Abstract
The sequence of events and the signals that regulate the remobilization of nitrogen (N) reserves during senescence induced by N starvation were studied in leaf 3, the last fully expanded leaf, in 17-day-old wheat (Triticum aestivum L.) plants. The first event observed was a rapid decrease in the isopentenyl adenosine (iPA) concentration during the first 24 h of N starvation. No differences in t-zeatin riboside and dihydrozeatin riboside concentrations were observed until the end of the assay. During the following 6 days, a decrease in soluble amino acids, chlorophyll, and protein, as well as an increase in soluble sugar concentration and endoproteolytic activity, could be observed. At day 3 of the experiment, the abscisic acid (ABA) concentration in the leaves of N-deprived plants started to increase. After 6 days of N deprivation there was a rise in oxidative stress, as indicated by the increase in malondialdehyde concentration, as well as a decrease in the activities of antioxidant enzymes catalase and ascorbate peroxidase. To analyze interactions with leaf development, the first, second, third, and fourth leaves were studied. iPA concentration decreased in all the leaf stages, including leaf 4, which was not fully expanded. A linear correlation between iPA and protein concentration was determined. These results suggest that the sharp fall in iPA could be the earliest event that induces protein degradation during the development of senescence induced by N deficiency, and that only later is ABA accumulated and oxidative stress developed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Senescence is a genetically programmed process that involves a general dismantling of cellular structures, followed by a mobilization of the degradation products to other parts of the plant (Woo and others 2004). The nitrogen (N) remobilization from vegetative tissues constitutes the main source of N that accumulates in the grain, affecting grain protein concentration and flour-baking quality. In soils with low N availability, remobilization is very efficient, with very little N remaining in the straw at maturity. However, when N supply in the soil is abundant, N remobilization is inhibited and a high N concentration remains in the vegetative organs, decreasing the N harvest index and the efficiency of fertilization. The enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) represents the major fraction of the N content in mesophyll cells and its degradation is critical for an efficient N remobilization (Hörtensteiner and Feller 2002). However, the mechanisms by which this enzyme is degraded are far from clear.
Leaf senescence is controlled by many internal and external factors and can be accelerated or delayed by alteration of these signals (Feller and Fischer 1994). Among the internal factors there are plant growth regulators, C/N relationship, reactive oxygen species (ROS), and sugar concentration (Gan and Amasino 1997; Yang and others 2002; Buchanan-Wollaston and others 2003). Cytokinins are plant growth regulators that have a delaying effect on senescence. Physiologic studies have demonstrated that in many plant species, exogenous application of cytokinin causes a delay in senescence, and that the level of endogenous cytokinins decreases in most senescing tissues (Gan and Amasino 1997; Buchanan-Wollaston and others 2003). In contrast to cytokinins, abscisic acid (ABA) treatment often promotes senescence. Furthermore, it has been shown that levels of endogenous ABA increase in senescent leaves that are induced by different stresses (Noodén and others 1997; Yang and others 2002). ROS increase with the progression of senescence, a process generally triggered by a decline in the activity of antioxidant enzymes such as catalase and ascorbate peroxidase (Buchanan-Wollaston 1997; Pastori and del Rio 1997; Prochaskova and others 2001; Munné-Boch and Alegre 2004).
Nitrogen stress is one of the main causes of leaf senescence in field-grown wheat plants, promoting proteolysis and N remobilization and, ultimately, leaf senescence. In this article we subjected young wheat plants to N deficiency to understand the sequence of events and the signals that trigger protein degradation and N remobilization in leaves.
Materials and Methods
Plant Material and Growth Conditions
Wheat (Triticum aestivum L. var. Pro INTA Isla Verde) seeds were germinated on moist filter paper at 25°C in the dark. The seedlings were transferred to plastic pots containing vermiculite. Plants were kept in a growth chamber at 25°C with a photoperiod of 16 h and a photosynthetic photon flux density of 300 μmol m−2 s−1. Each pot was supplied with 50 ml of nutrient solution according to Hoagland and Arnon (1950) with 10 mM KNO3.
