Abstract
Genotype and cultural management determine the shape of peach [Prunus persica (L.) Batch] tree canopies in orchards. Not well understood, however, is the relationship between terminal growth, lateral branching, and shoot hormone levels that can fundamentally affect tree canopy development. In this experiment, two peach cultivars with widely differing growth habits (Pillar, KV930479 and Standard, ‘Harrow Beauty’) were budded on ‘Lovell’ rootstock, planted in the field in 1998, and characterized for shoot morphology and hormone concentrations in 2002 and 2003 (the fourth and fifth leaf, respectively). Auxin (indole-3-acetic acid) and cytokinins (largely trans-zeatin riboside, dihydrozeatin riboside, and isopentenyladenosine) were measured in shoot tips (2002) and current-year shoots (2003) using mass spectrometry. In 2002, Pillar trees had less sylleptic branching, more upright growth, and higher auxin and auxin-to-cytokinin ratios than Standard trees. In Pillar trees in 2003, auxin concentrations and shoot growth were highest in current year shoots; in pruned trees, only auxin levels increased. Peach tree growth habits may be the result of altered hormone metabolism. Growth forms leading to superior production efficiency may be developed by selection based on specific target hormone concentrations and ratios.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Fruit tree size and architecture can be managed genetically and culturally to increase tree yield and facilitate orchard management. Plant growth hormones are important signals in plant development that are likely to be affected by management practices. In this article we describe the morphological characteristics and associated auxin and cytokinin concentrations in shoots of two peach (Prunus persica) genotypes with genetically distinct growth habits, and the effect of pruning on these characteristics.
Breeding programs have produced or developed a peach tree with a columnar growth habit (Pillar), which has a narrow canopy diameter that requires few pruning cuts and produces less vigorous acropetal growth than Standard (conventional) trees (Bassi and others 1994; Scorza 1984; Scorza and others 2002). Tree growth habit and architecture can be critical to the success of orchard plantings because branch arrangement will affect leaf distribution and production efficiency (Sumida and Komiyama 1997). Tree architecture must be managed to control vegetative growth that can cause canopy shading, which limits production, particularly in high-density plantings (Chalmers and others 1981; Giulivo and others 1984; Hayden and Emerson 1973; Loreti and Massai 2002). Understanding processes that regulate development of peach tree architecture will assist efforts to improve peach orchard productivity (Bassi and others 1994).
Auxin and cytokinin concentrations influence tree architecture by regulating apical dominance and sylleptic shoot development (that is, the time and pattern of axillary bud growth of the current year’s shoot) (Bangerth and others 2000; Bubán 2000; Cline and Dong-Il 2002; Thomas and Blakesley 1987). Generally, auxin suppresses sylleptic branching of poplar, and cytokinin promotes it (Cline and Dong-Il 2002). Several cytokinins have been identified in shoots and xylem exudates of roots, and each may have a unique role in development of peach tree architecture (Moncaleán and others 2002; Sorce and others 2002). Because root tip number and root system dry weight are less in Pillar than Standard peach trees, root-produced cytokinins may also be less in Pillar trees (Tworkoski and Scorza 2001). It is possible that reduced cytokinins could reduce sylleptic shoots and branching response to pruning in Pillar.
Hormone relationships associated with peach tree architecture have not been established, and improved understanding of auxin and cytokinin concentrations in growth habits of peach can provide opportunities for genetic improvement and cultural management. Preliminary work in our laboratory indicated that the auxin-to-cytokinin ratio in young shoots of Pillar was approximately twice that of Standard trees (data not shown). We hypothesized that such hormone differences could affect shoot morphology and branching that, in part, determine tree growth habit. The objectives of this experiment were to (1) define the morphological differences in shoots of Pillar and Standard peach trees, (2) determine auxin and cytokinin quantities in shoot tips of Pillar and Standard peach trees, and (3) determine the interactive effects of pruning and growth habit on regrowth and concentrations of auxin and cytokinin in shoots.
MATERIALS AND METHODS
Trees
Pillar (KV930479) peach scion was obtained from the breeding program at the Appalachian Fruit Research Station, Kearneysville, West Virginia, USA, and budded to one-year-old ‘Lovell’ rootstock. The trees were planted in December 1998 in weed-free rows with 1.5 m spacing. Standard (‘Harrow Beauty’) trees budded to ‘Lovell’ rootstock were also planted at this time with a 6 m spacing. Rows were spaced 6 m apart. Insect and disease pressure was managed following regional extension-recommended practices (Pfeiffer 1998).
