. In this research study we have synthesized certain Fe-amino acid chelates, including Fe-arginine [Fe(Arg)2], Fe-glycine [Fe(Gly)2], and Fe-histidine [Fe(His)2], and evaluated their efficacy as an Fe source for two tomato cultivars (Lycopersicon esculentum Mill. cvs. ‘Rani’ and ‘Sarika’) grown in nutrient solution. Application of Fe-amino acid chelates significantly increased root and shoot dry matter yield of both tomato cultivars compared with Fe-EDTA. Tomato plants supplied with Fe-amino acid chelates also accumulated significantly higher levels of Fe, Zn, and N in their roots and shoots compared with those supplied with Fe-EDTA. In ‘Sarika’, the effect of Fe-amino acid chelates on shoot Fe content was in the order Fe(His)2 > Fe(Gly)2 > Fe(Arg)2. In ‘Rani’, the addition of all synthesized Fe-amino acid chelates significantly increased activity of ascorbate peroxidase (APX) in comparison with Fe-EDTA, whereas in ‘Sarika’, only Fe(His)2 increased shoot APX activity. The results obtained indicated that using Fe-amino acid chelates in the nutrient solution could supply a sufficient amount of Fe for plant uptake and also improve root and shoot growth of tomato plants, although this increase was cultivar-dependent. According to the results, Fe-amino acid chelates can be used as an alternative for Fe-EDTA to supply Fe in nutrient solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iron (Fe) is an important micronutrient that plays a role in several crop physiological processes such as photosynthesis, respiration, and synthesis of heme proteins, DNA, RNA, and hormones (Curie and others 2009; Rivero and others 2003). In nutrient solution cultures, synthetic Fe chelates are widely used to maintain a desirable concentration of this element for the plant (Parker and Norvell 1999; Vadas and others 2007). The most common Fe sources used in nutrient solutions are Fe-EDTA and Fe-DTPA (Vadas and others 2007). Although these chelates maintain Fe solubility in hydroponic solutions, after Fe uptake by the plant, the concentration of free ligands is increased in the nutrient solution and, as a result, the possibility of complex formation between free ligands and other micronutrients (that is, Zn, Cu, and Mn) in the solution increases. Complexation with EDTA, EDDS, or DTPA reduces the concentrations of free metal cations and thereby decreases their availability for plant uptake (Albano and Miller 2001; Vadas and others 2007).
On the other hand, EDTA, EDDS, and DTPA are easily photodegradable compounds (Metsarinne and others 2004; Nowack and Baumann 1998). Significant photodegradation of Fe-EDTA in natural waters (Nowack and Baumann 1998) and of Fe-EDTA, Fe-DTPA, or Fe-EDDS in Fe-containing nutrient solutions (Metsarinne and others 2001) by sunlight and particularly ultraviolet (UV) light has been reported. The half-lives of Fe-EDTA and Fe-DTPA photodegradation under high light density conditions have been reported to be 8 and 11 min, respectively (Svenson and others 1989). Photodegradation of the Fe-chelates in plant growth nutrient solutions in the presence of blue and UV lights has also been reported by Albano and Miller (2001). Some harmful compounds may be produced from photolytic degradation of Fe-chelates in nutrient solutions (Vadas and others 2007). For example, Hangarter and Stasinopoulos (1991) found that Fe-EDTA was decomposed in an agar growth solution under white fluorescent lamps and produced glyoxylic acid and formaldehyde, two compounds that inhibit plant growth. Metsarinne and others (2004) also reported photolytic degradation of DTPA into diethylenetriaminetriacetic acid and diethylenediaminetriacetic acid.
The chelators used in nutrient solutions may also be transported into the plant tissue (Vadas and others 2007) probably via an undeveloped casparian band at the root tip (Bell and others 1991). High concentrations of chelates can remove calcium (Ca2+) from the cell membrane and impair root membrane integrity (Vassil and others 1998).
Supplying a sufficient amount of available Fe for plant growth in hydroponic nutrient solutions used in laboratory studies and commercial facilities is a great challenge. Considering the problem associated with the synthesized chelates currently used in nutrient solutions, finding a proper alternative of Fe compounds is necessary.
It has been shown that use of some amino acids in nutrient solutions improves Fe uptake by crops (Sánchez and others 2005). Advances in the understanding of the metabolic responses to Fe deficiency have also highlighted the key role of amino acids in both Strategy I and Strategy II plants (Zuchi and others 2009). Amino acids have the ability to coordinate metal ions (such as Fe) via their carboxyl groups (Aravind and Prasad 2005). On the other hand, amino acids are less sensitive to photodegradation and their degradation is completely biological (Jones and Hodge 1999). There are some results that indicate negligible degradation of amino acids in nutrient solutions (Jämtgård and others 2008). The degradability of metal–amino acid complexes is also less than free amino acids (Brynhildsen and Rosswall 1995; Renella and others 2004). Therefore, Fe–amino acid complexes seem to be stable in hydroponic nutrient solutions and prevent Fe precipitation.
In addition, amino acids are nitrogen sources for plant nutrition (Tida and others 2009). Most plants can directly absorb amino acids and use them in their physiological structures and processes (Jämtgård and others 2008; Näsholm and others 2009; Wu and others 2005). This may result in less accumulation of free ligand in the media and further impairment of other micronutrient balance.