N Deficiency and Senescence in Leaf 3 Seventeen days after sowing (day 0), plants were divided into two groups: one group remained under the same nutritive condition (N+ plants), whereas the second one was deprived of NO −3 supply (N− plants). The third leaf from the base of the five plants in each pot was collected at different times and pooled. Samples were weighed, immediately frozen in liquid nitrogen, and stored at −50°C until used for biochemical determinations. When significant differences in biochemical determinations between N+ and N− plants were found at day 3, experiments were repeated and plants sampled at shorter intervals (1, 2, and 3 days).
N Deficiency and Senescence of the Whole Plant Seventeen-day-old plants were treated, sampled, weighed, and stored as indicated above, but the first, second, third, and fourth leaves from the base were collected.
Chlorophyll, Protein, Amino Acids, Soluble Sugars and Endoproteolytic Activity Analysis
Leaf tissue was homogenized (2.5 ml buffer g−1 fresh weight) with 50 mM Tris-HCl buffer pH 7.5 containing 1% (w/v) polyvinylpolypyrrolidone (PVPP). The homogenates were filtered and centrifuged at 10,000g for 20 min. The supernatant was used for determination of soluble protein and endoproteolytic activity. All the procedures were performed at 4°C. For amino acids and sugar analysis, aliquots of homogenate were quickly precipitated with trichloroacetic acid (TCA) or boiled respectively. Protein concentration was determined according to Bradford (1976) using bovine serum albumin as a standard. Total free amino acids were quantified with ninhydrin reagent (Yemm and Cocking 1955). Soluble sugar concentration was determined with anthrone reagent following Yemm and Willis (1954). Endoproteolytic activity was measured using azocasein as substrate, as previously described by Roberts and others (2002). One enzymatic unit (EU) of azocaseinolytic activity was defined as the amount of protein causing a 0.01 increase in A340 over the 0 time values (the reaction stopped immediately after starting). Chlorophyll content was determined in the filtered homogenates before centrifugation according to Arnon (1949).
Catalase and Ascorbate Peroxidase (APX) Activities
Catalase and APX were measured in soluble extract obtained following the above-mentioned procedure, although using a different extraction buffer (50 mM potassium phosphate buffer pH 7.8, 5 mM ascorbate, 1 mM EDTA, and 1% PVPP). Catalase activity was measured according to Aebi (1984) by recording the changes in optical density (OD) at 240 nm. The enzymatic activity was expressed as μmol H2O2 reduced per min per g fresh weight (FW) using the extinction coefficient of 38 M−1 cm−1. The APX activity was monitored by the decrease in absorbance at 290 nm as described by Nakano and Asada (1981). The enzymatic activity was expressed as ascorbate oxidized per min per g FW using the extinction coefficient of 2.8 mM−1 cm−1.
SDS-PAGE
Protein extracts were analyzed by 15% SDS-PAGE according to Laemmli (1970). The same amount of extract corresponding to 3 mg of leaf fresh weight was loaded in each lane. For visualization of proteins, gels were stained with 0.1% (w/v) Coomassie Brilliant Blue R-250.
Ascorbate and Malondialdehyde (MDA) Analysis
Ascorbate and MDA were extracted from leaf tissues (0.3 g FW) with 3 ml 20% TCA and quantified spectrophotometrically according to the method of Law and others (1983) and Heath and Packer (1968), respectively.
Plant Growth Regulators Analysis
Isopentenyl adenosine (iPA), zeatin riboside (t-ZR), dihydrozeatin riboside (DHZR), and ABA were extracted from leaf tissue according to He and Loh (2002). Leaf tissue (1 g FW) was homogenized in 4 ml 80% ethanol followed by 2 h of incubation at 4°C. After centrifugation at 1670g for 3 min, the supernatant was transferred to another centrifuge tube. The tissues were then re-extracted with 2 ml 80% ethanol and centrifuged, and the supernatants were pooled together. The total extracts were dried under vacuum and dissolved with 1 ml Tris-buffered saline, 25 mM Tris pH 7.5, 0.1 mM NaCl, 1 mM MgCl2, and 3 mM sodium azide. The solutions were then filter-sterilized (Millipore, 0.2 μm) and the samples were stored at −20°C. iPA, t-ZR, DHZR, and ABA concentrations were determined by ELISA using monoclonal antibodies (Phytodetek, Agdia, Elkhart, IN, USA) following the protocols provided by the manufacturer.