Study 1: Morphological and hormonal traits in Pillar and Standard peach trees
Whole-season shoot growth and branching characteristics
Four one-year-old shoots per tree were measured at the time of bud break, 28 March 2002. Shoots were sampled at 90° intervals around the tree canopy, 2 m above the ground, from exterior canopy locations in Pillar and Standard trees that were 4.1 and 3.4 m tall, respectively. Each one-year-old shoot was comprised of a main stem axis and sylleptic branches. For each main stem axis, measurements included proximal diameter, number of flower buds, number of internodes, and total length. For associated lateral branches, total length and number of internodes were measured. To evaluate apical dominance, the number of internodes and stem length from the terminal bud to the first lateral branch were measured. Finally, dry weights of lateral branches and the main stem axis were measured. The four shoots per tree were subsamples, and five replications (trees) were arranged in a randomized design. Growth habit effects were evaluated by the general linear model procedure, and means were separated using Duncan’s multiple range test (SAS 2001).
On 17 April 2002 10 one-year-old shoots were selected from 1.5 to 3.3 m above the ground from each of five trees of each growth habit to measure branch angle from the horizontal position. A protractor was held horizontal on a carpenter’s level, and the branch angle of 5-cm segments of the proximal and the distal ends of each one-year-old shoot were measured. The angle of the new, current season shoot (approximately 1- to 2-cm long) growing from the distal end of the one-year-old shoot was also measured. Branch angles were evaluated as described above, but with 10 subsamples in each of five replications.
Monthly changes in growth, auxin, and cytokinin in Pillar and Standard peach trees
Each month the length of current season growth was measured on 10 branches from each of five trees of each growth habit. The 10 branches were subsamples per replication (tree), randomly selected from separate trees each month. Trees were measured in 2002 on 28 March, 24 April, 23 May, 20 June, 2 August, and 22 August (0, 29, 57, 85, 128, and 148 days after bud break). Measurements included the length of the main stem axis and the number and length of all the lateral branches on 10 current-year shoots sampled from the entire canopy of each tree. There were two growth habits, five replications (trees) per growth habit, and 10 subsamples (current year branches) per tree. Growth habits were evaluated statistically as previously described. Shoot tips are potential sites of active hormone metabolism. In 2002, shoot tips within a tree canopy were harvested, pooled, and analyzed for auxin and cytokinins. The most distal shoot tip (less than 1 cm long) was sampled from separate 2-year-old branches in the same tree. The shoot tips were immediately frozen in liquid N, lyophilized, ground with dry ice, and stored at −80°C until further analysis. Hormones from each tree were measured by harvesting vegetative tissue on the following dates: 200 swollen buds on 28 March; 100 tips (1 cm long leaves) on 11 April; 20 tips (leaves less than 4 cm long) on 26 April, 10 May, 24 May, 4 June, and 30 July; and 100 tips with buds on 23 October. (A few flower buds were inadvertently included in the sampling on 28 March.) Three trees (replications) from each growth habit were sampled on each date. Samples were collected evenly throughout the tree, and each tree was sampled only once.
Auxin extraction and purification, and preparation for GC-MS
One-gram samples were extracted overnight at −20°C with 80% methanol (fortified with stable isotope, phenyl-13C6 indole-3-acetic acid, Cambridge Isotope Laboratories, as internal standard and with 16 mg butylhydroxytoluene (BHT)/liter and 10 mg ascorbic acid/liter as antioxidants). Samples were centrifuged, decanted, re-extracted for 1 h in cold 80% methanol and centrifuged, after which the supernatants were pooled. The supernatants were rotary flash evaporated (RFE), diluted with 0.1 M K2HPO4 (pH 8), separated against ethyl acetate (retaining aqueous phase), slurried with insoluble polyvinylpyrrilidone (PVPP), centrifuged, and decanted. The extracts were adjusted to pH 3, separated on C18 columns (2 g Alltech high load), that were preconditioned with 10 ml methanol then 20 ml of pH 3 water, washed with 20 ml of pH 3 water, eluted with 80% methanol, and dried with RFE. The samples were then methylated with ethereal diazomethane (Cohen 1984), evaporated, reconstituted in ethyl acetate, and quantified by gas chromatography-mass spectrometry (GC-MS), correcting for losses with the internal standard.
