Abstract
A number of novel brassinosteroid analogues were synthesized and subjected to the rice leaf lamina inclination bioassay. Modified B-ring analogues included lactam, thiolactone, cyclic ether, ketone, hydroxyl, and exocyclic methylene derivatives of brassinolide. Those derivatives containing polar functional groups retained considerable bioactivity, whereas the exocyclic methylene compounds were devoid of activity. Analogues containing normal alkyl and cycloalkyl substituents at C-24 (in place of the isopropyl group of brassinolide) showed an inverse relationship between activity and chain length or ring size, respectively. The corresponding cyclopropyl and cyclobutyl derivatives were significantly more active than brassinolide and appear to be the most potent brassinosteroids reported to date. When synergized with the auxin indole-3-acetic acid (IAA), their bioactivity can be further enhanced by 1–2 orders of magnitude. The cyclopropyl derivative, when coapplied with the auxin naphthaleneacetic acid, gave a significant increase in yield of wheat in a field trial. Certain 25- and 26-hydroxy derivatives are known metabolites of brassinosteroids. All of the C-25 stereoisomers of 25-hydroxy, 26-hydroxy, and 25,26-dihydroxy derivatives of brassinolide were prepared and shown to be much less active than brassinolide. This indicates that they are likely metabolic deactivation products of the parent phytohormone. A series of methyl ethers of brassinolide was synthesized to block deactivation by glucosylation of the free hydroxyl groups. The most significant finding was that the compound where three of the four hydroxyl groups (at C-3, C-22, and C-23) had been converted to methyl ethers retained substantial bioactivity. This type of modification could, in theory, allow brassinolide or 24-epibrassinolide to resist deactivation and thus offer greater persistence in field applications. A series of nonsteroidal mimetics of brassinolide was designed and synthesized. Two of the mimetics showed significant bioactivity and one had bioactivity comparable to brassinolide, but only when formulated and coapplied with IAA. They thus represent the first nonsteroidal analogues possessing brassinosteroid activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Brassinolide (1) is a naturally occurring plant hormone with a highly oxygenated steroidal structure (Grove and others 1979). It manifests biological activity at doses as low as 1 ng/plant in certain bioassays (vide infra) and has been reported to improve the yields of various commercially important crops at remarkably low concentrations (for selected reviews of brassinosteroids, see Cutler and others 1991; Fujioka and Sakurai 1997; Clouse and Sasse 1998; Khripach and others 1999; Sakurai and others 1999). Consequently, much effort has been invested in the synthesis of 1 and a host of other naturally occurring brassinosteroids, as well as in the preparation of novel analogs for the purpose of elucidating structure–activity relationships (SAR). Despite the synthetic efforts of several groups (for reviews, see Back 1995; Khripach and others 1999; McMorris 1999), brassinolide and its natural and synthetic congeners generally remain too expensive for large-scale commercial applications. This has prompted the search for more efficacious analogues than 1, which is generally considered the most potent of the naturally occurring brassinosteroids, as well as recent efforts toward the design of novel, nonsteroidal structures, based on our growing understanding of brassinosteroid SAR. The potentially simpler structures and greater ease of synthesis of such mimetics offers the prospect of cheaper compounds that would be more suitable for commercial exploitation than 1 or other brassinosteroids. This review summarizes our recent work in the area of brassinosteroid SAR, our attempts to prepare more efficacious brassinosteroids, and our efforts toward the design, synthesis, and bioassay of novel nonsteroidal mimetics of 1.
Early SAR studies established that several key structural features were required for strong bioactivity in brassinosteroids. In the interest of brevity, only a brief summary of the most salient early observations follows; for more detailed information on SAR, the reader is directed to selected key articles and reviews (Wada and Marumo 1981; Thompson and others 1982; Takatsuto and others 1983; Adam and Marquardt 1986; Mandava 1988; Yokota and Mori 1992; Brosa 1997, 1999; Khripach and others 1999). Several bioassays have been developed for measuring the activity of brassinosteroids (for reviews of bioassays, see Kohout and others 1991; Brosa 1997; Khripach and others 1999; Takatsuto and Yokota 1999). The rice leaf lamina inclination bioassay, using intact rice seedlings (Takeno and Pharis 1982), appears to be the most widely used method, although the bean second internode assay, which was used in the initial studies by Grove and coworkers (1979), has also seen frequent use. The bean first internode assay, as well as other assays utilizing radish, tomato, wheat, and other plants, has also been employed. Because a given brassinosteroid can elicit varying responses in different types of bioassays, direct comparison of data derived from dissimilar bioassays must be undertaken with caution. Similarly, the relative activity of individual compounds does not necessarily remain constant over the entire dosage range in a given type of assay. Thus, data measured over a broad range of doses is more informative than single-dose studies.