Due to several disadvantages of Fe-EDTA (for example, toxic side effects on plants and impaired micronutrient balance), finding a suitable alternative for Fe-EDTA in hydroponic nutrient solutions is of great importance. There is limited information on the possibility of using Fe–amino acid complexes as a plant growth stimulator and Fe source in nutrient solution cultures. Therefore, this research was performed to synthesize three Fe–amino acid chelates and evaluate their efficacy as Fe sources for tomato plants grown in nutrient solution. Arginine (Arg), glycine (Gly), and histidine (His) were chosen as ligand amino acids. The L-enantiomers (natural forms in plants) of amino acids were used, and some factors considered in the selection of these amino acids were abundance in the plant rhizosphere, significance in plant and human nutrition, and stability of their Fe complexes in water.
Materials and Methods
Synthesis of Fe-Amino Acid Chelates
Iron chelates have been prepared using arginine (Arg), glycine (Gly), and histidine (His) amino acids as complexing agents. A solution of Arg, Gly, or His (2 mmol) in 5 ml distilled water was slowly added to a solution of FeSO4 (1 mmol) in 2 ml distilled water. The mixture was heated at reflux temperature for 2 h while being stirred vigorously. Evaporation of solvent at room temperature yielded brown microcrystals of Fe-amino acid chelates. The products were washed with cold ethanol followed by diethyl ether and air-dried. All complexes were characterized by different analytical techniques.
Analyses
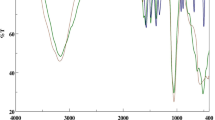
A PerkinElmer 2400 CHNS elemental analyzer was used to quantify nitrogen (N) and sulfur (S) in various operating modes. Atomic absorption measurements of Fe were recorded with atomic absorption spectrometry (PerkinElmer 3030; PerkinElmer, Wellesley, MA, USA). The FTIR spectra were measured with a JASCO FTIR 460 spectrophotometer (JASCO, Easton, MD, USA) over KBr pellet in the 4000–400 cm−1 range.
Plant Culture
Seeds of two tomato cultivars (Lycopersicon esculentum Mill. cvs. ‘Rani’ and ‘Sarika’), most commonly grown in Iran, were thoroughly rinsed with distilled water and germinated on moist filter paper in an incubator at 28°C. Uniform-size seedlings were transferred to PVC lids that fit tightly over 2-L polyethylene containers in a greenhouse under controlled conditions, with an 8-h light period at intensity of 390 μmol m−2 s−1, 25/20°C day/night temperature, and 65–75% relative humidity. The pots were wrapped with black polyethylene to prevent light from reaching the roots and solution. Two plants were planted in each pot. A basic nutrient solution was prepared in double-deionized water (electrical resistivity = 18 MΩ cm−1). The nutrient solution contained 1.0 mM KNO3, 1.0 mM Ca(NO3)2, 1.0 mM NH4H2PO4, 1.0 mM MgSO4, 50 μM KCl, 25 μM H3BO3, 2.0 μM MnSO4, 2.0 μM ZnSO4, 0.5 μM CuSO4, 1.0 μM NiSO4, and 0.02 μM H2Mo7O4 adjusted to pH 6 with NaOH or HCl as a buffer. Iron was supplied from four different sources: Fe-EDTA, Fe(Arg)2, Fe(Gly)2, and Fe(His)2. The Fe level in the nutrient solution was 100 μM. All solutions were renewed every day.
Plants were harvested approximately 4 weeks after seeding and divided into shoot and roots. Shoot and root dry matter yields were determined for each pot.
Elemental Analyses
The plant materials were dried immediately in a forced-air oven at 70°C to a constant weight and ground to a fine powder in a Wiley mill to pass through a 20-mesh sieve. Dry samples (1 g) were placed into ceramic vessels and combusted in a muffle furnace at 550°C for 8 h. The ashed samples were removed from the muffle furnace, cooled, and then dissolved in 2 M HCl (Chapman and Pratt 1961). The final solution was diluted to meet the range requirements of the analytical procedures. Analyses of Fe and Zn were carried out with an atomic absorption spectrophotometer (model 3400, PerkinElmer). Shoot nitrogen concentration was measured using Autotech (model 300) according to the Kjeldahl method (Bremmer and Mulvancey 1982). The total amount of Fe and Zn was calculated via multiplying their concentrations by the weight of dry matter.
Enzyme Assay
The plant leaf samples (buffer volume:fresh weight = 3:1) were homogenized with mortar and pestle with 100 mM Tris–HCl buffer (pH 8) containing 2 mM EDTA, 5 mM DL-dithiothreitol, 10% glycerol, 100 mM sodium borate, 4% (w/v) insoluble polyvinylpyrrolidone (PVP), and 1 mM phenylmethylsulfonyl fluoride (PMSF). The homogenate was filtered through four layers of muslin cloth and centrifuged at 12,000 g for 40 min. The supernatant was stored in separate aliquots at –80°C prior to enzyme analyses. Total protein was determined using the Bradford method (Bradford 1976).
Catalase (CAT)
Catalase (EC 1.11.1.6) activity of the leaves was determined according to Cakmak and Marschner (1992). The reaction mixture contained 25 mM sodium phosphate buffer (pH 7.0) plus 10 mM H2O2 in a total volume of 3 ml. The reaction was initiated by the addition of 100 μl of leaf extracts to the reaction mixture, and the enzyme activity was determined by measuring the initial rate of disappearance of H2O2 at 240 nm (E = 39.4 mM−1 cm−1) for 30 s.