Statistical Treatment
ANOVA and regression analyses of the data were performed with Statgraphics® software (StatPoint, Inc., Herndon, VA, USA). All the experiments were repeated independently at least twice. Data from each sampling date were analyzed separately. The results from the different experiments were very similar; therefore, only one is reported in this article.
Results
N Deficiency and Senescence in Leaf 3
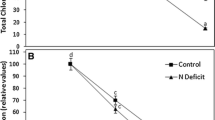
Chlorophyll and protein concentration in leaf 3 of N− plants decreased after plants were exposed to N deprivation and continued to diminish until the end of the assay, when this concentration reached the minimal value (Figure 1A, B). No significant differences in chlorophyll and protein concentration between the N− and N+ plants could be detected up to the second day of sampling (Figure 1A, B, insets). The endoproteolytic activity increased from day 6 until day 12, when it reached almost 150% of the initial value (Figure 1C).
Amino acids concentration in the leaves of N− plants decreased rapidly at day 2 (Figure 2A, inset) and then continued to diminish at a lower rate (Figure 2A). The leaf soluble sugars concentration increased 2.3-fold at day 3 in the N− plants and continued to increase until day 9 (Figure 2B).
Rubisco Degradation
When extracts of leaves subjected to N deprivation were analyzed by SDS-PAGE, a decrease in the intensity of all polypeptide bands could be observed, including both Rubisco subunits (Figure 3B). No substantial changes could be observed in the protein profile of the leaves of N+ plants (Figure 3A).
Development of Oxidative Stress
The activity of catalase and APX, two enzymes responsible for H2O2 scavenging, declined in leaves of N− plants (Figure 4). Catalase activity was similar up to day 6 in N+ and N− plants. Thereafter this activity decreased in N− plants, while remaining almost constant in N+ plants (Figure 4A). APX activity slowly decreased up to day 9 and rapidly decreased from then on until day 12 in the leaves of N− plants. The difference between the two treatments started to be significant from day 9 (Figure 4B). Ascorbate concentration (a nonenzymatic antioxidant) increased slowly up to day 6 in N− plants and from then on increased more rapidly, reaching values almost four-fold higher than the initial value at day 12 (Figure 4C). Lipid peroxidation, as shown by MDA accumulation, increased slowly up to day 6 in N− plants and thereafter showed a rapid increase up to 4.5-fold at the end of the experiment (Figure 4D).
Changes in Plant Growth Regulator Levels
The cytokinins t-ZR and DHZR showed no significant changes in leaf 3 due to N deprivation (Figure 5B, C). However, iPA concentration sharply decreased in leaves of N− plants at 24 h of N deprivation (Figure 5A, inset) and remained constant during the rest of the experiment (Figure 5A). In leaves of N+ plants, the concentration remained almost constant up to day 9 and from then on it began to decrease (Figure 5A). On the other hand, ABA concentration was higher in the N-deficient leaves than in the nondeficient only at day 3 of N deprivation (Figure 5D, inset) and continued to increase until day 12 (Figure 5D).
N Deficiency and Senescence of the Whole Plant
The changes in soluble protein concentration observed in leaves in response to N deficiency varied with the leaf level. At time 0 the leaf protein concentration decreased with leaf age, leaf 1 showing the lowest concentration and the not fully expanded leaf 4 the highest (Figure 6). With the progression of the experiment the protein concentration showed a slow decrease in the N-fed plants, except in leaf 4, where it initially increased, whereas in the N-starved plants protein concentration decreased in all leaves (Figure 6). No differences in the fresh weight/dry weight ratio were detected until day 12 (data not shown).
When iPA concentration was measured in leaves at different developmental stages, its concentration was highest in the younger leaves and decreased with leaf age. After 24 h of N deprivation a decrease in iPA concentration in all leaf stages was observed (Figure 7A).
iPA concentration in leaves at different developmental stages from N+ (
 ) and N− (
) and N− ( ) wheat plants after 24 h of treatment (A). Relation between iPA and protein concentration in leaves 1–4 (
) wheat plants after 24 h of treatment (A). Relation between iPA and protein concentration in leaves 1–4 (
 ) at time 0 (B) and in leaves of N+ and N− wheat plants after 24 h of treatment (C). Bars indicate ± SE of the mean, n = 4. No bar indicates SE smaller than the symbol
) at time 0 (B) and in leaves of N+ and N− wheat plants after 24 h of treatment (C). Bars indicate ± SE of the mean, n = 4. No bar indicates SE smaller than the symbol
When iPA concentration in leaves was plotted against the soluble protein concentration in every leaf level, a linear relation was observed at day 0 and day 1 in N+ and N− plants (Figure 7B, C).
