Abstract
Nitrogen fixing bacteria, collectively referred to as rhizobia, are able to trigger the organogenesis of a new organ on legumes, the nodule. The morphogenetic trigger is a Rhizobium-produced lipochitin-oligosaccharide called the Nod factor, which is necessary, and in some legumes sufficient, for triggering nodule development in the absence of the bacterium. Because plant development is substantially influenced by plant hormones, it has been hypothesized that plant hormones (mainly the classical hormones abscisic acid, auxin, cytokinins, ethylene and gibberellic acid) regulate nodule development. In recent years, evidence has shown that Nod factors might act in legumes by changing the internal plant hormone balance, thereby orchestrating the nodule developmental program. In addition, many nonclassical hormonal signals have been found to play a role in nodule development, some of them similar to signals involved in animal development. These compounds include peptide hormones, nitric oxide, reactive oxygen species, jasmonic acid, salicylic acid, uridine, flavonoids and Nod factors themselves. Environmental factors, in particular nitrate, also influence nodule development by affecting the plant hormone status. This review summarizes recent findings on the involvement of classical and nonclassical signals during nodule development with the aim of illustrating the multiple interactions existing between these compounds that have made this area so complicated to analyze.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Bacteria of the genus Rhizobium are capable of infecting the roots of host plants, resulting in the development of novel organs called nodules. Nodule development involves the induction of cortical and pericycle cell divisions and their subsequent differentiation into a vascularized organ with a meristem. Concurrently, infection by the bacteria into root hairs and cortical cells in a so-called infection thread occurs until their eventual release into the developing nodule. Within the nodule, the invading bacteria differentiate into nitrogen-fixing bacteroids that provide reduced nitrogen to the plant in exchange for carbohydrates and shelter (for recent reviews see Crespi and Galves 2000; Stougaard 2001; Kistner and Parniske 2002).
Precise interactions between phytohormones and various other signalling compounds are imperative for plant organogenesis, and in no case is this more apparent than in the process of nodulation. In this symbiosis, various signalling molecules are exchanged between the plant and the infecting bacteria to regulate nodule initiation, differentiation and functioning, as well as the number of nodules that develop. Nodule numbers are limited by at least two pathways. One pathway is a local regulation of infection in the root zone susceptible for infection (Vasse and others 1993), while the second pathway is a negative feedback process termed “autoregulation” during which existing nodule meristems trigger a signal in the shoot that inhibits further nodule development on the root system (Delves and others 1986). For this to occur, the timing and concentrations of hormones and other signalling compounds is crucial, as alterations to either can result in the abortion of nodulation. The following review culminates much of what is known about the various signalling elements involved in nodulation and attempts to identify possible links between them. Due to the size of the topic, we have concentrated on the signals involved in nodule organogenesis and have had to ignore many of the early signals, for example calcium, known to act in the root hair following Nod factor perception. However, a recent review by Lhuissier and others (2001) covers this topic.
SIGNALLING INTERACTIONS OF THE CLASSIC HORMONES
Earlier work on nodulation investigated hormones individually in an attempt to elucidate a role for each. For example, Thimann (1936) was one of the first to propose involvement of hormones in nodule formation and implicated auxin in the process. Later, the finding that many soil bacteria, including rhizobia, synthesize plant hormones (reviewed by Costacurta and Vanderleyden 1995), initially seemed to suggest that rhizobia could provide the hormones that subsequently stimulate nodule formation (for example, Phillips and Torrey 1972), although, this did not explain the specificity between legumes and their specific symbionts. Since then, nodule initiation has been shown to occur spontaneously in some legumes (Truchet and others 1989) and can be triggered by altering the hormone balance, thus illustrating that the hormones act independently of the bacteria. In addition, the application of Nod factors can induce pseudonodule structures on certain hosts (Truchet and others 1991), possibly by altering hormone levels within the host tissue. However, because Nod factor-induced nodule primordia typically fail to develop into differentiated nodules, it is possible that hormones or other signals produced by the bacteria during the infection process are also required.
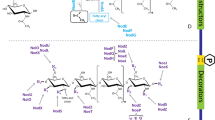
During root nodule development, rhizobia stimulate differentiated cortex cells to re-enter the cell cycle, divide and differentiate. In 1973, Libbenga and others recognized the need to assess hormone interactions during nodule development and suggested that gradients of both auxin and cytokinins are required for cortex proliferation and thus nodule initiation. Since the work of Libbenga and others (1973), much has been discovered about the complex signalling network required for nodule organogenesis. A central question in nodulation research is how changes in the hormone balance can affect the location (radially and longitudinally along the root), initiation, number and functioning of nodules on the root system. The following section discusses many of these findings and identifies the current knowledge of hormone signalling interactions in nodulation (summarized in Figure 1).