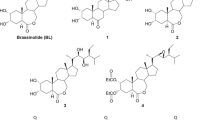
The above caveats notwithstanding, some general conclusions regarding the SAR of brassinosteroids are possible. The presence of the 2α,3α-diol moiety is necessary for optimum biological activity. The absence of either A-ring hydroxyl group, or a change in their configurations, is accompanied by a significant loss of bioactivity. The seven-membered B-ring lactone is needed for the highest level of activity, although some modifications to the B-ring are tolerated. For example, castasterone (2), with a 6-keto function, is strongly active, but less so than brassinolide (1), while typhasterol (3), which contains only one hydroxyl group in the A-ring, as well as a 6-keto moiety, is the least active of the three compounds. The complete absence of an oxygen function in the B-ring, as in the carbocyclic compound 4, results in very low or insignificant activity. Lactone (5), the 6-oxa-7-oxo isomer of 1, shows lower activity than brassinolide. The side chain hydroxyl groups also play an important role in the bioactivity of brassinosteroids. In general, a vicinal diol moiety with the 22R,23R configuration, as in 1, affords optimum activity. Considerable variation is possible in the side-chain alkyl substituents. Thus, 24-epibrassinolide (6) and 28-homobrassinolide (7) display relatively strong bioactivity, although generally lower than that of 1. However, 6 and 7 are cheaper to synthesize than 1 and so have seen frequent use in field trials. Moreover, brassinosteroids lacking the C-26, C-27, or C-28 methyl group also retain substantial bioactivity, as do compounds containing methylidene or ethylidene groups in place of the C-24 methyl substituent. For the structures of 1–7, see Figure 1.
Although most of the SAR studies reported to date have involved the synthesis of analogues containing systematically modified structures, followed by appropriate bioassays, molecular modeling has also been used to further refine our understanding of these phenomena. In particular, Brosa has developed modeling protocols designed to rationalize the SAR of brassinosteroids as well as explain the nature of their interactions with brassinosteroid receptors (Brosa 1996, 1997, 1999).
BIOASSAY PROTOCOLS
In our work on brassinosteroid SAR, we have relied upon the rice leaf lamina inclination bioassay, using the cultivar Tan-ginbozu (Takeno and Pharis 1982), because it provides rapid, convenient, and highly reproducible measurements. Typically, doses ranged from subnanogram to 1000- or 10000-ng levels. In general, 36 rice plants were used for each data point, with 24 plants employed at doses of 1000 ng or higher. Because brassinosteroids show synergy with auxins (for example, see Takeno and Pharis 1982; Mandava 1988; Sasse 1991), all new compounds were tested both alone and in the presence of indole-3-acetic acid (IAA, a naturally occurring auxin). The IAA was applied at a dose of 1000 ng, unless otherwise indicated. The results obtained with the coapplication of IAA are shown only in selected plots herein. When thus synergized with IAA, strongly active brassinosteroids such as 1 consistently show significant bioactivity at doses as low as 10 pg/individual plant. Furthermore, new analogues were always run simultaneously alongside a known standard (usually 1), as well as against controls consisting of solvent only and IAA plus solvent. We should note here that IAA (1000 ng) applied alone has no significant effect on leaf lamina bending in this intact plant bioassay. Bioassay results are presented here as plots of leaf lamina angle versus the dose of brassinosteroid in ng on a logarithmic scale. The leaf lamina angle in untreated controls is typically 160°–170° (almost upright) and decreases in response to an active brassinosteroid, reaching values of 60° or less with relatively high doses of brassinolide or the most potent analogues.
NOVEL B-RING ANALOGUES OF BRASSINOLIDE
In an effort to gain additional insight into the role played by the B-ring lactone moiety of brassinolide in its bioactivity, we synthesized and bioassayed the novel analogues 8–13 (Figure 2) (Back and others 1997a; Baron and others 1998). Some previous work on B-ring lactams, thiolactams, and cyclic ethers in the 28-homobrassinolide series (in some cases containing 22,23-diepi configurations) has shown these compounds to be only weakly active or inactive in the rice bioassay (Okada and Mori 1983; Anastasia and others 1984, 1986; Kishi and others 1986). However, because brassinolide is generally considered the benchmark for brassinosteroid activity, we wished to determine the bioactivity of such compounds when they retain the brassinolide side chain. Although brassinolide derivatives are more difficult to prepare than their 28-homo analogues, a new synthetic approach (Back and others 1997b) has allowed us to prepare 1 and 2 in batches of approximately 5 g, thus providing adequate material for further transformation into new analogues. The bioassay results of 8–11 are shown in Figure 3, along with that of 1 for comparison. Compounds 12 and 13 displayed no significant activity and are not shown in Figure 3. It can be seen that compounds 8–11 all showed substantial activity, although significantly lower (by about an order of magnitude) than that of 1. These results indicate that neither the oxygen atom at the 7-position nor the C-6 carbonyl group of brassinolide is essential for activity. However, the lack of activity of carbocycles 12 and 13 demonstrates that a polar functional group is necessary in the B-ring. Conversely, the presence of a trigonal planar center at C-6 is not sufficient for activity if it is part of a nonpolar exocyclic alkene, even if it emulates the flattened geometry associated with a carbonyl group. Coapplication of 8–11 with IAA (not shown) rendered these compounds comparable to 1 alone, but less active than 1 coapplied with IAA. Derivatives 12 and 13 remained essentially inactive even when IAA was present. The bioactivity of 11 was subsequently reexamined by Seto and coworkers (1999a), who confirmed our results and indicated that 11 was approximately 1/20th as active as 1. They also found that the corresponding 7-keto isomer showed weaker activity than the corresponding 6-ketone 11, and the 8-membered B-ring homologue of 11 was almost inactive (Seto and others 1999a, 2002).
Bioassay of compounds 8–11 vs. 1. Data taken from Figures 4 and 5 of Baron and others (1998).