Cytokinin extraction, purification, and preparation for GC-MS and HPLC-MS
The procedure of Moritz and Sundberg (1996) was followed with modification. One gram dry weight of tissue was extracted overnight in 20 ml of extraction solution (80% methyl alcohol, 20% 0.02 M potassium phosphate buffer, plus 16 mg BHT/liter and 10 mg ascorbic acid/liter) at −20°C. The extraction solution was spiked with stable isotopes of each cytokinin (see below). The solution was centrifuged, decanted, re-extracted with 10 ml of extraction solution, and centrifuged, after which the supernatants were pooled. The supernatants were evaporated to an aqueous residue with RFE, treated with phosphatase (0.04 units/ml acid phosphatase; EC 3.1.3.2; Sigma P-3627) and incubated for 30 min at 37°C. The sample was then loaded on a strong anion exchange column (10 g SAX, Varian) linked to a C18 column (2 g Alltech high load) that had been preconditioned with 15 ml methanol followed by 20 ml of 0.02 M potassium phosphate buffer (pH 7.2). After the sample was loaded, the columns were washed with 40 ml of 0.02 M potassium phosphate buffer, the columns disconnected and the C18 column was washed with 15 ml of water. Cytokinins were eluted from the C18 column with 10 ml of 80% methanol (the first 2 ml were considered void volume and discarded). The eluant was evaporated to near dryness with RFE, re-suspended in 1 ml of 0.01 M ammonium acetate buffer (pH 3), loaded onto a strong cation exchange column (0.5 g Varian) that was preconditioned with 10 ml of 0.01 M ammonium acetate buffer (pH 3). The column was washed with 10 ml of 0.01 M ammonium acetate buffer (pH 3), and the cytokinins were eluted with 2 M ammonia in methanol and evaporated to dryness with RFE. If the sample was to be analyzed quantitatively for cytokinins, it was reconstituted in HPLC buffer and assayed by HPLC-MS. If the sample was to be analyzed qualitatively for cytokinins, it was permethylated in preparation for GC-MS.
Permethylation was conducted under nitrogen with freshly prepared reagents with modification of the method of Morris (1977) and Chen and others (1997). The sample was evaporated to dryness under a stream of nitrogen and, in an N-enriched environment of a glove bag, 250 μl of 1 M dimethyl sulphenyl carbanion was mixed with the sample and 50 μl of methyl iodide which reacted for 20 min. The reaction was stopped by adding 250 μl of water.
Chloroform (250 μl) was added to the reaction mixture and centrifuged to separate phases; the organic phase was then removed. The chloroform separation was repeated and the chloroform phases were pooled and dried in a Speed-Vac (Savant) concentrator. The permethylated cytokinins were then dissolved in 75 μl of ethyl acetate and qualitatively analyzed for cytokinins by GC-MS.
For cytokinin analysis, samples were fortified in the extraction solution with [15N] trans-zeatin ([15N]-tZ), [15N-N6] isopentenyladenosine ([15N-N6]-iPA), [2H3] dihydrozeatin ([2H3]-dhZ), [2H6] N6-isopentenyladenine ([2H6] N6-iP), [2H5] trans-zeatin riboside ([2H5]-tZR), [2H3] dihydrozeatin riboside ([2H3]-dhZR), and [2H5] trans-zeatin-9-glucoside ([2H5]-tZG) as internal standards (Olchemim, P.O. Box 22, Slechtitelu 27, 770 10 Olomouc, Czech Republic).
GC-MS for identification of auxin and cytokinins and quantitation of auxin
Auxin and cytokinins were analyzed with a gas chromatograph (5890 Series, Hewlett Packard) equipped with a 30 m × 0.320 mm × 0.25 μm column (DB5, J&W Scientific) and mass selective detector (5971, Hewlett Packard). For auxin (indole-3-acetic acid, IAA), chromatographic conditions were injector temperature of 250°C, detector temperature of 315°C, and oven temperature gradient from 60°C to 200°C (5°C / min), 200°C to 300°C (30°C / min), hold at 300°C for 10 min, then 300°C to 60°C (50°C / min). IAA eluted at 24.9 min with m/z fragments M+ 189, Base 130, 131 (12% height of Base ion), 103 (9%), and 77 (13%). IAA quantitation was accomplished by monitoring authentic IAA (m/z 130 and 189) and 13C6-IAA (m/z 136, 195) with selective ion monitoring (100 msec dwell per ion) (Cohen and others 1986). The limit of IAA quantitation was 29 pmole with a linear range from 29 to 143 pmoles, and the recovery average was 30%.
For cytokinin identification, chromatographic conditions were injector temperature of 250°C, detector temperature of 315°C, and oven temperature gradient from 60°C to 250°C (40°C / min), 250°C to 300°C (4°C / min), hold at 300°C for 10 min, then 300°C to 60°C (50°C/min). Based on full scan evaluation, four native cytokinins were observed: trans-zeatin (tZ), Rt 8.7 min, with m/z fragments M+ 261, Base 230, 231 (10%), 188 (22%), and 162 (7%); isopentenyladenosine (iPA), Rt 13.5 min, with m/z fragments M+ 391, Base 174, 348 (20%), 216 (60%), and 202 (90%); dihydrozeatin riboside (dhZR) Rt 14.8 min, with m/z fragments M+ 423, Base 162, 392 (35%), 250 (45%), and 176 (45%); and trans-zeatin riboside (tZR), Rt 15.8 min, with m/z fragments M+ 421, Base 216, 390 (71%), 348 (6%), and 174 (17%). The limit of cytokinin detection in SCAN mode was 30 pmoles.