Discussion
When the effect of N deficiency in leaf 3 was followed, we were able to observe a sequence of events with different timings. The first event detected was a sharp drop in iPA levels that occurred only after 24 h of N deprivation (Figure 5A). It is generally assumed that cytokinin is one of the major regulators of plant senescence. Treatment with cytokinins has been shown to delay leaf senescence in many plants (Yang and others 2002; Noodén and others 1997). Transgenic tobacco plants expressing a cytokinin biosynthesis gene (isopentenyl transferase, ipt) under the control of the senescence-specific promoter SAG12 remained green and nonsenescent for an extended period of time (Gan and Amasino 1995; Wingler and others 1998). Recently, He and others (2005) compared hormonal concentration of different senescence types of maize and found that the concentration of t-ZR, DHZR, and iPA in the leaves was higher in stay-green type than in the earlier senescent. They showed that t-ZR and DHZR, but not iPA concentration, decreased during grain filling in both cultivars.
We did not detect any significant changes in the concentration of t-ZR and DHZR during the experiments (Figure 5B, C). Similar results were reported by Yang and others (2003), who observed a very slow decrease in the concentration of zeatin + t-ZR during age-dependent senescence in wheat flag leaves. Cytokinins are structurally diverse and biologically versatile, and although their chemistry and physiology have been studied extensively, the regulation of cytokinin biosynthesis, metabolism, and signal transduction is still undefined (Mok and Mok 2001). Thus, it is possible that iPA and not t-ZR and DHZR has a physiologic function in the induction of leaf senescence. However, it should be regarded that other cytokinins not measured in this study could be involved in senescence regulation.
The second event observed was a decrease in protein, chlorophyll, Rubisco, and amino acid concentration, as well as the increase in sugar concentration in the third leaf of N− plants (Figures 1–3). This event occurred during the first 3 days. These results are in agreement with the ordered breakdown of the photosynthetic apparatus and nutrient recycling function of leaf senescence, which has been extensively reported by various authors (Noodén and others 1997; Buchanan-Wollaston and others 2003). Some amino acids are known to be transcriptional and post-transcriptional regulators of many intermediates of the N assimilatory pathway, such as nitrate transporters, nitrate (NR) and nitrite reductase (NiR), glutamine synthetase and glutamate dehydrogenase (Stitt and others 2002; Masclaux-Daubresse and others 2005). The decrease in soluble amino acids concentration observed in these experiments has been described as one of the earliest metabolic changes after N deprivation (Barneix and Causin 1996) and a consequence of the decrease in NR and NiR activities (Miller and Cramer 2004). These enzymes are induced by the presence of NO −3 , but in the presence of other N sources, NH +4 for instance, they are not induced and plants grow without any sign of senescence.
The third event that occurred in the third leaf of N− plants was the development of oxidative stress at day 9, as shown by MDA accumulation. The increased MDA concentration coincided with a decrease in the activities of the antioxidant enzymes APX and catalase, despite the increase in nonenzymatic antioxidant ascorbate concentration in leaves of N− plants (Figure 4A–C).
ABA, a growth regulator produced by the plant in response to different stress conditions, has also been associated with the induction of senescence (Yang and others 2002; He and others 2006). In the present experiments, increased ABA concentration in leaves of N-deprived plants occurred only at day 3 of N deficiency and continued to increase until day 12 (Figure 5D). Recent evidence has demonstrated that ABA accumulation triggers the generation of ROS in water-stressed plants (Jiang and Zhang 2002; Hu and others 2006). Accordingly, the oxidative stress in the N− plants occurred only after ABA started accumulating (Figures 4, 5D) when most protein had already been hydrolyzed. However, at this point we cannot be certain if the oxidative stress was triggered by an increased ABA concentration or if it was a consequence of the breakdown of components of the photosynthetic apparatus.