Proposed model for the interaction of hormones and other signals regulating the initiation of cell divisions and nodule development. See text for details. This figure summarizes interactions that have been analyzed separately and in different legume species. It should therefore not be seen as an accurate or complete overview for any particular legume. The flow diagram does not suggest a strict temporal but rather a functional overlap of interactions. Dashed arrows indicate that the interaction might be indirect and needs to be tested; see Conclusions and Outlook for details. The effect of nitrate on the signalling interactions is indicated in several places, but it will need to be tested as to whether some of the observed nitrate effects are indirect.
Abscisic Acid
The role of abscisic acid (ABA) in nodulation is poorly understood. Initially, ABA was thought to act as an inhibitor of nodule development, as application of the hormone reduced the number of nodules in Pisum sativum (pea) (Phillips 1971). ABA application to wild type Glycine max (soybean) and its supernodulating mutant line NOD1-3 also caused a decrease in nodule numbers and dry weights in addition to isoflavonoid levels (Cho and Harper, 1993). Moreover, Bano and Harper (2002) determined that nodule initiation, development and functioning were all inhibited by ABA in wild type and NOD1-3. Phillips (1971) speculated that ABA might act by reducing the cytokinin-stimulated cortical cell divisions associated with nodule formation, thus suggesting a putative ABA-cytokinin signalling interaction.
ABA and cytokinins have been shown to act in concert to affect numerous aspects of plant development, including root/shoot signalling (Davies and Zhang 1991) and symbiotic photosynthetic gas exchange (Goicoechea and others 1997). Since the work of Phillips (1971), the ratio of the two hormones has been positively correlated with nodule suppression and autoregulation (Caba and others 2000; Bano and others 2002). The root ABA/zeatin riboside (ZR) ratio was found to be consistently higher in wild type soybean relative to the supernodulating mutant nts382 (Caba and others 2000). Recently, Bano and others (2002) proposed a model to explain possible influences of plant ABA/ZR ratios in nodule autoregulation. In this model, inoculation induces an initial decrease in the xylem ABA/ZR ratio. These authors speculated that the hormones of this ratio are then translocated to the leaves where they promote the synthesis of ABA. The increased ABA then moves via the phloem to the root where it inhibits further nodule formation, thus regulating the number of nodules that form. In supernodulating mutants, this pathway is effectively non-functional, as the initial decrease in the xylem ABA/ZR ratio does not occur and thus proper regulation of nodule number is not achieved (Bano and others 2002). Caba and others (2000) demonstrated that a final rise in root ABA concentration is absent in the mutant, consistent with the model.
In further support of this model, Gresshoff and others (1988) illustrated via extrapolation that the concentration of ABA increased in the shoot at the onset of autoregulation in the wild type, but not in nts382. In addition, Bano and Harper (2002) demonstrated that the application of partially-purified phloem ABA-extracts, from either wild type or the supernodulating soybean mutant NOD1-3, inhibited nodule formation in the mutant. However, they found that phloem-ABA levels were similar in both lines and concluded that another signal may be present in the phloem that either inhibits nodule formation or counteracts the inhibitory effect of ABA in this autoregulatory process.
Further evidence supporting a negative role for ABA in nodule development was reported by Watts and others (1983) who analyzed the endogenous ABA content in nodules that form on the perennial Alnus glutinosa infected by the actinomycete Frankia. ABA levels were higher in nodules than in the surrounding root tissue, particularly in dormant compared with actively growing nodules. However, despite this finding, Watts and others (1983) were unable to determine any obvious correlations between nodule ABA content and growth rate.
The level of endogenous ABA is also reported to be higher in nodules of pea (Charbonneau and Newcomb 1985) and soybean (Williams and Sicardi De Mallorca 1982; Fedorova and others 1992) compared with that of the roots. Moreover, increased amounts of ABA are detected in shoots, roots and nodules of soybean plants bearing VA mycorrhiza associations when compared with nodulated nonmycorrhizal plants, suggesting that these fungal associations contribute to the ABA pool of the host, including the nodule (Murakami-Mizukami and others 1991). Because ABA had previously been shown to activate a carbohydrate sink during the seed fill phase of soybean, Murakami-Mizukami and others (1991) speculated that increased nodule ABA may act as a signal to induce a similar carbohydrate sink in the nodule. Thus, as opposed to acting as an inhibitory factor, ABA could play a role in allocating photosynthates to the nodule to be used as a source of energy for growth and development, rhizobial respiration and nitrogen fixation. Rhizobia synthesize ABA in culture when supplied with ABA-precursors (Dangar and Basu 1991) so perhaps this production is a mechanism used by the bacteria as a means of obtaining plant-derived carbohydrates. In the case of nitrogen fixation, however, nitrogenase activity has been shown to decrease with increasing endogenous ABA levels in some species (Dangar and Basu 1984, 1987). As well, the daily application of ABA significantly reduced the level of nitrogen fixation in pea (González and others 2001a), although this treatment may have exceeded an appropriate ABA concentration for optimum nodule functioning. This reduction in nitrogen fixation paralleled a decline in nodule leghemoglobin content, which the authors speculated resulted in a restriction of available oxygen required by the bacteroids for cellular respiration, thus inducing the decline in nitrogen fixation (González and others 2001b).