HPLC-MS for quantitation of cytokinins
The high performance liquid chromatography (HPLC) method used for separating the cytokinins was a modification of the method used by Suttle (1998). A 1 μl sample was injected to an HPLC equipped with a binary pump (G1321A, 1100 series, Agilent), a C18 guard column (Bio-Sil HL90-5, Bio-Rad), a C18 column (0.8 × 10 cm NovaPak; Waters Associates), and a mass spectrometer with electrospray ionization (G1956B, Agilent). The solvent flow rate was 0.5 ml/min with solvent A being 1% acetic acid and solvent B being acetonitrile. The solvent delivery was 5% B for 10 min, linear gradient to 30% B from 10 to 35 min, 30% B from 35 to 40 min, linear gradient to 100% B from 40 to 45 min, 100% B from 45 to 58 min, linear gradient to 5% B from 58 to 63 min. Cytokinin quantitation was based on a modification of the method used by Novak and others (2003). The electrospray conditions were capillary voltage +3.0 kV, cone voltage +100 V, desolvation temperature 350°C, nitrogen drying gas 12.0 l/min and nebulizer pressure of 35 psig. Quantitation was done using SIM of quasi-molecular ions ((M + H)+) of extracted and internal standard stable isotopes of each cytokinin: iP (204) and [2H6] N6-iP (210) at Rt 44.0 min; tZ (220) and [15N]-tZ (221) at Rt 27.2 min; iPA (336) and [15N-N6]-iPA (337) at 41.9 min; tZR (352) and [2H5]-tZR (357) at 29.5 min; and dhZR (354) and [2H3]-dhZR (357) at 30.0 min. Cytokinin recovery averaged between 30% and 40%.
Cytokinin quantitation included correction for losses with the isotope dilution/relative response method (EPA method 1625). Isotope ratios were determined for the quasi-molecular ions noted above from nonisotope standards alone, the stable isotope standards alone, and mixtures of nonisotope and stable isotope standards. The following mixtures of nonisotope cytokinin and isotope cytokinin were prepared (ng/μl): 25:0, 0:25, 25:10, 25:5, 25:1, and 25:0.5, brought to a final volume of 75 μl, and injected for each cytokinin and LC-MS run as a standard relative response versus concentration curve.
Relative response = (Ry − Rm) (Rx + 1)/(Rm − Rx) (Ry + 1). Where Rx = the quasi molecular ion ratio for the nonisotope cytokinin, Ry = the quasi molecular ion ratio for the isotope cytokinin, and Rm = the quasi molecular ion ratio for the mixtures of nonisotope cytokinin and isotope cytokinin prepared as described above.
Study 2: Growth and Hormonal Response to Pruning in Pillar and Standard Peach Trees
There were three main treatment effects during the 2003 growing season: growth habit and canopy position (upper, 4-m height, and lower, 2-m height, canopy in Pillar and upper, 2-m height, canopy in Standard), pruning (pruned and unpruned), and days after bud break. Pruning goals were to achieve a balance between structural and fruiting wood in a manner similar to that employed by a commercial grower. There was an average of 54 and 103 pruning cuts per tree, respectively, in Pillar and in Standard trees. Current-year shoot length, weight, number, and length of lateral branches, and auxin and cytokinin concentrations were measured during the growing season. Twenty shoots per tree were measured as subsamples; each tree was an experimental unit with three replications per treatment. Entire current season shoots were frozen, lyophilized, and ground, and subsamples were analyzed for auxin and cytokinin as described above. Measurements were taken at monthly intervals in April, May, June, and July of 2003.
RESULTS
Study 1 Morphological Traits of Pillar and Standard Peach Trees
Shoot growth measurements from the 2001 season illustrated differences between Standard and Pillar growth habits. Although main stem axis length, number of internodes, and dry weight were greater in Standard trees (Table 1), length and diameter of the main stem axis were similar in Pillar and Standard trees until the stem diameter was 11 mm, suggesting equal distributions of allometric growth (Figure 1). When stem diameter growth exceeded 11 mm, Standard trees sustained more branch growth (Table 1). The number of internodes from the main axis terminus to the first branch was similar in Pillar and Standard trees, but the internode distance was greater in Pillar trees, resulting in longer internodes (Table 1).