The 6α- and 6β-hydroxy reduced analogues of castasterone (14 and 15, respectively; see Figure 2) displayed significant and comparable activity, which was, however, lower than that of 1 or 2, and was strongly synergized by IAA (Sung and others 2000). During an investigation of the activity of biosynthetic precursors of 1 and 2, Fujioka and coworkers (1998) reported that the 6α-isomer 14 was intermediate in activity between 2 and the 6-deoxy derivative 4. These results further illustrate that considerable variation is possible in the B-ring without complete loss of bioactivity.
The A/B-trans ring junction has been widely regarded as necessary for bioactivity. Surprisingly, the 2,3-diepi-5β analogue 16 (Figure 2) showed relatively strong activity in the rice leaf lamina inclination bioassay (Brosa 1996). However, this discovery has aroused some controversy because it employed only a single, relatively high dose of 1 µg/plant (Seto and others 1998, 1999b). The 5α-hydroxy and 5α-fluoro derivatives in several brassinosteroid series have also been investigated. The presence of an alcohol substituent at C-5 generally diminishes bioactivity (Takatsuto and others 1987; Brosa and others 1998; Ramírez and others 2000), whereas fluorination gives rise to relatively potent analogues. For example, 5α-fluorotyphasterol is more active than typhasterol (3) (Ramírez and others 2000).
SIDE CHAIN ANALOGUES THAT ACT AS “SUPERBRASSINOLIDES”
As noted earlier, the brassinosteroid side chain tolerates considerable variation in its alkyl substituents. Indeed, even some compounds completely lacking a side chain at C-17 have been reported to show measurable activity in the bean second internode assay (for example, see Kohout and Strnad 1989). However, side chain analogues do not generally display bioactivity exceeding that of brassinolide (1). We were therefore intrigued by a report that 25-homobrassinolide (17) is more potent than 1 in the rice leaf lamina inclination bioassay (Mori and Takeuchi 1988). This prompted us to search for new analogues that might be similarly superior to 1 in this bioassay, compounds we refer to as “superbrassinolides.” Consequently, we prepared a series of novel derivatives containing chains of varying length (18–20) and cycloalkyl groups with different ring sizes (21–24) at C-24 (in lieu of the isopropyl group present at that position in 1). See Figure 4 for structures 17–24. The results obtained in the bioassay of 18–20, along with those of 1 and 17 for comparison, are shown in Figure 5 (Back and others 2000a). The bioactivity clearly increases inversely with the chain length. Thus, the n-dodecyl derivative 18 shows no activity, while the n-propyl analogue 20 is strongly active, but less so than 17 or 1. In our hands there was virtually no difference between the activities of the latter two compounds. A similar trend is seen with the cycloalkyl derivatives. Although the cyclohexyl analogue 21 was less active than 1 or 17, the cyclopentyl compound 22 was comparable to 1 (Figure 6), and the small-ring derivatives 23 and 24 were more potent than 1 or 17 (Figure 7). In particular, the superbrassinolides 23 and 24 are approximately 5–7 times more potent than 1 or 17 and appear to be the two most strongly active brassinosteroids reported to date (Back and others 2000a, 2001). Moreover, the cyclopropyl derivative, when coapplied with naphthaleneacetic acid, gave a significant increase in the yield of wheat in a field trial (Back and others 2001). One can speculate that the small-ring compounds 23 and 24 have fewer degrees of freedom than acyclic alkyl substituents, thus permitting tighter binding with their putative receptor(s). All of the brassinosteroids 17–24, except for 18, were strongly synergized by IAA, affording activities 1–2 orders of magnitude stronger than in the absence of the auxin.
Bioassay of compounds 17–20 vs. 1. Data taken from Figure 1 of Back and others (2000a).
Bioassay of compounds 21 and 22 vs. 1 and 17. Data taken from Figure 2 of Back and others (2000a).
Bioassay of compounds 23 and 24 vs. 1 and 17. Data taken from Figure 3 of Back and others (2000a).
ANALOGUES DESIGNED TO BLOCK METABOLIC DEACTIVATION OF BRASSINOSTEROIDS
A limitation to the commercial exploitation of brassinosteroids in crop production is that they are metabolized relatively rapidly by plants to less bioactive products. Repeated exogenous applications are therefore required to compensate for metabolic deactivation. Clearly, an improved understanding of the steps that comprise metabolic deactivation and the means to block such processes would be useful in devising more efficacious and persistent brassinosteroids for field applications. Recent work has shed considerable light on the pathways by which active brassinosteroids are metabolized in both cell cultures and intact plants (Yokota and others 1991; Adam and others 1996; Adam and Schneider 1999; Khripach and others 1999). For example, metabolism of exogenously applied 24-epibrassinolide (6) in cell cultures of Lycopersicon esculentum resulted in enzymatic hydroxylation at C-25 and C-26, followed by glucosylation of these sites (Schneider and others 1994; Hai and others 1995) (Figure 8). The glucosylated conjugates had low activity in bioassays. However, the hydroxylated aglycones 25 and 26 were described as having varying degrees of bioactivity, up to 10 times that of the parent compound 6 in the case of 25 (Adam and others 1996; Hai and others 1995; Voigt and others 1996). The metabolism of brassinolide (1) has also been studied. Voigt and others (1996) reported that 25-hydroxybrassinolide (27) displays comparable bioactivity to 1 in the rice leaf lamina inclination bioassay. In contrast, Seto and others (1999c) found relatively low activity for 27 and for (25S)-26-hydroxybrassinolide (28). Thus, although it is generally agreed that metabolic glucosylation of brassinosteroids results in deactivation, it appeared less clear whether enzymatic hydroxylation at C-25 and C-26 represents an activation or deactivation step. We therefore synthesized 27, 28, and its 25R-epimer 29, as well as both C-25 epimers (30 and 31, respectively) of the related 25,26-diol (Back and others 2000b); for structures 27–31, see Figure 9. All of these hydroxylated derivatives were subjected to the rice leaf lamina inclination bioassay and proved to have very low or no activity (Pharis and others 2001). We therefore conclude that hydroxylations at C-25 and C-26 comprise deactivation pathways in the case of brassinolide (1).