In Phaseolus vulgaris, ABA application increased the accumulation of lipoxygenase (LOX, Figure 2) mRNAS, which are enzymes associated with stress and development (Porta and others 1999). These authors detected LOX in developing, but not mature, nodules suggesting a role for LOX in nodule growth. Moreover, in situ hybridization revealed no exclusive LOX expression in the invasion zone of pea nodules; however, all LOX transcripts were expressed at the nodule apex (Wisniewski and others 1999), thus further suggesting a role for the enzymes in nodule growth and development rather than a more direct role in the plant-microbe interaction or in host defense. Also in pea, Charbonneau and Newcomb (1985) noted an increased amount of ABA in the apical region of the nodule, possibly indicating a link between elevated levels of nodule ABA and LOX (Figure 3). If indeed LOX is required for nodule development and ABA is required to up-regulate the level of nodule LOX, it can therefore be argued that ABA is actually required for nodule growth. Furthermore, a role for LOX has been implicated in nitrogen storage and assimilate partitioning (Stephenson and others 1998), which, if coupled with ABA, supports the hypothesis of Murakami-Mizukami and others (1991) that ABA could have a role in inducing a carbohydrate sink in the nodule.
Proposed model for the interaction of signals regulating defense responses and nodule functioning. As in Figure 1, interactions that have been analyzed separately and in different legume species are integrated in one diagram and should not be seen as an accurate or complete overview for any particular legume. The flow diagram does not suggest a strict temporal but rather a functional overlap of interactions. Dashed arrows indicate that it is unknown whether Rhizobium independently activates these responses via different signals (for example, Nod factors, exopolysaccharides, and so on) or whether Rhizobium induces one initial response that triggers further secondary events. This could be tested in mutants for ABA, SA, ethylene or NO.
Spatial changes in hormone signals in relation to nodule development. The figure shows an idealized cross-section through the root at the site of nodule formation, including the xylem poles (small circles) inside the stele, which is surrounded by the pericycle cell layer (p). A gradient of uridine (U) exists that emanates from the xylem. ACC oxidase (ACCO) is expressed opposite the phloem poles and might create local ethylene gradients that regulate possible sites for nodule initiation. Four developmental stages are shown in clockwise sequence: (1) initial infection of rhizobia at the site of root hair curling (rhc) accompanied by the induction of ethylene and reactive oxygen species (ROS) as well as ENOD40 induction in pericycle cells within hours of inoculation. (2) Precursor cells of the cortex, which will divide to become a nodule, show increased expression of GH3, ENOD40 and accumulation of specific flavonoids. (3) Early cortical cell divisions (ccd) show enhanced AUX1, GH3 and ENOD40 expression as well as flavonoid and cytokinin accumulation. (4) In a differentiating nodule, increased levels of ABA, auxin, GA and nitric oxide have been detected. AUX1, GH3 and ENOD40 expression are located in peripheral (probably vascular) tissue. Cytokinin, ABA and LOX levels are increased in the nodule meristem.
Additional evidence supporting a requirement of ABA in nodule development is the significantly reduced number of nodules that form on the ABA-deficient wilty mutant of pea (BJ Ferguson, JB Reid and JJ Ross unpublished). If the role of ABA in nodulation were of a strictly inhibitory nature, it would be expected that wilty would develop more nodules than its wild type. These findings, however, do not necessarily discredit the previously mentioned work regarding an inhibitory role for ABA in nodulation and it is possible that ABA has a dual role in nodule development: one in negatively regulating nodule numbers and one in positively regulating the growth and development of individual nodules. As such, an increase in ABA (for example, one brought about by exogenous application or stress) would directly inhibit nodule development, whereas a deficit of the hormone (as in wilty) would fail to induce signalling elements (such as LOX) required to meet the growth requirements of the nodule, thereby also inhibiting nodule formation. This hypothesis may explain why some reports of ABA application (for example, Bano and Hillman 1986) illustrate no effects of the hormone on nodule numbers.