Ascorbate Peroxidase (APX)
Ascorbate peroxidase (APX) activity was determined according to Nakano and Asada (1981). The reaction mixture, with a total volume of 3 mL, consisted of 25 mM sodium phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.25 mM ascorbate, 1.0 mM H2O2, and 100 μl of the leaf extract. H2O2-dependent oxidation of ascorbate was followed by a decrease in the absorbance at 290 nm (E = 2.8 mM−1 cm−1).
Statistical Analysis
The experiments were set up in a completely randomized factorial design; each treatment contained three replicates. A total of 24 pots were used in this experiment. Two plants were planted in each pot. Treatment effects were analyzed by analysis of variance using the GLM procedure. Means were compared using least significant differences (LSD) at p < 0.05 (SAS Institute, Cary, NC, USA, 2000).
Results
Characteristics of Fe-Amino Acid Chelates
The Fe–amino acid complexes were synthesized by reaction of FeSO4 and Arg, Gly, or His in a 1:2 mole ratio and the reaction yield was more than 84%. The amino acid ligands generally act as bidentate (N,O) chelates with respect to pH. The FTIR spectra of the complexes show an absorption pattern in the 4,000–400 cm−1 region, similar to amino acid ligands. Predominant vibrations for the complexes are associated with ν(CO), ν(C—O), ν(NH2), δ(NH2), and δ(CO). The observed vibrational bands for –NH2 groups around 3,100–3,350 cm−1 are very sensitive to the effect of intermolecular interaction in the solid state and these bands sometimes appear very broad. The carboxylate ion of amino acid coordinates to the iron ion as a unidentate mode. The C = O groups of the complexes have approximately the same frequency of around 1,590–1,690 cm−1 (data not shown).
Root Dry Matter Weight
In both tomato cultivars, Fe-amino acid chelates significantly enhanced root dry matter yield compared to Fe-EDTA (Fig. 1). The positive effect of Fe-amino acid chelates on root growth varied dependent on the crop cultivar and amino acid type. Fe(Arg)2 had no significant effect on root dry matter yield of ‘Sarika’ but there was significantly increased root dry matter yield of ‘Rani’. In ‘Sarika’ no significant difference was found in root growth between the Fe(Gly)2 and Fe(His)2 treatments. In contrast, the positive effect of Fe(Gly)2 on root dry matter yield of ‘Rani’ was greater than Fe(His)2. With the Fe-EDTA treatment, no significant difference was found in root growth between ‘Sarika’ and ‘Rani’, whereas in the presence of the Fe-amino acid chelates, ‘Rani’ produced higher root dry matter yield than ‘Sarika’.
Root and shoot dry matter weights of two tomato cultivars grown in nutrient solution containing Fe-EDTA, Fe-arginine [Fe(Arg)2], Fe-glycine [Fe(Gly)2], and Fe-histidine [Fe(His)2]. Error bars represent standard error (n = 3). Bars having different letters in root or shoot are significantly different at the 5% level by LSD
Shoot Dry Matter Weight
Application of Fe-amino acid chelates as an Fe source significantly increased shoot dry matter yield of both tomato cultivars compared with Fe-EDTA (Figs. 1, 2). In ‘Sarika’, the effect of Fe-amino acid chelates on shoot dry matter yield was in the order Fe(Arg)2 < Fe(Gly)2 < Fe(His)2. In contrast, in ‘Rani’ no significant difference was found in shoot growth of plants supplied with Fe(Arg)2, Fe(Gly)2, and Fe(His)2 complexes. At all Fe treatments except Fe(His)2, ‘Rani’ produced higher shoot dry matter yield than ‘Sarika’.
Root Fe Content
Application of Fe-amino acid chelates resulted in a significant increase of root Fe content in comparison with Fe-EDTA (Fig. 3). The positive effect of Fe-amino acid chelates on root Fe content was dependent on the tomato cultivar and amino acid type. Addition of the Fe(Arg)2 complex significantly increased Fe content in the roots of ‘Rani’, whereas it had no effect on root Fe content of ‘Sarika’. In ‘Rani,’ the magnitude of increase in root Fe content was Fe(Arg)2 > Fe(Gly)2 > Fe(His)2. In ‘Sarika’, the effect of Fe(His)2 on root Fe content was greater than that of Fe(Gly)2.
In the nutrient solutions containing Fe-EDTA and Fe(His)2, ‘Sarika’ accumulated higher amounts of Fe in its roots than did ‘Rani’ (Fig. 3), whereas in the presence of Fe(Arg)2, ‘Rani’ had higher root Fe content than ‘Sarika’. No significant difference in root Fe content was found between the two tomato cultivars at the Fe(Gly)2 treatment.
Shoot Fe Content
Tomato plants supplied with Fe-amino acid chelates accumulated significantly higher Fe in their shoots compared with those supplied with Fe-EDTA (Fig. 4). The positive effect of Fe(Gly)2 and Fe(His)2 on shoot Fe content was higher than Fe(Arg)2. In ‘Sarika’, addition of Fe(Arg)2 had no effect on shoot Fe content. In general, ‘Rani’ accumulated higher Fe in its shoots than did ‘Sarika’.
Root Zn Content
Addition of Fe-amino acid chelates resulted in higher root Zn content compared with Fe-EDTA (Fig. 5). The effect of Fe(Arg)2 and Fe(Gly)2 on root Zn content of ‘Rani’ was higher than that of Fe(His)2, whereas in ‘Sarika’, Fe(Arg)2 had no effect on root Zn content. In the nutrient solutions containing Fe-EDTA and Fe(His)2, ‘Sarika’ accumulated higher Zn in its roots than did ‘Rani’, whereas with the Fe(Arg)2 treatment, root Zn content of ‘Rani’ was greater than that of ‘Sarika’.