The initiation phase of the senescence mechanism may vary in different inducing conditions (Yoshida 2003). Yang and others (2002) observed that ABA first increased as soon as water deficit was imposed on rice plants, whereas zeatin + t-ZR showed almost no response during the initial treatment days. On the other hand, we observed that iPA concentration decreased on the first day of N starvation, whereas ABA increased at day 3. Although to our knowledge there are no reports that showed the decrease in iPA concentration and the increase in ABA concentration during N-stressed treatment, there are some reports that relate iPA and N availability. It has been reported that nitrate resupply to N-deficient plants induces an increase in cytokinin concentration and that plants grown with low N supply show a lower cytokinin concentration than those supplied with high N (Takei and others 2002; Kakimoto 2003). When we followed the senescence induced by N deficiency in the whole plant, we observed that the iPA concentration declined in all leaf stages (Figure 7A). Furthermore, iPA concentration decreased with age in both N+ and N− plants (Figure 7A), as did leaf protein concentration (Figure 6). Thus, we determined a lineal relation between iPA and protein concentration (Figure 7B). Given the time of occurrence of both events and the association observed between iPA and protein concentrations, we consider that a decrease in iPA content may act as a more sensitive signal for N remobilization in N-deficient plants than ABA. Unfortunately, the mutual crosstalk between cytokinins and ABA in the signaling of regulation of the senescence process is poorly documented.
It is known that leaves of many annual crop plants become predisposed to senesce near the time of full expansion and that the subsequent rate of senescence can be influenced by many factors, including source:sink ratio, growth regulators, and plant nutrition (Crafts-Brandner and others 1998). In agreement with these observations, the present experiments showed that the symptoms of senescence in leaf 3 of N+ plants were initiated after the leaf became fully expanded, as indicated by the decrease in chlorophyll and protein concentration, and that imposing N stress at this developmental stage enhanced the rate of leaf senescence (Figure 1). On the other hand, when we studied the protein concentration of leaf 1, 2, 3 and 4 we observed that leaf 4 (not fully expanded) had a different pattern. The protein concentration in leaf 4 of N+ plants increased up to day 3 and only then did it start to decrease (Figure 6D), but when we removed N from the nutrient solution the protein concentration already decreased at day 3, indicating the onset of the senescence process (Figure 6D). In this way we separated the effect of age-dependent senescence and N-deficiency-induced senescence in an intact plant system. Because in the N− plants iPA decreased in all leaf stages, including leaf 4 (Figure 7), we suggest that iPA concentration is a signal that triggers proteolysis induced by N starvation in leaves in all developmental stages.
The present results indicate that in wheat plants both leaf age and N status affect iPA concentration. iPA and protein concentration decrease with leaf age, whereas N-deficiency conditions induce a rapid decrease of iPA, and probably other cytokinins, that promotes degradation of macromolecules to supply nutrients to the younger organs. Only later is ABA accumulated and oxidative stress developed.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:1221–1226
Arnon DI (1949) Cooper enzymes in chloroplasts. Polyphenoloxydases in Beta Vulgaris. Plant Physiol 24:1–15
Barneix AJ, Causin HF (1996) The central role of amino acids on nitrogen utilization and plant growth. J Plant Physiol 149:358–362
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem 72:248–254
Buchanan-Wollaston V (1997) The molecular biology of leaf senescence. J Exp Bot 48:181–199
Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D (2003) The molecular analysis of leaf senescence – a genomics approach. Plant Biotechnol J 1:3–22
Crafts-Brandner SJ, Hölzer R, Feller U (1998) Influence of nitrogen deficiency on senescence and the amounts of RNA and proteins in wheat leaves. Physiol Plant 102:192–200
Feller U, Fischer A (1994) Nitrogen metabolism in senescing leaves. Crit Rev Plant Sci 13:241–273