In support of this hypothesis, Charbonneau and Newcomb (1985) reported that pea nodule ABA levels were high in the first 2 weeks of nodule development followed by a 2-week plateau and then a secondary period of elevated ABA. It is possible that the first rise in ABA is related to the regulation of nodule growth and number, the plateau corresponds to the period of nitrogen fixation and the second rise is associated with the onset of nodule senescence. These results suggest a putative third role for ABA in nodulation in which ABA increases in older nodules as part of a senescence-signalling pathway. In addition to pea, older nodules of Lens sp. (Dangar and Basu 1984), Phaseolus aureus (Dangar and Basu 1987), Samanea saman (Chattopadhyay and Basu 1989) and soybean (Fedorova and others 1992) have elevated amounts of ABA when compared with younger nodules, which the authors of these studies also suggested was related to nodule senescence. The elevated level of ABA in soybean nodules led Fedorova and others (1992) to speculate that ABA played a role in both the suppression of the formation of new nodule structures and in nodule senescence, which is consistent with our hypothesis.
Auxin
Auxin is a plant hormone with multiple roles in cell division, differentiation and vascular bundle formation, three processes that also occur during nodule formation. Auxin is synthesized mainly in the shoot and is transported to the roots by an active transport process involving import into the cell by an auxin import protein (AUX1) and active auxin export by an export protein (PIN1 and PIN2/AGR/EIR1; reviewed by Muday and DeLong 2001). Additional control stems from negative regulators of auxin export by auxin transport inhibitors that bind to proteins interacting with the auxin exporter (Muday and DeLong 2001). Thus, the plant has several targets for regulating auxin homeostasis tightly to control organogenesis.
Compared with the roots, auxins levels have been reported to be elevated in the nodules of a variety of plant species (for example, pea (Badenoch-Jones and others 1984), P. vulgaris (Fedorova and others 2000) and A. glutinosa (Wheeler and others 1979)). Increased auxin levels in legume nodules, and in nodule-like structures of non-legumes, have also been observed after application of the synthetic auxin, 2,4-dichlorophenoxyacetic acid (2,4-D) (for example, Ridge and others 1992). Early experiments suggested that the ratio of auxins to cytokinins in the root was responsible for the initiation of cortical cell divisions and nodule formation (for example, Libbenga and others 1973). In the soybean hypernodulating mutant nts386, the auxin:cytokinin balance was found to be lowered compared with the wild type, suggesting that the auxin:cytokinin ratio could be important for regulating nodule numbers (Caba and others 1998). These experiments suggested that rhizobia might manipulate auxin levels in the plant. In addition, sensitivity to auxin in Medicago sativa (alfalfa) lines correlates with the rate of spontaneous nodule formation, and nodulation efficiency can be increased by the introduction of Agrobacterium rol genes, which are known to affect auxin sensitivity and plant hormone levels (Kondorosi and others 1993).
A number of experiments suggest that rhizobia manipulate auxin transport thus changing the auxin:cytokinin ratio in the root. For example, direct measurements of auxin transport using labelled auxin showed that rhizobia inhibit acropetal auxin transport (from the root base to the tip) capacity in Vicia sativa (vetch) roots (Boot and others 1999). In addition, the expression of the auxin responsive promoter GH3 fused to the GUS reporter gene was reduced towards the root tip between 12 and 24 h following rhizobia inoculation or ballistic microtargeting of Nod factors in Trifolium repens (white clover; Mathesius and others 1998a).
High GH3-GUS expression levels were then seen 24–48 h following inoculation (Mathesius and others 1998a) and in soybean, increased auxin levels were measured 48 h after inoculation (Caba and others 2000). These results are consistent with the auxin burst hypothesis of nodulation which states that subsequent to the initial induction of nodule primordia, shoot-derived auxin export into the root is stimulated, resulting in elevated auxin levels that inhibit further nodule primordia initiations, thus controlling nodule numbers (Gresshoff 1993). This auxin burst is assumed to be defective in supernodulation mutants, where increased auxin levels following inoculation could not be detected (Caba and others 2000). Altogether, it is likely that auxin plays (at least) a dual role during nodulation: in the early stages, auxin transport inhibition might result in a reduced auxin:cytokinin ratio to allow cell division to start, and later divisions are inhibited by super optimal auxin levels (Figure 1).
The application of synthetic polar auxin transport inhibitors (PATIs), which interfere with the hormone balance, can induce pseudo-nodule structures on the root and are also sufficient to induce some of the nodulin genes inside pseudo-nodules, including ENOD2 and ENOD12 (Hirsch and others 1989; Scheres and others 1992; Wu and others 1996). More recently, it has been shown that PATIs mimic the action on Nod factors on the repression of calmodulin expression in P. vulgaris (Camas and others 2002).