Shoot Zn Content
Tomato plants supplied with Fe-amino acid chelates accumulated significantly higher Zn in their shoots compared with those supplied with Fe-EDTA (Fig. 6). In ‘Rani’, Fe(Arg)2 and Fe(Gly)2 resulted in higher shoot Zn content in comparison with Fe(His)2, whereas the effect of Fe(Arg)2 on shoot Zn content of ‘Sarika’ was less than that of Fe(His)2 and Fe(Gly)2. With the Fe-EDTA and Fe(His)2 treatments, no significant difference was found in shoot Zn content between the two tomato cultivars, whereas with the Fe(Arg)2 and Fe(Gly)2 treatments, ‘Rani’ accumulated higher Zn in its shoots than did ‘Sarika’.
Shoot N Content
In both tomato cultivars, plants supplied with Fe-amino acid chelates had higher shoot N content compared with those supplied with Fe-EDTA (Fig. 7). The effect of Fe-amino acid chelates on shoot N content varied significantly depending on the crop cultivar and amino acid type. Application of Fe(Arg)2 had no effect on the shoot N content in ‘Sarika’, whereas it increased it in ‘Rani’. With the Fe-EDTA treatment, no significant difference was found in the shoot N content between the two tomato cultivars, whereas with the Fe-amino acid chelate treatments, ‘Rani’ had higher N in its shoots than did ‘Sarika’.
Activity of CAT in Shoots
In both tomato cultivars, addition of Fe(Arg)2 significantly increased the shoot activity of CAT in comparison with Fe-EDTA (Fig. 8). The effect of Fe(Gly)2 on shoot CAT activity was dependent on the tomato cultivar. Addition of Fe(Gly)2 increased the shoot CAT activity in ‘Rani’, whereas it had no effect on CAT activity in ‘Sarika’. In all treatments except Fe(His)2, the activity of CAT was greater in ‘Rani’ than ‘Sarika’.
Activity of CAT in the leaves of two tomato cultivars grown in nutrient solution containing Fe-EDTA, Fe-arginine [Fe(Arg)2], Fe-glycine [Fe(Gly)2], and Fe-histidine [Fe(His)2]. Error bars represent standard error (n = 3). Bars having different letters are significantly different at the 5% level by LSD
Activity of APX in Shoots
The effect of Fe-amino acid chelates on the shoot activity of APX was cultivar-dependent (Fig. 9). In ‘Rani’, addition of all synthesized Fe-amino acid chelates significantly increased the activity of APX in comparison with Fe-EDTA (Fig. 9). In ‘Sarika’, the addition of Fe(His)2 and Fe(Gly)2 increased the shoot APX activity compared with Fe-EDTA, whereas no such effect was found for Fe(Arg)2. In the presence of Fe(Arg)2, the activity of APX in the shoots of ‘Sarika’ was greater than that in ‘Rani’, whereas with the Fe-EDTA and Fe(His)2 treatments, ‘Rani’ had higher activity of APX in its shoots than ‘Sarika’.
Activity of APX in the leaves of two tomato cultivars grown in nutrient solution containing Fe-EDTA, Fe-arginine [Fe(Arg)2], Fe-glycine [Fe(Gly)2], and Fe-histidine [Fe(His)2]. Error bars represent standard error (n = 3). Bars having different letters are significantly different at the 5% level by LSD
Discussion
The iron-amino acid chelates were synthesized using proper amounts of Arg, Gly, or His. These amino acids are abundant in the plant rhizosphere (Jones and other 2004; Rothstein 2009; Werdin-Pfisterer and others 2009) and play significant roles in plant and human nutrition (Amin and others 2011; Li and others 2007). The stability of metal complexes of these amino acids is also high in water.
The results obtained from our study indicated that using Fe-amino acid chelates in the nutrient solution could supply sufficient amounts of Fe for plant uptake (Figs. 3, 4) and also improve root and shoot growth of tomato plants (Figs. 1, 2). The stimulating effect of amino acids on the yield and quality of crops is due to increased mRNA transcription, activation of sugar synthesis, and increasing protein content (Keutgen and Pawelzik 2008; Nassar and others 2003; Rashad and others 2003). Amino acids induce biosynthesis of chlorophyll and thereby improve the photosynthesis rate (Amin and others 2011; Zeid 2009). Several studies (Amin and others 2011; Wang and others 2007; Zeid 2009) have reported the growth-stimulating effect of amino acids. For example, soil application of tryptophan and methionine improved plant growth via increasing auxin and ethylene production in soil and plant tissues and/or increasing the population of beneficial microorganisms (Arshad and Frankenberger 1990; Arshad and others 1995). Furthermore, some authors indicated that the level of free methionine in plants is rate limiting for ethylene production as a component of the complex regulation mechanism for the onset of the Fe-deficiency response (Zuchi and others 2009). Wang and others (2007) found that replacing N–NO3 by 20% in nutrient solution with Arg resulted in a significant increase of fresh and dry matter weight of pak-choi (Brassica chinensis L.) shoots. Replacement of N–NO3 in nutrient solution with glutamic acid and aspartic acid reduced accumulation of excess NO -3 in plant tissues and, as a result, improved crop quality (Wang and others 2007).