It is of interest to design novel brassinosteroids in which metabolic deactivation is precluded by structural features capable of blocking hydroxylation at C-25 and C-26. Toward this effect, we prepared the 25-fluoro, 25-aza, and 25-methoxy derivatives 32–34, respectively (Figure 9). Compounds 32 and 33 proved completely inactive, although 34 displayed strong activity in the rice bioassay (Back and others 1999). Coapplication of 34 with IAA produced a stronger bending response than was observed with 1 alone, but not as strong as that displayed by 1 when IAA was coapplied (Figure 10). At the time of this discovery, the lack of activity of 32 and 33 suggested that hydroxylation at C-25 was a required step in the biosynthesis of active brassinosteroids and that this hydroxylation was blocked by the presence of a heteroatom. The activity of 34 was taken as an indication that alkoxy groups can act as surrogates for free alcohols at C-25. However, in view of the subsequent negative bioassay results observed with an authentic sample of 27 (vide supra), this hypothesis appears to be erroneous. In hindsight, the lack of activity of 32 and 33 is probably related to their relatively poor solubilities (and therefore low bioavailability) instead of their refractory nature toward enzymatic hydroxylation.
Bioassay of compound 34 vs. 1 with and without coapplied IAA. Data taken from Figure 1 of Back and others (1999).
Glucosylation of existing hydroxyl groups at C-2, C-3, and C-23 of brassinosteroids represents, inter alia, another metabolic deactivation pathway (Yokota and others 1991; Adam and others 1996; Adam and Schneider 1999; Khripach and others 1999). It has been demonstrated that brassinosteroids containing 22,23-epoxy groups and ester functions at C-2 and C-3 exhibit greater persistence in the field than natural brassinosteroids. One such compound, TS303 (35; Figure 11), is particularly efficacious in this regard (Kamuro and Takatsuto 1999). Although this may be partly due to its gradual conversion by hydrolysis into more active brassinosteroids, it is also likely that TS303 resists glucosylation, which contributes to its long-lived activity. We considered the possibility that glucosylation could be blocked by converting the free hydroxyl groups of brassinolide into the corresponding methyl ethers. Because ethers are hydrolytically more stable than epoxides or esters, such compounds might have exceptional persistence, provided that O-methylation did not destroy the bioactivity associated with the free alcohol groups of 1 in the first place. The significant bioactivity observed with 34 (Figure 10) provided some reassurance in this regard. Furthermore, the systematic O-methylation of brassinolide’s hydroxyl groups was expected to provide information on the role that these groups play in manifesting biological activity through, for example, hydrogen bonding to the brassinosteroid receptor(s).
We therefore prepared a series of methyl ethers 36–45 (in addition to 34) and subjected them to the rice leaf lamina inclination bioassay (Luo and others 1998; Back and others 2002). Figure 11 shows the structures of 35–45, whereas Figure 12 shows that methylation of the hydroxyl group at C-23 (37) is more deleterious to bioactivity than methylation of the hydroxyl group at C-22 (36). Surprisingly, methylation of both side-chain alcohol groups resulted in the strongly active analogue 38, which is comparable to 24-epibrassinolide (6) across the entire dosage range (Luo and others 1998). Evidently, free hydroxyl groups on the brassinosteroid side chain are not essential for bioactivity. We next examined the bioactivity of compounds containing methyl ether groups at C-2 and C-3, with and without methylation of the side chain hydroxyl groups (39–43, respectively) (Back and others 2002). Figure 13 shows that compounds 40 and 42 (methyl ethers at C-3) are more active than 39 and 41 (methyl ethers at C-2), suggesting that a free hydroxyl group at C-2 is more crucial for bioactivity than at C-3. It is therefore possible that the C-2 alcohol functions as a hydrogen bond donor when interacting with a putative receptor, and that methylation of this group blocks the required interaction. All of the above methyl ethers were synergized by IAA and coapplication of 40 or 42 with the auxin produced activity approaching or comparable to that observed with 1 alone, but lower than that obtained with 1 plus IAA. In contrast to 25-methoxybrassinolide (34), which was quite strongly active (Figure 10), additional methylation of hydroxyl groups at either C-2 or C-3 resulted in compounds 44 and 45, respectively, with very low activity (Back and others 2002). The tetramethyl derivative 43 was essentially inactive, with or without IAA (Luo and others 1998). The relatively strong bioactivity of the trimethylated analogue 42, particularly when it is coapplied with IAA, is particularly noteworthy as this compound is expected to be resistant to glucosylation at two of the key sites (that is, hydroxyl groups at C-3 and C-23) where glucosylation is known to occur. It remains to be seen, though, whether this finding will be translated into greater persistence and efficacy in the field.