In addition to PATIs, the inhibition of auxin transport could be achieved by regulating the number of auxin efflux carriers in the cells transporting auxin. Alternatively, Nod factors or chitin oligosaccharides could affect the affinity of endogenous auxin transport regulators to their binding site, similar to the effect of ethylene (Suttle 1988), and/or Nod factors could induce the synthesis or release of an endogenous auxin transport inhibitor. Other plant compounds, including ethylene, cytokinins and flavonoids (for example, Brown and others 2001; Jacobs and Rubery 1988; Murphy and others 2000; Stenlid 1976), can also inhibit auxin transport and can regulate various peroxidases and IAA oxidases, the enzymes that break down auxin (Burgh and Burgh 1966; Lee 1971), thus leading to local shifts in the plant auxin:cytokinin ratio.
Peroxidase activity is elevated in P. vulgaris nodules, presumably to limit an auxin increase in maturing nodules (Fedorova and others 2000). A temporal and spatial correlation was found between the accumulation of specific flavonoids that inhibit auxin breakdown by a peroxidase and the accumulation of GH3:GUS expression in nodule primordia (Mathesius 2001). Furthermore, the accumulation of other flavonoids that stimulate auxin breakdown was detected in cells that exhibit low GH3:GUS activity, further suggesting that a local accumulation of specific flavonoids could regulate auxin levels.
The expression of flavonoid genes (for example, PAL (phenylalanine-ammonia lyase) and CHS (chalcone synthase)) is enhanced in nodules (for example, Estabrook and Sengupta-Gopalan 1991; Djordjevic and others, 1997), and rhizobia and Nod factors can induce flavonoid gene expression and localized flavonoid accumulation (for example, Djordjevic and others 1997; Lawson and others 1996; Mathesius and others 1998b; Schmidt and others 1994). Therefore, it has been suggested that Nod factors could have a role in inducing flavonoid accumulation at the infection site, followed by changes in the auxin balance (Hirsch 1992; Mathesius and others 1998a). By micro-targeting flavonoids into roots of white clover carrying the GH3:GUS construct, it was shown that flavonoids had similar effects on auxin distribution as Nod factors and synthetic auxin transport inhibitors. Although this suggests that flavonoids could mimic Nod factor action, it remains unclear if the exact flavonoids induced by rhizobia in the root would mediate this response in the concentration present in the tissue, and whether these flavonoids would be sufficiently mobile to reach their binding site.
There is also evidence that auxin distribution is regulated locally in nodule primordia and mature nodules, which would allow for spatial control of cell division in the root (Figure 3). Direct measurements of auxin (that is, indole acetic acid, IAA) contents in P. vulgaris roots and nodules showed increased IAA levels in roots preceding nodule formation and during the early stages of nodule emergence, whereas auxin levels dropped in mature nodules (Fedorova and others 2000). In white clover, expression patterns of GH3:GUS indicated that auxin levels and/or sensitivity are increased in early dividing cortical cells (Mathesius and others 1998a). GH3:GUS expression then decrease in the differentiating nodule primordium and remain only in developing vascular tissue, consistent with a role of auxin in triggering cell division and vascular bundle formation. Recent studies by de Billy and others (2001) have expanded this idea by showing that in Medicago truncatula AUX1-related genes (termed MtLAX) are induced in early nodule primordia and developing vascular tissue. These expression sites mirrored those of GH3:GUS in white clover (Figure 3), which suggests that auxin might increase in early nodule primordia by regulation of auxin import into these cells.
The role of auxin in nodulation is tightly linked to the development of other root structures, including lateral roots and root galls, which require similar induction of new cell divisions and differentiation as nodules. Auxin transport is required for lateral root induction (Bhalerao and others 2002) and auxin appears to accumulate not only in nodule but also lateral root primordia (Himanen and others 2002) and root galls caused by nematodes (Goverse and others 2000; Hutangura and others 1999). Expression levels of GH3:GUS were very similar in developing nodule and lateral root primordia (Mathesius and others 1998a). These similarities are likely due to auxin-induced activation of cell cycle genes that are required for the induction of new cell divisions during organogenesis (Doerner and others 1996; John and others 1993). A genetic link between regulation of root system architecture and nodulation has been found in the Lotus japonicus (lotus) har1 (hypernodulation aberrant root formation) and the soybean nts mutants (Wopereis and others 2000; Searle and others 2003, respectively), which are both supernodulating mutants that show increases in the number of lateral roots in the uninoculated state and altered activities of the root apical meristem. Because auxin affects both lateral root, nodule and meristem formation, it is tempting to speculate, and pertinent to test, whether autoregulation exerts some of its effects via changes in auxin homeostasis, or whether additional, or different, signals are involved. The fact that lateral root frequency is not affected in the supernodulation mutant astray in L. japonicus suggests the existence of nodule specific regulators in addition to regulation of all root meristems (Nishimura and others 2002b).