Although amino acids used in the present study stimulated plant growth, it is not easy to dissect out whether the effect is due to better Fe uptake, more nitrogen supplied in the form of amino acids, or the hormonal effect of amino acids. Considering the positive and significant correlation between shoot dry matter yield and Fe and N uptake in both tomato cultivars, it seems that both enhanced Fe and N uptakes play roles in improvement of tomato growth. Without a control-free Fe treatment, it is impossible to differentiate the effects of amino acids and Fe on plant growth. Using just the amino acid ligands as the control for such a study would not be useful due to severe growth damage under free Fe conditions. Supplying Fe from Fe-EDTA or other sources was also difficult because of possible interactions between added Fe, amino acids, and other ligands. For example, Sánchez and others (2005) reported a significant interaction between free amino acids added to the nutrient solution and Fe-EDDHA in Fe uptake and plant growth. There were some restrictions to foliar spray of Fe to overcome this problem (to use just amino acid control treatment) because of the differential growth response of plants to various application methods for Fe. The possibility also exists that Fe and amino acid interactions at the root surface significantly affect plant growth as well as amino acid-N and Fe uptake by plants.
In this study, the effect of Fe-amino acid chelates on growth depended on the amino acid type and tomato cultivar (Figs. 1, 2). The growth-stimulating effect of Fe-amino acid chelates on ‘Rani’ was greater than that on ‘Sarika’. In contrast to Fe(Gly)2 and Fe(His)2, addition of Fe(Arg)2 had no effect on shoot and root dry matter weight of the ‘Sarika’ cultivar, whereas it significantly increased shoot and root growth of the ‘Rani’ cultivar. The different response to Fe(Arg)2 application in two tomato cultivars is due to genetic diversity and/or different nutrient requirements of these cultivars. With all treatments, the shoot and root dry matter yield of ‘Rani’ was greater than that of ‘Sarika’. The fact that there was no significant effect of Fe(Arg)2 on growth and shoot Fe and N uptake in ‘Sarika’ is probably because of a lower ability of this cultivar to absorb Fe(Arg)2. The uptake rate of amino acids is dependent on the plant cultivar and amino acid characteristics (Falkengren-Grerup and others 2000; Okamoto and Okada 2004; Svennerstam and others 2008). Okamoto and others (2003) reported a higher ability of sorghum and rice plants to absorb organic nitrogen forms from soil solution than maize and pearl millet. Reeve and others (2009) reported that the effect of Gly addition on N uptake varied among modern and classic wheat cultivars. The greater N uptake by modern wheat cultivars seems to be due to a higher demand for N or greater root-to-shoot transport of this nutrient element in these cultivars (Reeve and others 2009). Large variations among strawberry cultivars in their amino acid uptake have been reported by Reeve and others (2008). Kielland (1994) found that Gly, aspartic acid, and glutamic acid comprised 80% and less than 10% of the total N absorbed by Ledum palustre and Eriophorum angustifolium, respectively. Variation among plant species with respect to amino acid uptake could be due to differences in the number and type of amino acid transporters. Amino acids are taken up by plants via certain transporters, for example, lysine-histidine transporter 1 (LHT1), amino acid permease 1(AAP1), and amino acid permease 5 (AAP5) (Ortiz-Lopez and others 2000; Svennerstam and others 2008). Svennerstam and others (2008) found that reduced expression of LHT1 caused a rapid decrease in Gly and His uptake, whereas it had no effect on Arg uptake. Arginine uptake is dependent on the activity of AAP5 (Svennerstam and others 2008). Therefore, lower plant uptake of Fe(Arg)2 compared with Fe(Gly)2 and Fe(His)2 in the present study could partly be due to differences in the number and type of amino acid transporters in the root cell membrane. On the other hand, the effect of amino acids on plant growth is dependent on their characteristics. Svennerstam and others (2007) found the growth response of Arabidopsis sp. to glutamine application was greater than that to the other amino acids applied. Wang and others (2007) reported that certain amino acids reduced nitrate uptake and thereby inhibited plant growth. In contrast, the addition of some other amino acids improved N uptake and plant growth. In the present study, shoot growth of both tomato cultivars was significantly correlated with shoot N content (Table 2). Tomato plants supplied with Fe(His)2 and Fe(Gly)2 had higher N in their shoots and thus produced greater biomass compared with those plants supplied with Fe(Arg)2. Another possible reason for the smaller response of tomato plants to Fe(Arg)2 is its larger molecular size (Table 1) and, thus, the lower uptake of this amino acid chelate compared with the other amino chelates used. This suggestion needs to be tested in further experiments, particularly considering that the estimated diameter of Fe-amino chelate molecules is smaller than the cell wall pore diameter. Thus, there seems to be no limitation on the movement of amino chelates via these free spaces toward the cell membrane.