Bioassay of compounds 36–38 vs. 1 and 6. Data taken from Figure 2 of Luo and others (1998).
Bioassay of compounds 39–42 vs. 1. Data taken from Figures 5 and 6 of Back and others (2002).
SEARCH FOR NONSTEROIDAL MIMETICS OF BRASSINOLIDE
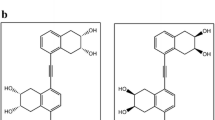
The high cost of brassinosteroids has severely curtailed their commercial exploitation. However, in view of the considerable data available in brassinosteroid SAR, we considered that it might be possible to design nonsteroidal molecules that would be easier to synthesize but would still emulate the bioactivity of brassinosteroids. Our approach was based on the supposition that mimetics with structures that superimpose effectively on that of 1 would interact similarly with putative receptors in plants and therefore manifest similar bioactivity. The design of such mimetics consisted of building subunit structures that contain the required functional groups of 1, with a geometry that approximates their disposition in 1. The subunits were then joined by rigid linkers that hold the respective functionalities the necessary (as determined by molecular modeling) distance apart, thus rendering the mimetic molecules superimposable with 1. The modular design of such molecules (subunits and linkers), as well as their symmetrical nature, makes their preparation relatively simple and concise. To date, two out of a series of ten potential nonsteroidal mimetics that we synthesized showed significant bioactivity in the rice leaf lamina inclination bioassay (Andersen and others 2001). Figure 14 shows how the key functional groups (shown in bold) of the two compounds (46 and 47) resemble their counterparts in the minimum energy conformation of brassinolide (1). The most active of these mimetics (47) yielded significant bioactivity at doses as low as 10 pg/plant, thus making it comparable to the most potent brassinosteroids. These compounds represent the first nonsteroidal mimetics reported to date. However, in contrast to brassinosteroids, which are active alone and are also highly synergized by coapplied IAA, the mimetics were active only in the presence of the coapplied auxin and when applied in an aqueous solution with an appropriate formulating agent (Andersen and others 2001). The bioactivity of 47 is illustrated in Figure 15, where 47 is inactive on its own but gives an increasingly strong bending response with the coapplication of 1000 and 5000 ng of IAA. The gradual decrease in activity at higher doses of 47 is not clearly understood but may be due to saturation and/or toxicity effects. Both 46 and 47 were prepared and tested as inseparable mixtures of stereoisomers and it is not known which stereoisomers are the most bioactive. Their independent stereoselective synthesis, as well as the design of even simpler and more efficacious mimetics, is currently in progress.
Bioassay of 47 with 0 ng, 1000 ng, and 5000 ng of IAA. Data taken from Figure 3 of Andersen and others (2001).
CONCLUSIONS
Since the discovery of brassinolide (1) in 1979, considerable progress has been achieved in elucidating the SAR of brassinosteroids. This has led, inter alia, to the discovery of more potent analogues than 1, such as 23 and 24. When strongly active brassinosteroids are coapplied with a synergistic auxin such as IAA, the threshold of significant bioactivity in the rice leaf lamina inclination bioassay drops a further 1–2 orders of magnitude, approaching 10 pg/plant. The synthesis and bioassay of 25- and 26-hydroxylated derivatives and related congeners of brassinolide (27–31) has confirmed that, when formed in vivo, they are the products of metabolic deactivation, not activation, in plants. The search for more persistent analogues that resist deactivation by glucosylation of existing hydroxyl groups resulted in the discovery of T303 (35) in Japan, whereas a systematic investigation of O-methylated brassinosteroids in our laboratory revealed that 42, where glucosylation is presumably blocked at the key hydroxyl groups at C-3 and C-23 (as well as at the less critical C-22), retains considerable bioactivity in the rice bioassay. Finally, compounds 46 and 47 represent the first nonsteroidal mimetics of brassinolide to date. Compound 47 gives strong bioactivity when applied under optimized conditions and demonstrates that it is possible to prepare biologically active nonsteroidal analogues of brassinolide consisting of a relatively rigid scaffolding that holds key functional groups in the required spatial orientation to mimic the structure and geometry of 1 and related brassinosteroids. The simpler structures of such mimetics should result in lower costs of production, thereby expediting commercial applications that are currently precluded by the high cost of brassinosteroids.