Cytokinins
Cytokinins are a class of plant hormones having diverse roles in cell cycle regulation and differentiation. Re-activation of the cell cycle initiates nodule primordium formation (Foucher and Kondorosi 2000; Goormachtig and others 1997; Yang and others 1994) and cytokinins, together with auxin and ethylene, play a major role in cell cycle progression in plants (D’Agostino and Kieber 1999). Therefore, it is likely that cytokinins are also necessary for new cortical cell divisions initiated by Rhizobium. However, even though cytokinins have been reported to be synthesized by different bacteria, including rhizobia (Phillips and Torrey 1970, 1972), it is unlikely that cytokinins provided by rhizobia are the main factors necessary for nodule initiation, because purified Nod factors are sufficient to induce nodules in some legume species. Instead, it is more likely that Nod factors trigger changes in cytokinin synthesis, turnover or sensitivity in the roots during nodule initiation.
Either way, several pieces of evidence suggest that rhizobia do induce changes in the cytokinin balance of the root. Nodule cytokinin levels are reported to be elevated in numerous plant species when compared with the roots (for example, pea (Badenoch-Jones and others 1987), Phaseolus mungo (Jaiswal and others 1981), Myrica gale (Rodriguez-Barrueco and others 1979), and Vicia faba (Hensen and Wheeler 1976)). In pea, Newcomb and others (1976) showed that nodule cytokinin levels were highest in young, developing nodules and decrease with maturity. Syono and others (1976) demonstrated that the highest cytokinin levels in the pea nodule were located in the meristem (Figure 3). This agrees with the role of cytokinin in cell division and differentiation and supports the results of Newcomb and others (1976) as young nodules would be the most mitotically active and thus one would expect them to contain elevated levels of the hormone.
The application of cytokinins induces the formation of pseudo-nodule structures on legumes and non-legumes, including Nicotiana tabacum (tobacco) (Arora and others 1959), A. glutinosa (Rodriguez-Barrueco and Bermudez de Castro 1973), pea (Libbenga and others 1973), Macroptilium atropurpureum (siratro) (Relic and others 1994) and alfalfa (Cooper and Long 1994; Bauer and others 1996). Cooper and Long (1994) transferred the Agrobacterium trans-zeatin secretion gene into E. coli and nodulation deficient mutants of R. meliloti, and showed that synthesis of the cytokinin zeatin of these bacteria is sufficient to induce nodule-like structures in alfalfa. However, it is important to note that the concentration of cytokinins is important in determining whether a stimulating or inhibiting effect on nodulation occurs (Lorteau and others 2001).
The roles of cytokinins during nodule development include, as expected, the activation of the cell cycle and genes associated with it (Jelenska and others 2000). For example, cytokinins induce the expression of Msgbl, which is expressed in dividing cells of alfalfa, including those of the nodule primordia, and may be involved in hormone-mediated cell division including having a putative signal transduction role during nodule organogenesis (McKhann and others 1997). Cytokinins may also be important for activating a number of early nodulin genes. For example, ENOD2, a gene expressed in nodules and nodule primordia, can be induced by cytokinins in Sesbania rostrata (Dehio and deBrujin 1992) and in alfalfa (Cooper and Long 1994; Bauer and others 1996). ENOD12A, coding for a hydroxyproline-rich glycoprotein that is expressed during nodule organogenesis, can also be induced by cytokinins in addition to Nod factor treatment (Bauer and others 1996). Another early nodulin gene that may have an important role in organ formation is ENOD40 (see Signalling Peptides section below), which is also induced by both Rhizobium and cytokinins in alfalfa (Fang and Hirsch 1998; Mathesius and others 2000; Sinvany and others 2002). Screening of molecular markers in alfalfa identified seven nodulin genes regulated by cytokinins, four of which were also inducible by auxin, suggesting partial overlaps between auxin and cytokinin regulated pathways during nodulation (Jimenez-Zurdo and others 2000). Cytokinins have further been shown to affect ethylene levels in pea roots (Lorteau and others 2001). However, Lorteau and others (2001) were unable to demonstrate a direct correlation between cytokinin-induced ethylene and nodule inhibition, as inhibitors of ethylene synthesis did not restore nodulation in plants treated with high levels of cytokinin.
Cytokinins probably also play a role in setting up a carbohydrate sink for the developing nodule as they can induce starch formation in the root cortex, similar to that of Rhizobium infection (Bauer and others 1996). The use of a split root system in vetch has shown that cytokinin treatment of a root can also induce acidification of the growth medium around a separate root of the same plant (van Brussel and others 2002). These authors suggest that while cytokinins do not appear to be the autoregulation signal, they might create a sink in the inoculated root, which sends a signal to the shoot that regulates metabolism, including acid secretion, in the uninoculated roots. This cytokinin-induced root signal could play a role in autoregulation, in addition to the so far unidentified autoregulation signal from the shoot, which requires actively dividing cortex cells (van Brussel and others 2002).