No difference was observed between the growth habits in percentage of bud break on one-year-old (2002) shoots (Table 1). Approximately 74% of all buds in the distal 10 cm of one-year-old shoots had broken, and new season growth was 1 to 2 cm long by 3 April 2002. The high proportion of bud break suggested little or no correlative inhibition by current-year shoots of early proleptic growth (lateral buds that grow out after a period of rest) on one-year-old shoots in either growth habit. Pillar shoots were more upright, with a greater branch angle from the horizontal position than Standard shoots, regardless of the branch angle of the one-year-old shoot from which each one grew (Table 2). More upright growth in Pillar was observed whether the current-year branch angle was compared with the branch angle at the proximal or the distal end of one-year-old shoots (data not shown). Overall there were more branches with horizontal orientation in Standard trees than in Pillar trees.
The 2001 season branch angle shoot measurements were collected at a 2-m height that was 60% and 50% the full heights of Standard and Pillar trees, respectively. In 2002, shoot growth was measured from branches selected from throughout the tree canopies (1.5–4 m height). Prior to 84 days after bud break, growth of main stems was greater in Pillar than in Standard trees, and beyond 84 days after bud break Standard trees grew more (Table 3). Despite greater early growth, Pillar trees averaged less growth over the whole season, with less sylleptic growth than Standard trees (Table 3). However, during 2002 we observed that growth of Pillar trees was greater from the canopy tops than from lower in the canopy. Also, during 2002, we observed increased shoot growth and branching in both growth habits in response to pruning. Effects of pruning and canopy position were quantitatively evaluated during 2003, as discussed below.
Auxin, cytokinins, and the auxin-to-cytokinin ratio differed significantly with growth habit and days after bud break. Significant interactions were not found except for tZ (growth habits × days after bud break, p > F = 0.01). Auxin concentrations were greater and cytokinin concentrations were lower in Pillar than in Standard trees (Table 4). The highest auxin-to-cytokinin ratio and the lowest cytokinin concentrations were found in both growth habits at 57 days after bud break. The low cytokinin concentrations were associated with decreasing concentrations of iPA and tZR (Table 5). Sylleptic branch growth began by 84 days after bud break (Table 3). Reduced auxin-to-cytokinin ratios in shoot tips of Standard trees appeared to be associated with greater sylleptic growth. The ratio of auxin and cytokinin has been associated with bud break and branch patterns in plants (Bangerth and others 2000).
Study 2: Growth and Hormone Concentration in Current-year Shoots of Pruned and Unpruned Trees and from Two Canopy Locations in Pillar from April to July 2003
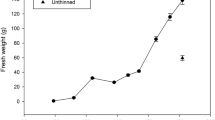
In 2003, there were three-way interaction effects of pruning, canopy location-growth habit, and days after bud break on growth of main stems and sylleptic branches. In unpruned trees, growth from the Pillar upper canopy and from Standard trees was similar and was greater than growth from the lower canopy of Pillar trees (Figure 2). Pruning stimulated growth in both growth habits and canopy positions. However, by 87 days after bud break, growth in pruned trees was greater from the upper canopy of Pillar than from Standard or the lower canopy of Pillar trees. Greater growth in the upper canopy of Pillar trees was accompanied by greater sylleptic branch growth at 87 days after bud break (data not shown).
Growth of main stems in two canopy positions of Pillar and the canopy of Standard peach trees that were unpruned (A) and pruned (B) during the 2003 growing season. Within each pruning treatment and time, values followed by the same letter do not differ at the 0.05 level according to Duncan’s multiple range test. Each value is the mean of 20 subsamples per tree and three trees per canopy position-growth habit combination, and time.
Hormone concentrations measured in whole current-year shoots during 2003 were lower than concentrations measured in shoot tips during 2002, and they decreased with time (Table 6). The lower hormone concentrations in 2003 may be due, in part, to greater mass of the whole shoot that was sampled and may have diluted hormone concentrations. One interesting similarity between 2002 and 2003 was the highest auxin-to-cytokinin ratio occurred about 58 days after bud break before declining. This decrease in auxin-to-cytokinin ratio coincided approximately with the time of first measured sylleptic branch growth.
Auxin concentration was greater in pruned than unpruned trees. Cytokinin concentration was not affected by pruning (Table 6). In Pillar trees, hormone concentrations were not different in shoots from the upper or the lower canopy. However, auxin levels were lower and cytokinin levels were higher in Standard trees than in shoots from either canopy location in Pillar trees. This growth habit difference was consistent in pruned and unpruned trees, as the effects of pruning did not interact with growth habit and canopy location to affect hormone concentrations (data not shown).
Of the cytokinins measured, only tZ and tZR were greater in Standard than in Pillar trees, at 107 and 91 and 27 and 7 pmol/g, respectively. Three other cytokinins, iP, iPA, and dhZR, did not differ due to growth habit, averaging 21, 52, and 2 pmol/g, respectively. Individual cytokinins were not affected by pruning (data not shown).