References
G Adam V Marquardt (1986) ArticleTitleBrassinosteroids. Phytochemistry 25 1787–1799 Occurrence Handle10.1016/S0031-9422(00)81151-6 Occurrence Handle1:CAS:528:DyaL28XlsV2ltbk%3D
Adam, G, Porzel, A, Schmidt, J, Schneider, B, Voigt, B (1996) “New developments in brassinosteroid research.” In: Atta-ur-Rahman (Ed.)., Studies in natural products chemistry, Vol. 18., Elsevier, Amsterdam, pp 495–549
G Adam B Schneider (1999) Uptake, transport and metabolism. A Sakurai T Yokota SD Clouse (Eds) Brassinosteroids: steroidal plant hormones. Springer-Verlag Tokyo 113–136
M Anastasia P Allevi MG Brasca P Ciuffreda A Fiecchi (1984) ArticleTitleSynthesis of (2R,3S,22S,23S)-2,3,22,23-tetrahydroxy-B-homo-6-aza-5α-stigmastan-7-one, an aza-analogue of brassinolide. Gazz Chim Ital 114 159–161 Occurrence Handle1:CAS:528:DyaL2cXlvFahur0%3D
M Anastasia P Allevi P Ciuffreda A Fiecchi A Scala (1986) ArticleTitleSynthesis of (2R,3S,22S,23S)-2,3,22,23-tetrahydroxy-B-homo-7-aza-5α-stigmastan-6-one, an aza-analogue of homobrassinolide. J Chem Soc Perkin Trans 1 2117–2121
DL Andersen TG Back L Janzen K Michalak RP Pharis GCY Sung (2001) ArticleTitleDesign, synthesis and bioactivity of the first nonsteroidal mimetics of brassinolide. J Org Chem 66 7129–7141 Occurrence Handle10.1021/jo015832+ Occurrence Handle1:CAS:528:DC%2BD3MXmvVKit7g%3D Occurrence Handle11597241
TG Back (1995) Stereoselective synthesis of brassinosteroids. Atta-ur-Rahman (Ed.), Studies in natural products chemistry, Vol. 16. Elsevier Amsterdam 321–364
Back TG, Baron DL, Luo W, Nakajima SK, Janzen L, Pharis RP. 1997a. A concise synthesis of brassinolide and the preparation and bioactivity of some novel analogues. Proceedings of the 24th Annual Meeting of the Plant Growth Regulation Society of America. Atlanta, p 107–110.
TG Back DL Baron W Luo SK Nakajima (1997b) ArticleTitleConcise improved procedure for the synthesis of brassinolide and some novel side-chain analogues. J Org Chem 62 1179–1182 Occurrence Handle1:CAS:528:DyaK2sXovVynsw%3D%3D
TG Back L Janzen SK Nakajima RP Pharis (1999) ArticleTitleSynthesis and biological activity of 25-methoxy, 25-fluoro and 25-azabrassinolide and 25-fluorocastasterone. Surprising effects of heteroatom substituents at C-25. J Org Chem 64 5494–5498 Occurrence Handle10.1021/jo990312o Occurrence Handle1:CAS:528:DyaK1MXjvFGiu7c%3D Occurrence Handle11674612
TG Back L Janzen SK Nakajima RP Pharis (2000a) ArticleTitleEffect of chain length and ring size of alkyl and cycloalkyl side chain substituents upon the biological activity of brassinosteroids. Preparation of novel analogues with activity exceeding that of brassinolide. J Org Chem 65 3047–3052 Occurrence Handle1:CAS:528:DC%2BD3cXisVKksLY%3D
TG Back SK Nakajima J Zhu (2000b) ArticleTitleSynthesis of 25-hydroxy, 26-hydroxy and 25,26-dihydroxybrassinolide. Synlett . 1649–1651
Back TG, Pharis RP, Nakajima SK. 2001. Brassinosteroid Analogs Useful as Plant Growth Regulators, U.S. Patent 6,239,073, issued May 29, 2001.
TG Back L Janzen RP Pharis Z Yan (2002) ArticleTitleSynthesis and bioactivity of C-2 and C-3 methyl ether derivatives of brassinolide. Phytochemistry 59 627–634 Occurrence Handle10.1016/S0031-9422(02)00019-5 Occurrence Handle1:CAS:528:DC%2BD38XhsFagur8%3D Occurrence Handle11867094
DL Baron W Luo L Janzen RP Pharis TG Back (1998) ArticleTitleStructure–activity studies of brassinolide B-ring analogues. Phytochemistry 49 1849–1858 Occurrence Handle10.1016/S0031-9422(98)00367-7 Occurrence Handle1:CAS:528:DyaK1MXhvFGisg%3D%3D
C Brosa (1996) ArticleTitleBrassinosteroids: a new way to define the structural requirements. Tetrahedron 52 2435–2448 Occurrence Handle10.1016/0040-4020(95)01065-3 Occurrence Handle1:CAS:528:DyaK28XhtVajtbo%3D
C Brosa (1997) Biological effects of brassinosteroids. EJ Parish WD Nes (Eds) Biochemistry and function of sterols CRC Press Boca Raton, FL 201–220
C Brosa L Soca E Terricabras JC Ferrer A Alsina (1998) ArticleTitleNew synthetic brassinosteroids: a 5α-hydroxy-6-ketone analog with strong plant growth promoting activity. Tetrahedron 54 12337–12348 Occurrence Handle10.1016/S0040-4020(98)00743-1 Occurrence Handle1:CAS:528:DyaK1cXmtlSmu7s%3D
C Brosa (1999) Structure–activity relationship. A Sakurai T Yokota SD Clouse (Eds) Brassinosteroids: steroidal plant hormones Springer-Verlag Tokyo 191–222