Legume mutants such as R50 (pea) and MN1008 (alfalfa) also provide valuable tools for investigating the roles of cytokinins in nodulation. R50 develops abnormal infection threads that twist and bulge as opposed to properly progressing into the inner cortex (Lorteau and others 2001). Lorteau and others (2001) demonstrated that this characteristic could also be induced in wild type pea upon cytokinin application. Interestingly, nodulation is rescued in R50 by the application of inhibitors of ethylene biosynthesis or action. However, as stated above, the same ethylene inhibitors were unable to reverse the effects of cytokinin application on wild type pea.
The application of cytokinins to the Rhizobium and Nod factor resistant MN1008 overcomes the nodulation block in this mutant (Hirsch and others 1997), suggesting that this plant has low levels of the hormone or is unable to increase its cytokinin levels to meet the requirements for nodule initiation. PATIs were also reported to induce pseudo-nodules in this mutant (Hirsch and Fang 1994), suggesting again that the cytokinin:auxin ratio rather than cytokinins alone might be important for nodulation. The mutated gene in MN1008 was recently cloned and identified as a receptor kinase (Endre and others 2002).
Further evidence that cytokinins play a role in cell division and autoregulation comes from the receptor kinase mutant har1 of L. japonicus (Krussel and others 2002; Nishimura and others 2002a). The har1 mutant has a short root phenotype that can be mimicked in the wild type by application of cytokinin. However, in the presence of the ethylene synthesis inhibitor aminoethoxyvinylglycine (AVG), cytokinin caused root elongation in the mutant in excess of untreated wild type levels, suggesting that har1 has an altered response or sensitivity to cytokinin that is not mediated by ethylene (Wopereis and others 2000).
Ethylene
Ethylene is a gas with multiple roles in plant development and defense. Its role in nodulation has recently been reviewed by Guinel and Geil (2002) and Wang and others (2002). Ethylene might have a dual effect on nodulation in that it causes a local inhibition of nodule formation in most legumes but might be required at certain levels for proper infection by the bacteria. The application of ethylene, or ethylene-releasing compounds, is inhibitory to nodule organogenesis in numerous species including P. vulgaris (Grobbelaar and others 1971), pea (Drennon and Norton 1972; Lee and LaRue 1992c), white clover (Goodlass and Smith 1971), Melilotus alba (sweet clover) (Lee and LaRue 1992c), M. truncatula (Penmetsa and Cook 1997), L. japonicus, and siratro (Nukui and others 2000). Grobbelaar and others (1971) found that ethylene also reduced the level of nitrogen fixation in P. vulgaris. In pea, Lee and LaRue (1992c) determined that ethylene concentrations as low as 0.07 µL/L are able to inhibit nodule formation. It appears, however, that soybean is less sensitive to the hormone as nodulation of this species is not affected by applied ethylene (Lee and LaRue 1992c; Schmit and others 1999; Nukui and others 2000). This finding suggests that different species display different requirements and regulatory mechanisms for hormones, a point that must be considered for any hormone when investigating its roles in processes such as nodulation.
Inoculation of roots with rhizobia has been reported to induce increases in the local ethylene concentration in alfalfa (Ligero and others 1986), vetch (van Workum and others 1995), and soybean (Suganuma and others 1995), but this increase was not detected in pea (Lee and LaRue 1992b). These increases are likely due to an initial defense response elicited by the invading bacteria, which, interestingly, also synthesize the hormone (Billington and others 1979).
The application of inhibitors of ethylene synthesis (for example, AVG) or perception (for example, silver ions) increased the number of nodules that formed on pea (for example, Lee and LaRue 1992a), alfalfa (Peters and Crist-Estes 1989; Caba and others 1998), L. japonicus and siratro (Nukui and others 2000). These compounds also partially restored the nodulation phenotype of low nodulating mutants of pea including sym5 (Fearn and LaRue 1991), brz (Guinel and LaRue 1992) and sym21 (Markwei and LaRue 1997) and completely restored that of sym16 (Guinel and Sloetjes 2000). Surprisingly, the nodulation phenotype of sym17, a pea mutant thought to overproduce the hormone, is not rescued with the application of ethylene inhibitors (Lee and LaRue 1992a). Interestingly, Yuhashi and others (2000) illustrated that Bradyrhizobium elkani-produced rhizobitoxine, which acts as an inhibitor of ethylene synthesis, also enhances the nodulation of siratro, possibly by helping the bacteria overcome ethylene’s inhibitory effects on nodulation. Additionally, Roddam and others (2002) recently illustrated that the role of ethylene in nodulation can depend on the infecting Rhizobium cultivar as the application of AVG to Trifolium subterraneum (subterranean clover) enhanced the nodulation by some, but not all, strains of R. leguminosarum.