Regardless of crop cultivar, tomato plants supplied with Fe-amino acid chelates accumulated higher amounts of Fe in their shoots compared with those supplied with Fe-EDTA (Fig. 4). To better understand the effect of Fe-amino acid chelates on the nutritional status of Fe in tomato, the activity of CAT and APX was measured (Figs. 8, 9). Based on results obtained from several studies (Dasgan and others 2003; Ruiz and others 2000), biochemical indices such as activity of Fe-containing enzymes are much better indices to show the plant nutritional status of Fe than is shoot Fe concentration. The effect of Fe-amino acid chelates on the shoot activity of CAT varied with tomato cultivar and amino acid type (Fig. 8). Although Fe(Gly)2 increased the activity of CAT in the shoots of ‘Rani’, it had no effect on the activity of this enzyme in the shoots of ‘Sarika’. CAT activity was greater in both cultivars, whereas shoot Fe uptake was smaller in plants treated with Fe(Arg)2 than in plants treated with Fe(His)2 and Fe(Gly)2. Higher activity of CAT in the presence of Fe(Arg)2 is probably due to the role of Arg in the expression of genes encoding the CAT enzyme. It has been reported that Arg plays a role in several enzymatic activities within plants. Arginine and polyamines are involved in the structure and function of several proteins and antioxidant enzymes, particularly the CAT enzyme (Drolet and others 1986; Kuznetsov and Shevyakova 2007; Lovass 1991). In ‘Sarika’, increasing shoot Fe content was associated with elevated APX activity in the leaves. No significant difference was found in leaf activity of APX between plants fed with the Fe(Arg)2 and those fed with Fe-EDTA (Fig. 9). In addition, application of Fe(Arg)2 had no significant effect on shoot Fe content of the ‘Sarika’ cultivar. This result suggests greater dependency of APX activity on the Fe nutritional status of tomato compared with CAT. Elevated shoot Fe content and activity of CAT and APX in the presence of Fe-amino acid chelates indicates improvement of plant nutritional Fe status in comparison with Fe-EDTA treatment. In line with our results, Sánchez and others (2005) reported that addition of amino acids in the nutrient solution improved Fe uptake by tomato. Amino acids can form soluble complexes with Fe and thereby play an important role in maintaining Fe availability for the plant (Zhou and others 2007). It has been shown that due to a larger molecular size, plant uptake of synthesized chelates (for example, Fe-EDTA and Fe-DTPA) is much lower than that of free metal cations (Marschner 1995). As mentioned before, the molecular structure of our synthesized Fe-amino acid chelates, designed using Hyperchem software, indicated that the molecular diameter of Fe-amino acid chelates is much smaller than the size of the cell wall pores (<5 nm) (Marschner 1995). Therefore, cell wall pores have no inhibitory effect on the movement of Fe-amino acid chelates into the free apoplasmic spaces, and thus Fe-amino acid chelates can pass easily through the cell wall and enter into the free spaces of the root apoplasm.
Improved Fe nutritional status of tomato plants supplied with Fe-amino acid chelates compared with Fe-EDTA-supplied plants could also be related to improved N nutritional status. Nitrogen nutritional management affects the number and activity of Fe-carrier proteins on the root cell membranes and thereby increases uptake and translocation of Fe in the plant tissues (Curie and others 2009; Murata and others 2008). Some field and pot experiments (Cakmak and others 2010; Shi and others 2010) indicated an improved concentration of Fe in wheat shoots and grain with the addition of N. An elevated Fe and Zn content in the shoots of wheat by N nutrition has also been reported by Kutman and others (2010). Although the plants absorb N in the form of N–NO3 or N–NH4, there is some evidence showing direct absorption of amino acids by plants (Jämtgård and others 2008; Näsholm and others 2009; Tida and others 2009). For example, Arg is an essential amino acid containing several N atoms (Abdul-Qados 2009). Glycine is easily converted to ammonium, amide, and aliphatic compounds and can be used as an N source (Schmidt and Stewart 1999).
Although the net influx and translocation of Fe and N in tomato plants were not measured in the present study, the close correlation between shoot N and Fe contents (Table 2) suggests a role of synthesized Fe-amino acid chelates in the translocation of Fe from roots to shoots. Translocation of amino acids in the plant is an important process (Ortiz-Lopez and others 2000). In contrast to assimilated carbon that is translocated and restricted to the phloem, amino acid translocation occurs in both the phloem and xylem. This translocation of amino acids in phloem and xylem helps nitrogen recycling between the roots and shoots and hastens retranslocation of nutrient elements, particularly immobile elements (for example, Fe and Zn) in the plant (Caputo and Barneix 1997; Owen and Jones 2001). In a trend similar to that of Fe, shoot Zn content was enhanced in the presence of amino acid chelates (Fig. 6).
Conclusion
Results obtained from the present study showed that in general, the Fe–amino acid complexes Fe(Arg)2, Fe(His)2, and Fe(Gly)2 improved uptake and translocation of Fe, Zn, and N in comparison with Fe-EDTA and thus resulted in higher root and shoot growth of tomato. Elevated activity of CAT and APX confirmed the improvement of plant nutritional status of Fe. According to the results, Fe-amino acid chelates can be used as an alternative to Fe-EDTA to supply Fe in nutrient solutions. Although transport into the plant tissues is considered a potential loss of the chelator from the hydroponic solution, this problem can be resolved by frequent addition of amino acid chelates into the nutrient solution. Further research is needed to investigate the fate of these Fe-amino acid chelates in commercial hydroponic nutrient solutions where solutions are recycled for economic and environmental reasons.