SD Clouse JM Sasse (1998) ArticleTitleBrassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49 427–451 Occurrence Handle1:CAS:528:DyaK1cXjvVShu7o%3D
HG Cutler T Yokota G Adam (Eds) (1991) Brassinosteroids: chemistry, bioactivity and applications. ACS Symp Ser 474. American Chemical Society Washington, DC
S Fujioka A Sakurai (1997) ArticleTitleBrassinosteroids. Natural Prod Rep 14 1–10 Occurrence Handle1:CAS:528:DyaK2sXhvVyjsbY%3D
S Fujioka T Noguchi S Takatsuto S Yoshida (1998) ArticleTitleActivity of brassinosteroids in the dwarf rice lamina inclination bioassay. Phytochemistry 49 1841–1848 Occurrence Handle10.1016/S0031-9422(98)00412-9 Occurrence Handle1:CAS:528:DyaK1MXhvFGjuw%3D%3D
MD Grove GF Spencer WK Rohwedder N Mandava JF Worley JD Warthen Jr GL Steffens JL Flippen–Anderson JC Cook Jr (1979) ArticleTitleBrassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281 216–217 Occurrence Handle1:CAS:528:DyaL3cXht1CrtLY%3D
T Hai B Schneider G Adam (1995) ArticleTitleMetabolic conversion of 24-epi-brassinolide into pentahydroxylated brassinosteroid glucosides in tomato cell cultures. Phytochemistry 40 443–448 Occurrence Handle10.1016/0031-9422(95)00224-U Occurrence Handle1:CAS:528:DyaK2MXot1Gitro%3D
Y Kamuro S Takatsuto (1999) Practical application of brassinosteroids in agricultural fields. A Sakurai T Yokota SD Clouse (Eds) Brassinosteroids: steroidal plant hormones Springer-Verlag Tokyo 223–241
VA Khripach VN Zhabinskii AE de Groot (1999) Brassinosteroids: a new class of plant hormones. Academic Press San Diego, CA
T Kishi K Wada S Marumo K Mori (1986) ArticleTitleSynthesis of brassinolide analogs with a modified ring B and their plant growth-promoting activity. Agric Biol Chem 50 1821–1830 Occurrence Handle1:CAS:528:DyaL28XmtVClt70%3D
L Kohout M Strnad (1989) ArticleTitleBrassinolide analogues without side chain. Collect Czech Chem Commun 54 1019–1027 Occurrence Handle1:CAS:528:DyaK3cXnslym
L Kohout M Strnad M Kaminek (1991) Types of brassinosteroids and their bioassays. HG Cutler T Yokota G Adam (Eds) Brassinosteroids: chemistry, bioactivity and applications. ACS Sym Ser 474. American Chemical Society Washington, DC 56–73
W Luo L Janzen RP Pharis TG Back (1998) ArticleTitleBioactivity of brassinolide methyl ethers. Phytochemistry 49 637–642 Occurrence Handle10.1016/S0031-9422(97)00881-9 Occurrence Handle1:CAS:528:DyaK1cXmsF2qtLg%3D
NB Mandava (1988) ArticleTitlePlant growth-promoting brassinosteroids. Ann Rev Plant Physiol Plant Mol Biol 39 23–52 Occurrence Handle10.1146/annurev.pp.39.060188.000323 Occurrence Handle1:CAS:528:DyaL1cXlsVKhtrY%3D
TC McMorris (1999) Chemical synthesis of brassinosteroids. A Sakurai T Yokota SD Clouse (Eds) Brassinosteroids: steroidal plant hormones. Springer-Verlag Tokyo 69–90
K Mori T Takeuchi (1988) ArticleTitleSynthesis of 25-methyldolichosterone, 25-methyl-2,3-diepidolichosterone, 25-methylcastasterone and 25-methylbrassinolide. Liebigs Ann Chem . 815–818
K Okada K Mori (1983) ArticleTitleSynthesis of brassinolide analogs and their plant growth-promoting activity. Agric Biol Chem 47 89–95 Occurrence Handle1:CAS:528:DyaL3sXitFaqsbg%3D
RP Pharis L Janzen SK Nakajima J Zhu TG Back (2001) ArticleTitleBioactivity of of 25-hydroxy, 26-hydroxy, 25,26-dihydroxy- and 25,26-epoxybrassinolide. Phytochemistry 58 1043–1047 Occurrence Handle10.1016/S0031-9422(01)00381-8 Occurrence Handle1:CAS:528:DC%2BD3MXos1Wrsbw%3D Occurrence Handle11730867
JA Ramírez EG Gros LR Galagovsky (2000) ArticleTitleEffects on bioactivity due to C-5 heteroatom substituents on synthetic 28-homobrassinosteroid analogs. Tetrahedron 56 6171–6180 Occurrence Handle1:CAS:528:DC%2BD3cXlvVKksrg%3D
A Sakurai T Yokota SD Clouse (Eds) (1999) Brassinosteroids: steroidal plant hormones. Springer-Verlag Tokyo
JM Sasse (1991) The case for brassinosteroids as endogenous plant hormones. HG Cutler T Yokota G Adam (Eds) Brassinosteroids: chemistry, bioactivity and applications. ACS Symp Ser 474. American Chemical Society Washington, DC 158–166
B Schneider A Kolbe A Porzel G Adam (1994) ArticleTitleA metabolite of 24-epibrassinolide in cell suspension cultures of Lycopersicon esculentum. Phytochemistry 36 319–321 Occurrence Handle10.1016/S0031-9422(00)97068-7 Occurrence Handle1:CAS:528:DyaK2cXksFCjsL8%3D
H Seto S Fujioka H Koshino T Suenaga S Yoshida T Watanabe S Takatsuto (1998) ArticleTitleEpimerization at C-5 of brassinolide with sodium methoxide and the biological activity of 5-epibrassinolide in the rice leaf lamina inclination test. J Chem Soc Perkin Trans 1 3355–3358 Occurrence Handle10.1039/a805945d