DISCUSSION
Branch Development and Growth Habit
Sylleptic branches grew from buds that were at least 17 internodes from the shoot terminus in both Pillar and Standard trees. However, fewer sylleptic shoots were measured in Pillar than in Standard trees in 2002. Less branching in Pillar trees was associated with shorter current-year shoots and longer internodes from the shoot terminus to the first sylleptic branch than in Standard trees. Fewer sylleptic branches suggested that Pillar trees had stronger apical dominance than Standard trees. Auxin inhibits and cytokinin promotes growth of axillary buds, and their relative concentrations affect apical dominance (Shimizu-Sato and Mori 2001). Auxin concentrations and the auxin-to-cytokinin ratio was higher in Pillar trees than in Standard trees in shoot tips measured in 2002 and in current-year shoots in 2003. In Pillar, the higher auxin concentration likely inhibited sylleptic branch growth, and greater distance may have been needed from the shoot terminus in Pillar than Standard to achieve a sufficiently low auxin concentration to permit sylleptic growth to occur. In apple, a change in hormonal gradients with increased distance from the apex may lead to bud burst (Costes and Guédon 2002). The differences in apical dominance that we found in peach tree growth habits maybe explained, in part, by auxin/cytokinin ratios. Pillar trees have fewer roots than Standard trees, and roots may be a significant source of cytokinins (Davies and others 1987; Jackson 1993; Skene 1975; Tworkoski and Scorza 2001; Van Staden and others 1988). It is possible that fewer roots resulted in less cytokinin supply to shoots in Pillar trees. In addition to hormones, other factors such as resource availability, bud maturity, sink competition, and parent shoot growth rate may affect apical dominance and growth of sylleptic branches (Génard and others 1994; McIntyre 1987; Tromp 1996). The low root/shoot ratio in Pillar trees may reduce mineral or water resources as well as root-produced hormones (Tworkoski and Scorza 2001).
In the present experiment, the branch angle of the elongating shoots was consistently more vertical in Pillar than in Standard trees, whether growing from pre-existing one-year-old branches with an upright or horizontal orientation. Gravitropic plant responses are complex, and auxin can play a role in upright growth of shoots (Blancaflor and Masson 2003; Zimmermann and Brown 1971). Higher auxin concentrations found in Pillar trees may thus have influenced branch orientation. The modification of tree form in peach may be due to selection of genotypes with altered auxin metabolism or sensitivity. It is possible that the auxin response (that is, auxin metabolism or sensitivity to auxin) could be genetically engineered to achieve a branch angle in peach that can influence growth form and reduce the need for pruning.
Branch Development and Canopy Position
Growth habit of Pillar trees was characterized by more upright shoots throughout the canopy, appreciable sylleptic growth in the top of the canopy, and less sylleptic growth in the mid and lower canopy. Pillar trees had greater auxin concentrations than Standard trees throughout the canopy, which may account for stronger anti-gravitropic growth in Pillar. However, the auxin concentration and the auxin-to-cytokinin ratio was the same in current-year shoots from the upper and lower canopy of Pillar trees, indicating that mechanisms other than these hormone ratios may be important in branch development in different parts of the canopy. The effects of canopy position in our experiment were similar to those found by Baraldi and others (1994) in ‘Nectagrand’ peach. There was nearly twice the shoot length, shorter internodes, and twice the number of lateral shoots in the top than the bottom of the canopy (Baraldi and others 1994). In the lower canopy, reduced growth was associated with reduced PAR and R/FR light that induced a growth pattern of reduced resource import (Baraldi and others 1994). This pattern of reduced resource import in the lower canopy was accompanied by increased apical dominance. In our experiment, greater growth in the upper canopy may be associated with greater resource availability (for example, PAR) or environmental cues (R/FR ratios) that may affect growth.
Branch Development and Pruning
Reduced planting distances are required in some peach plantings, and branches with upright orientations (low angle of insertion on the central leader) can be achieved genetically and by pruning and training (Loreti and Massai 2002). Peach growth habits with upright branches responded to pruning with excessive shoot regrowth that adversely affected fruit yield and quality (Miller and Scorza 2002). In the current experiment, pruning stimulated growth of the main stem and sylleptic branches in the canopy of Pillar and Standard trees. Pruning also increased auxin, but not cytokinin, concentrations in the current year shoots in both Pillar and Standard peach. Whole shoot auxin and cytokinin concentrations were measured in 2003 to average hormone effects for the current-year growth. These measurements do not provide insight into growth features at the individual bud level, and it is possible that cytokinin concentrations were higher in buds of pruned trees that had greater sylleptic growth. However, greater auxin concentrations and auxin-to-cytokinin ratios in current-year shoots of pruned trees suggest that increased sylleptic growth may not have been a cytokinin-induced effect.