Gan S, Amasino RM (1995) Inhibition of leaf senesce by autoregulated production of cytokinin. Science 270:1986–1988
Gan S, Amasino RM (1997) Making sense of senescence. Molecular genetic regulation and manipulation of leaf senescence. Plant Physiol 113:313–319
He P, Osaki M, Takebe M, Shinano T, Wasaki J (2005) Endogenous hormones and expression of senescence-related genes in different senescent types of maize. J Exp Bot 56:1117–1128
He YW, Loh CS (2002) Induction of early bolting in Arabidopsis thaliana by triacontano, cerium and lanthanum is correlated with increased endogenous concentration of isopentenyl adenosine (iPAdos). J Exp Bot 53:505–512
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. (. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 25:189–198
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Calif Agric Exp Sta Circ 347:1–39
Hörtensteiner S, Feller U (2002) Nitrogen metabolism and remobilization during senescence. J Exp Bot 53:927–937
Hu X, Zhang A, Zhang J, Jiang M (2006) Abscisic acid is a key inducer of hydrogen peroxide production in leaves of maize exposed to water stress. Plant Cell Physiol 47:1484–1495
Jiang M, Zhang J (2002) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53:2401–2410
Kakimoto T (2003) Perception and signal transduction of cytokinins. Annu Rev Plant Biol 54:605–627
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem J 210:899–903
Masclaux-Daubresse C, Carrayol E, Valadier M-H (2005) The two nitrogen mobilisation- and senescence-associated GS1 and GDH genes are controlled by C and N metabolites. Planta 221:580–588
Miller AJ, Cramer MD (2004) Root nitrogen acquisition and assimilation. Plant Soil 274:1–36
Mok DWS, Mok MC (2001) Cytokinin metabolism and action. Ann Rev Plant Physiol Plant Mol Biol 52:89–118
Munné-Bosch S, Alegre L (2004) Die and let live: leaf senescence contributes to plant survival under drought stress. Funct Plant Biol 31:203–216
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Noodén LD, Guiamét JJ, John I (1997) Senescence mechanisms. Physiol Plant 101:746–753
Pastori GM, del Rio LA (1997) Natural senescence of pea leaves. An activated oxygen-mediated function for peroxisomes. Plant Physiol 113:411–418
Prochaskova D, Sairam PK, Srivastava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci 161:765–771
Roberts I, Fernández Murray P, Passeron S, Barneix AJ (2002) The activity of the 20S proteasome is maintained in detached wheat leaves during senescence in darkness. Plant Physiol Biochem 40:161–166
Stitt M, Müller C, Matt P, Gibon Y, Carillo P, Morcuende R, Scheible W-R, Krapp A (2002) Steps towards an integrated view of nitrogen metabolism. J Exp Bot 53:959–970
Takei K, Takahashi T, Sugiyama T, Yamaya T, Sakakibara H (2002) Multiple routes communicating nitrogen availability from roots to shoots: a signal transduction pathway mediated by cytokinin. J Exp Bot 53:971–977
Wingler A, von Schaewen A, Leegood RC, Lea PJ, Quick WP (1998) Regulation of leaf senescence by cytokinin, sugars, and light. Plant Physiol 116:329–335
Woo HR, Kim JH, Nam HG, Lim PO (2004) The delayed leaf senescence mutants of Arabidopsis, ore1, ore3 and ore9 are tolerant to oxidative stress. Plant Cell Physiol 45:923–932
Yang J, Zhang J, Wang Z, Zhu Q, Liu L (2002) Abscisic acid and cytokinins in the root exudates and leaves and their relationship to senescence and remobilization of carbon reserves in rice subjected to water stress during grain filling. Planta 215:645–652
Yang J, Zhang J, Wang Z, Zhu Q, Liu L (2003) Involvement of abscisic acid and cytokinins in the senescence and remobilization of carbon reserves in wheat subjected to water stress during grain filling. Plant Cell Environ 26:1621–1631
Yemm EW, Cocking EC (1955) The determination of amino-acids with ninhydrin. Analyst 80:209–213
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:508–514
Yoshida S (2003) Molecular regulation of leaf senescence. Planta 6:79–84
Acknowledgments
This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Criado, M.V., Roberts, I.N., Echeverria, M. et al. Plant Growth Regulators and Induction of Leaf Senescence in Nitrogen-Deprived Wheat Plants. J Plant Growth Regul 26, 301–307 (2007). https://doi.org/10.1007/s00344-007-9016-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-007-9016-5