References
Abdul-Qados AMS (2009) Effect of arginine on growth, yield and chemical constituents of wheat grown under salinity condition. Acad J Plant Sci 2:267–278
Albano JP, Miller WB (2001) Photodegradation of FeDTPA in nutrient solutions. I. Effects of irradiance, wavelength and temperature. Hortscience 36:313–316
Amin AA, Gharib AEF, El-Awadia M, Rashad ESM (2011) Physiological response of onion plants to foliar application of putrescine and glutamine. Sci Hortic 129:353–360
Aravind P, Prasad MNV (2005) Cadmium-induced toxicity reversal by zinc in Ceratophyllum demersum L. (a free floating aquatic macrophyte) together with exogenous supplements of amino- and organic acids. Chemosphere 61:1720–1733
Arshad M, Frankenberger WTJ (1990) Response of Zea mays and Lycopersicon esculentum to the ethylene precursors, L-methionine and L-ethionine applied to soil. Plant Soil 122:219–227
Arshad M, Hussain A, Shakoor A (1995) Effect of soil applied L-tryptophan on growth and chemical composition of cotton. J Plant Nutr 18:317–329
Bell PF, Chaney RL, Angle JS (1991) Free metal activity and total metal concentrations as indices of micronutrient availability to barley (Hordeum vulgare L.) Klages. Plant Soil 130:51–62
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bremmer JM, Mulvancey CS (1982) Total Nitrogen. In: Page AL, Miller RH, Keeney DR (eds) Method of Soil Analysis part II. Madison, WI, ASA and SSSA, pp 599–622
Brynhildsen L, Rosswall T (1995) Effects of metals on the microbial mineralization of organic acids. Water Air Soil Poll 94:45–57
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Cakmak I, Kalayci M, Kaya Y, Torun AA, Aydin N, Wang Y, Arisoy Z, Erdem H, Gokmen O, Ozturk L, Horst WJ (2010) Biofortification and localization of zinc in wheat grain. J Agr Food Chem 58:9092–9102
Caputo C, Barneix AJ (1997) Export of amino acids to the phloem in relation to N supply in wheat. Physiol Plantarum 101:853–860
Chapman HD, Pratt PF (1961) Methods of analysis for soils, plants, and waters. Priced Publication 4034. Division of Agriculture Sciences, University of California, Berkeley
Curie C, Cassin G, Couch D, Divol F, Higuchi K, Jean ML, Misson J, Schikora A, Czernic P, Mari S (2009) Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot 103:1–11
Dasgan HY, Römheld V, Cakmak I, Abak K (2002) Physiological root responses of iron deficiency susceptible and tolerant tomato genotypes and their reciprocal F1 hybrids. Plant Soil 241:97–104
Dasgan HY, Ozturk L, Abak K, Cakmak I (2003) Activities of iron-containing enzymes in leaves of two tomato genotypes differing in their resistance to Fe chlorosis. J Plant Nutr 26:1997–2007
Drolet G, Dumbroff EB, Legg RL, Thompson JE (1986) Radical scavenging properties of polyamines. Phytochemistry 25:367–371
Falkengren-Grerup U, Månsson KF, Olsson MO (2000) Uptake capacity of amino acids by ten grasses and forbs in relation to soil acidity and nitrogen availability. Environ Exp Bot 44:207–219
Hangarter RP, Stasinopoulos TC (1991) Effect of Fe-catalyzed photooxidation of EDTA on root-growth in plant culture media. Plant Physiol 96:843–847
Jämtgård S, Näsholm T, Huss-Danell K (2008) Characteristics of amino acid uptake in barley. Plant Soil 302:221–231
Jones DL, Hodge A (1999) Biodegradation kinetics and sorption reactions of three differently charged amino acids in soil and their effects on plant organic nitrogen availability. Soil Biol Biochem 31:1331–1342
Jones DL, Hodge A, Kuzyakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163:459–480
Keutgen A, Pawelzik E (2008) Contribution of amino acids to strawberry fruit quality and their relevance as stress indicators under NaCl salinity. Food Chem 111:642–647
Kielland K (1994) Amino acid absorption by arctic plants: implications for plant nutrition and nitrogen cycling. Ecology 75:2373–2383
Kutman UB, Yildiz B, Ozturk L, Cakmak I (2010) Biofortification of durum wheat with zinc through soil and foliar applications of nitrogen. Cereal Chem 87:1–9
Kuznetsov VV, Shevyakova NI (2007) Polyamines and stress tolerances of plants. Plant Stress 1:50–71
Li P, Yin YL, Li D, Kim SW, Wu G (2007) Amino acids and immune function. Br J Nutr 98:237–252
Lovass E (1991) Antioxidative effects of polyamines. J Am Oil Chem Soc 68:353–358
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, San Diego, CA
Metsarinne S, Tuhkanen T, Aksela R (2001) Photodegradation of ethylenediaminetetraacetic acid (EDTA) and ethylenediamine disuccinic acid (EDDS) within natural UV radiation range. Chemosphere 45:949–955
Metsarinne S, Rantanen P, Aksela R, Tuhkanen T (2004) Biological and photochemical degradation rates of diethylenetriaminepentaacetic acid (DTPA) in the presence and absence of Fe(III). Chemosphere 55:379–388
Murata Y, Harada E, Sugase K, Namba K, Horikawa M, Ma JF, Yamaji N, Ueno D, Nomoto K, Iwashita T, Kusumoto S (2008) Specific transporter for iron(III)-phytosiderophore complex involved in iron uptake by barley roots. Pure Appl Chem 80:2689–2697
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Näsholm T, Kielland K, Ganeteg U (2009) Uptake of organic nitrogen by plants. New Phytol 182:31–48
Nassar AH, El-Tarabily KA, Sivasithamparam K (2003) Growth promotion of bean (Phaseolus vulgaris L.) by a polyamine-producing isolate of Streptomyces griseoluteus. Plant Growth Regul 40:97–106