Pruning appears to have affected hormone ratios in the shoots of the pruned trees by invigorating growth and stimulating auxin production at rates that were specific to the growth habit. It is possible that bud burst within a branch may enforce latency on other buds due to sink competition or another form of antagonism between buds in the branching zone (Costes and Guédon 2002). Hormones also may influence apical control by regulating relative sink strength among branches (Wilson 2000). Auxin and cytokinin concentrations in current-year shoots did not differ between the upper and lower parts of the canopy of Pillar trees, but growth was greater in the upper canopy. Branches are not completely autonomous but are interdependent with other branches (Sprugel 2002). Hallé and others (1978) proposed that shoots pass from a less vigorous state to a more vigorous state, and at that threshold, branching tended to be more sylleptic than proleptic. Our study tends to support this proposal in that growth rate, not hormone concentrations alone, affected apical dominance, as illustrated by increased sylleptic branching after pruning, particularly in the upper part of the canopy of Pillar trees.
CONCLUSIONS
Auxin and cytokinin concentrations appeared to significantly affect canopy architecture in peach trees by regulating branch angle and influencing sylleptic branching. The upright growth of Pillar consistently had higher auxin concentrations than the more spreading growth of Standard trees. However, canopy position strongly affected growth in Pillar so that shoots and sylleptic branches grew more vigorously in the upper canopy than the lower canopy. In the upper canopy, a high auxin-to-cytokinin ratio did not inhibit sylleptic growth of Pillar peach trees. One interpretation of these results is that auxin and cytokinin have greater influence on aspects of sylleptic branch development when growth is constrained by sink competition or by limited resource availability. The results support the hypothesis that different growth habits were associated with auxin and cytokinin differences and that hormone differences were not altered by pruning. Peach tree breeding and genetic engineering programs should be able to use auxin and cytokinin expression as selection criteria to achieve branch orientation for a desired growth habit.
References
Bangerth F, Li CJ, Gruber J. 2000. Mutual interaction of auxin and cytokinin in regulating correlative dominance. J Plant Growth Regul 32:205–217
Baraldi R, Rossi F, Facini O, Fasolo F, Rotondi A, and others. 1994. Light environment, growth and morphogenesis in a peach canopy. Physiol Plant 91:339–345
Bassi D, Dima A, Scorza R. 1994. Tree structure and pruning response of six peach growth forms. J Amer Soc Hort Sci 119:378–382
Blancaflor EB, Masson PH. 2003. Plant gravitropism. Unraveling the ups and downs of a complex process. Plant Physiol 133:1677–1690
Bubán T. 2000. The use of benzyladenine in orchard fruit growing: a mini review. J Plant Growth Regul 32:381–390
Chalmers DJ, Mitchell PD, van Heek L. 1981. Control of peach tree growth and productivity by regulated water supply, tree density, and summer pruning. J Am Soc Hort Sci 106:307–312
Chen W-S, Huang K-L, Yu H-C. 1997. Cytokinins from terminal buds of Euphoria longana during different growth stages. Physiol Plant 99:185–189
Cline MG, Dong-Il K. 2002. A preliminary investigation of the role of auxin and cytokinin in sylleptic branching of three hybrid poplar clones exhibiting contrasting degrees of sylleptic branching. Ann Bot 90:417–421
Cohen JD. 1984. Convenient apparatus for the generation of small amounts of diazomethane. J Chrom 303:193–196
Cohen JD, Baldi BG, Slovin JP. 1986. 13C6-[benzene ring]-indole-3-acetic acid. A new internal standard for quantitative mass spectral analysis of indole-3-acetic acid in plants. Plant Physiol 80:14–19
Costes E, Guédon Y. 2002. Modeling branching patterns on 1-year-old trunks of six apple cultivars. Ann Bot 89:513–524
Davies WJ, Blackman PG, Lodge TR, da Costa AR, Metcalfe J. 1987. Root to shoot communication of the effects of soil drying, flooding or increase salinity. A case for the involvement of plant growth regulators in a multiple chemical signal. In Tenhunen JD, Catarino FM, Lange OL, Oechel WC (eds) Plant Response to Stress, Springer-Verlag, Berlin, Germany, p 201–221
EPA method 1625. Revision B—Semivolatile Organic Compounds by Isotope Dilution GC/MS available at http://www.epa.gov/waterscience/methods/guide/methods.html