H Seto S Fujioka H Koshino H Hayasaka T Shimizu S Yoshida T Watanabe (1999a) ArticleTitleSynthesis and biological activity of 6a-carbabrassinolide: B-ring homologation of 6-oxo-steroid to 6-oxo-7a-homosteroid with trimethylsilyldiazomethane-boron trifluoride etherate. Tetrahedron Lett 40 2359–2362 Occurrence Handle1:CAS:528:DyaK1MXhvVemt7c%3D
H Seto S Fujioka H Koshino T Suenaga S Yoshida T Watanabe S Takatsuto (1999b) ArticleTitle2,3,5-Tri-epi-brassinolide: preparation and biological activity in rice leaf lamina inclination test. Phytochemistry 52 815–818 Occurrence Handle1:CAS:528:DC%2BD3cXhtFequg%3D%3D
H Seto S Fujioka H Koshino S Yoshida M Tsubuki T Honda (1999c) ArticleTitleSynthesis and biological evaluation of extra-hydroxylated brassinolide analogs. Tetrahedron 55 8341–8352 Occurrence Handle1:CAS:528:DyaK1MXksVSgurc%3D
H Seto S Hiranuma S Fujioka H Koshino T Suenaga S Yoshida (2002) ArticleTitlePreparation, conformational analysis, and biological evaluation of 6a-carbabrassinolide and related compounds. Tetrahedron 58 9741–9749 Occurrence Handle10.1016/S0040-4020(02)01247-4 Occurrence Handle1:CAS:528:DC%2BD38XovVSmur0%3D
GCY Sung L Janzen RP Pharis TG Back (2000) ArticleTitleSynthesis and bioactivity of 6α- and 6β-hydroxy analogues of castasterone. Phytochemistry 55 121–126 Occurrence Handle10.1016/S0031-9422(00)00259-4 Occurrence Handle1:CAS:528:DC%2BD3cXntF2js74%3D Occurrence Handle11065287
S Takatsuto N Yazawa N Ikekawa T Takematsu Y Takeuchi M Koguchi (1983) ArticleTitleStructure–activity relationship of brassinosteroids. Phytochemistry 22 2437–2441 Occurrence Handle10.1016/0031-9422(83)80135-6 Occurrence Handle1:CAS:528:DyaL2cXhsFeqsrw%3D
S Takatsuto N Ikekawa T Morishita H Abe (1987) ArticleTitleStructure–activity relationship of brassinosteroids with respect to the A/B ring functional groups. Chem Pharm Bull 35 211–216 Occurrence Handle1:CAS:528:DyaL2sXktVClsL4%3D
S Takatsuto T Yokota (1999) Biochemical analysis of natural brassinosteroids. A Sakurai T Yokota SD Clouse (Eds) Brassinosteroids: steroidal plant hormones Springer-Verlag Tokyo 47–68
K Takeno RP Pharis (1982) ArticleTitleBrassinosteroid-induced bending of the leaf lamina of dwarf rice seedlings: an auxin-mediated phenomenon. Plant Cell Physiology 23 1275–1281 Occurrence Handle1:CAS:528:DyaL3sXivVal
MJ Thompson WJ Meudt NB Mandava SR Dutky WR Lusby DW Spaulding (1982) ArticleTitleSynthesis of brassinosteroids and relationship of structure to plant growth-promoting effects. Steroids 39 89–l05 Occurrence Handle10.1016/0039-128X(82)90129-5 Occurrence Handle1:CAS:528:DyaL38XlvFynt78%3D Occurrence Handle7080117
B Voigt A Porzel D Golsch W Adam G Adam (1996) ArticleTitleRegioselective oxyfunctionalization of brassinosteroids by methyl(trifluoromethyl)dioxirane: synthesis of 25-hydroxybrassinolide and 25-hydroxy-24-epibrassinolide by direct C-H insertion. Tetrahedron 52 10653–10658 Occurrence Handle10.1016/0040-4020(96)00587-X Occurrence Handle1:CAS:528:DyaK28XkvV2ksrc%3D
K Wada S Marumo (1981) ArticleTitleSynthesis and plant growth-promoting activity of brassinolide analogues. Agric Biol Chem 45 2579–2585 Occurrence Handle1:CAS:528:DyaL38XotFWrug%3D%3D
T Yokota K Mori (1992) Molecular structure and biological activity of brassinolide and related brassinosteroids. M Bohl WL Duax (Eds) Molecular structure and biological activity of steroids CRC Press Boca Raton, FL 317–340
T Yokota Y Ogino H Suzuki N Takahashi H Saimoto S Fujioka A Sakurai (1991) Metabolism and biosynthesis of brassinosteroids. HG Cutler T Yokota G Adam (Eds) Brassinosteroids: chemistry, bioactivity and applications. ACS Symp Ser 474. American Chemical Society Washington, DC 86–96
Acknowledgements
We thank the Natural Sciences and Engineering Research Council of Canada (Research, Strategic, and CRD grants), the Environmental Science and Technology Alliance of Canada, Agritope Inc. (now Exelixis Plant Sciences Inc.), and CIDtech Research Inc. for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Back, T.G., Pharis, R.P. Structure–Activity Studies of Brassinosteroids and the Search for Novel Analogues and Mimetics with Improved Bioactivity . J Plant Growth Regul 22, 350–361 (2003). https://doi.org/10.1007/s00344-003-0057-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-003-0057-0