Nowack B, Baumann U (1998) Biodegradation of the photolysis products of Fe(III)EDTA. Acta Hydroch Hydrob 26:104–108
Okamoto M, Okada K (2004) Differential responses of growth and nitrogen uptake to organic nitrogen in four gramineous crops. J Exp Bot 55:1577–1585
Okamoto M, Okada K, Watanabe T, Ae N (2003) Growth responses of cereal crops to organic nitrogen in the field. Soil Sci Plant Nutr 49:445–452
Ortiz-Lopez A, Chang HC, Bush DR (2000) Amino acid transporters in plants. Biochim Biophys Acta 1465:275–280
Owen AG, Jones DL (2001) Competition for amino acids between wheat roots and rhizosphere microorganisms and the role of amino acids in plant N acquisition. Soil Biol Biochem 33:651–657
Parker DR, Norvell WA (1999) Advances in solution culture methods for plant mineral nutrition research. In: Advances in Agronomy. San Diego, CA: Academic Press, pp 561–566
Rashad ESM, El-Abagg HM, Amin AA (2003) Physiological effects of some bioregulators on growth and productivity of two broad bean cultivars. Egypt J Appl Sci 18:132–149
Reeve JR, Smith JL, Carpenter-Boggs L, Reganold JP (2008) Soil-based cycling and differential uptake of amino acids by three species of strawberry (Fragaria spp.) plants. Soil Biol Biochem 40:2547–2552
Reeve JR, Smith JL, Carpenter-Boggs L, Reganold JP (2009) Glycine, nitrate, and ammonium uptake by classic and modern wheat varieties in a short-term microcosm study. Biol Fert Soils 45:723–732
Renella G, Landi L, Nannipieri P (2004) Degradation of low molecular weight organic acids complexed with heavy metals in soil. Geoderma 122:311–315
Rivero RM, Sánchez E, Ruiz JM, Romero L (2003) Iron metabolism in tomato and watermelon plants: influence of nitrogen source. J Plant Nutr 26:2413–2424
Rothstein DE (2009) Soil amino acid availability across a temperate-forest fertility gradient. Biochemistry 92:201–215
Ruiz JM, Baghour M, Romero L (2000) Efficiency of the different genotypes of tomato in relation to foliar content of Fe and the response of some bioindicators. J Plant Nutr 23:1777–1786
Sánchez AS, Juárez M, Sánchez-Andreu J, Jordá J, Bermúdez D (2005) Use of humic substances and amino acids to enhance iron availability for tomato plants from applications of the chelate FeEDDHA. J Plant Nutr 28:1877–1886
Schmidt S, Stewart GR (1999) Glycine metabolism by plant roots and its occurrence in Australian plant communities. Aust J Plant Physiol 26:253–264
Shi R, Zhang Y, Chen X, Sun Q, Zhang F, Römheld V, Zou C (2010) Influence of long term nitrogen fertilization on micronutrient density in grain of winter wheat (Triticum aestivum L.). J Cereal Sci 51:165–170
Svennerstam H, Ganeteg U, Bellini C, Näsholm T (2007) Comprehensive screening of Arabidopsis mutants suggests the Lysine Histidine Transporter 1 to be involved in plant uptake of amino acids. Plant Physiol 143:1853–1860
Svennerstam H, Ganeteg U, Bellini C, Näsholm T (2008) Root uptake of cationic amino acids by Arabidopsis depends on functional expression of amino acid permease 5. New Phytol 180:620–630
Svenson A, Kaj L, Bjorndal H (1989) Aqueous photolysis of the iron(III) complexes of NTA, EDTA and DTPA. Chemosphere 18:1805–1808
Tida G, Song S, Roberts P, Jones DL, Huang D, Iwasaki K (2009) Amino acids as a nitrogen source for tomato seedlings: The use of dual-labeled (13C, 15N) glycine to test for direct uptake by tomato seedlings. Environ Exp Bot 66:357–361
Vadas TM, Zhang X, Curran AM, Ahner BA (2007) Fate of DTPA, EDTA and EDDS in hydroponic media and effects on plant mineral nutrition. J Plant Nutr 30:1229–1246
Vassil AD, Kapulnik YI, Salt DE (1998) The role of EDTA in lead transport and accumulation by Indian mustard. Plant Physiol 117:447–453
Wang HJ, Wu LH, Wang MY, Zhu YH, Tao QN, Zhang FS (2007) Effects of amino acids replacing nitrate on growth, nitrate accumulation, and macroelement concentrations in pak-choi (Brassica chinensis L.). Pedosphere 17:595–600
Werdin-Pfisterer NR, Kielland K, Boone RD (2009) Soil amino acid composition across a boreal forest successional sequence. Soil Biol Biochem 41:1210–1220
Wu L, Mo L, Fan Z, Tao Q, Zhang F (2005) Absorption of glycine by three agricultural species under sterile sand culture conditions. Pedosphere 15:286–292
Zeid IM (2009) Effect of arginine and urea on polyamines content and growth of bean under salinity stress. Acta Physiol Plant 31:65–70
Zhou Z, Zhou J, Li R, Wang H, Wang J (2007) Effect of exogenous amino acids on Cu uptake and translocation in maize seedlings. Plant Soil 292:105–117
Zuchi S, Cesco S, Varanini Z, Pinton R, Astolfi S (2009) Sulphur deprivation limits Fe-deficiency responses in tomato plants. Planta 230:85–94
Acknowledgment
This research was financially supported by Support Box of Iranian Researcher (Project No. 88002077).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghasemi, S., Khoshgoftarmanesh, A.H., Hadadzadeh, H. et al. Synthesis of Iron-Amino Acid Chelates and Evaluation of Their Efficacy as Iron Source and Growth Stimulator for Tomato in Nutrient Solution Culture. J Plant Growth Regul 31, 498–508 (2012). https://doi.org/10.1007/s00344-012-9259-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-012-9259-7