Génard M, Pagès L, Kervellia L. 1994. Relationship between sylleptic branching and components of parent shoot development in the peach tree. Ann Bot 74:465–470
Giulivo C, Pomina A, Costa G. 1984. Effects of planting density on peach and nectarine productivity. J Amer Soc Hort Sci 109:287–290
Hallé F, Oldeman RAA, Tomlinson PB. 1978. Tropical Trees and Forests: An Architectural Aanalysis. Springer-Verlag, New York, NY, USA, p 441
Hayden RA, and Emerson FH. 1973. Close ranks for more peaches. Am Fruit Grower (Dec.):13–15
Jackson MB. 1993. Are plant hormones involved in root to shoot communication? Adv Bot Res 19:103–187
Loreti F, Massai R. 2002. The high density peach planting system: present status and perspectives. Acta Hort 592:377–390
McIntyre GI. 1987. The role of water in the regulation of plant development. Can J Bot 55:1287–1298
Miller S, Scorza R. 2002. Training and performance of Pillar, Upright, and Standard form peach trees: early results. Acta Hort 592:391–399
Moncaleán P, Rodríguez A, Fernández B. 2002. Plant growth regulators as putative physiological markers of developmental stage in Prunus persica. J Plant Growth Regul 36:27–29
Moritz T, Sundberg B. 1996. Endogenous cytokinins in the vascular cambial region of Pinus sylvestris during activity and dormancy. Physiol Plant 98:693–698
Morris RO. 1977. Mass spectroscopic identification of cytokinins: glucosyl zeatin and glucosyl ribosylzeatin from Vinca rosea crown gall. Plant Physiol 59:1029–1033
Novak O, Tarkowski P, Tarkowska D, Dolezal K, Lenobel R.et al 2003. Quantitative analysis of cytokinins in plants by liquid chromatography-single quadrupole mass spectrometry. Anal Chim Acta 480:207–218
Pfeiffer DG. Bulletin coordinator. 1998. Virginia-West Virginia-Maryland Commercial Tree Fruit Spray Bulletin. Virginia Coop Ext Publ 456–419
SAS. 2001. Version 8.02. SAS Institute, Cary, NC, USA
Scorza R. 1984. Characterization of four distinct peach tree growth types. J Amer Soc Hort Sci 109:455–457
Scorza R, Bassi D, Liverani A. 2002. Genetic interactions of pillar (columnar), compact, and dwarf peach tree genotypes. J Amer Soc Hort Sci 127:254–261
Shimizu-Sato S, Mori H. 2001. Control of outgrowth and dormancy in axillary buds. Plant Physiol 127:1405–1413
Skene KGM. 1975. Cytokinin production by roots as a factor in the control of plant growth. In Torrey JG, Clarkson DT (eds) The Development and Function of Roots. Academic Press, New York, NY, USA, p 365–396
Sorce C, Massai R, Picciarelli P, Lorenzi R. 2002. Hormonal relationships in xylem sap of grafted and ungrafted Prunus rootstocks. Sci Hort 93:333–342
Sprugel DG. 2002. When branch autonomy fails: Milton’s law of resource availability and allocation. Tree Physiol 22:1119–1124
Sumida A, Komiyama A. 1997. Crown spread patterns for five deciduous broad-leaved woody species: ecological significance of the retention patterns of larger branches. Ann Bot 80:759–766
Suttle JC. 1998. Postharvest changes in endogenous cytokinins and cytokinin efficacy in potato tubers in relation to bud endodormancy. Physiol Plant 103:59–69
Thomas TH, Blakesley D. 1987. Practical and potential uses of cytokinins in agriculture and horticulture. Br Plant Growth Regul Group 14:69–83
Tromp J. 1996. Sylleptic shoot formation in young apple trees exposed to various soil temperature and air humidity regimes in three successive periods of the growing season. Ann Bot 77:63–70
Tworkoski T, Scorza R. 2001. Root and shoot characteristics of peach trees with different growth habits. J Amer Soc Hort Sci 126:785–790
Van Staden J, Cook EL, Nooden LD. 1988. Cytokinins and senescence. In Nooden LD, Leopold AC (eds), Senescence and Aging in Plants. Academic Press, New York, NY, USA, p 281–328
Wilson BF. 2000. Apical control of branch growth and angle in woody plants. Am J Bot 87:601–607
Zimmermann MH, Brown CL. 1971. Trees Structure and Function. Springer-Verlag, New York, NY, USA, p 336
Acknowledgments
The authors thank Bill Blake, Tom Millay, Larry Crim, and Jonathan Degenfelder for their technical assistance. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tworkoski, T., Miller, S. & Scorza, R. Relationship of Pruning and Growth Morphology with Hormone Ratios in Shoots of Pillar and Standard Peach Trees. J Plant Growth Regul 25, 145–155 (2006). https://doi.org/10.1007/s00344-005-0123-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-005-0123-x