The mechanism of ethylene action as an inhibitor of nodulation is not known. One proposal is that ethylene induces plant chitinases, which subsequently destroy Nod factors and thereby limit the extent of nodule initiation (Mellor and Collinge 1995; Staehelin and others 1994).
Oldroyd and others (2001) postulated that a block in nodulation induced by ethylene could occur very early during the signal transduction cascade. Evidence for this came from the finding that the sensitivity of root hair cells to Nod factors is significantly increased in the skl mutant, and that modulation of ethylene synthesis in the wild type had comparable effects on the sensitivity of Nod factor perception. Ethylene appears to influence a component at, or upstream of, calcium spiking in the Nod factor signal transduction pathway leading Oldroyd and others (2001) to propose that, in addition to inhibiting the frequency of calcium spiking, the hormone determines the Nod factor concentration required for the root hair Ca2+ spiking response. These authors also illustrated that in M. truncatula, ethylene regulates the expression of the early nodulin genes ENOD11 and RIP1 and thus might effect events downstream of the early influence on calcium spiking.
Guinel and Geil (2002) proposed a model in which the rhizobia would not come into contact with ethylene in the root until after the epidermis, as this cell layer contains no ACC oxidase (the enzyme that catalyzes the conversion of 1-aminocyclopropane-1-carboxylic acid (ACC) to ethylene) and does not appear to perceive the hormone. Consistent with this model is evidence that in pea, ethylene appears to block rhizobial entry into the root cortex, rather than the number of infection events (Lee and LaRue 1992c). This finding is supported by work with the brz mutant of pea, which has a third less infection events than its wild type. Although nodulation in brz is partially restored by ethylene inhibitors, the number of infection events is only slightly increased (Guinel and LaRue 1992).
Contrary to these findings with pea, ethylene does appear to negatively regulate rhizobial colonization of M. truncatula as the application of AVG increased the number of infection events, whereas ACC decreased them (Oldroyd and others 2001). In addition, the ethylene insensitive skl mutant of M. truncatula has a significantly increased number of infection events compared with that of its wild type (Penmetsa and Cook 1997; Oldroyd and others 2001). The skl mutant is also unable to regulate the number of these events that develop into fully functional nodules and as such it hypernodulates (Penmetsa and Cook 1997). Although ethylene is unlikely to be involved in systemic autoregulation (Nishimura and others 2002c; Wopereis and others 2000), it is likely that ethylene plays a role in regulating infection events locally in the susceptible root zone, as demonstrated in the skl mutant.
Ethylene may positively influence infection thread development as the number of infection threads aborted in skl is very low (Penmetsa and Cook 1997; Oldroyd and others 2001). Guinel and Geil (2002) suggested that in pea, ethylene could affect the cytoskeleton, preinfection thread and infection thread formation. Using pea and vetch, Heidstra and others (1997) demonstrated that ethylene is also likely to be involved in determining the positioning of nodule primordium development around the stele (Figure 3). These authors showed that the expression of ACC oxidase is elevated in the inner cortical cells located in front of the root phloem poles. These locations are between the positions at which nodules preferentially arise opposite the root xylem poles. In addition, inoculation of vetch with R. leguminosarum induces ethylene-related responses, including a thick and short root phenotype and abnormal nodule positioning on the root system, which is restored following AVG application (Zaat and others 1989; van Spronsen and others 1995).
Interestingly, ethylene was also discovered to change the phenotype of nodules of Sesbania rostrata, a legume that grows in waterlogged soils and therefore likely to be exposed to varying levels of ethylene (Fernáandez-López and others 1998). The authors found that in the absence of ethylene (perception), nodules were of the indeterminate type, whereas in the presence of ethylene, determinate nodules with a terminal meristem were formed, suggesting a role for ethylene in meristem differentiation.
Gibberellins
Little is known about the signalling involvement of gibberellins (GAs) in nodulation. Early work focused on applying the hormone (generally GA3) to the plant, which resulted in a decline in nodule formation (Thurber and others 1958; Galston 1959; Fletcher and others 1959; Mes 1959). In 1952, Nutman demonstrated that the removal of root tips and mature nodules from various red clover sp. promoted the formation of new nodules, presumably by removing the source of a compound inhibitory to nodulation. Based on the results of Nutman (1952), and evidence that nodules of pea and P. vulgaris contain elevated levels of GAs, Radley (1961) speculated that GAs regulate nodule formation. Since then, nodules of Lupinus luteus (Dullaart and Duba 1970), A. glutinosa (Henson and Wheeler 1977), Phaseolus lunatus (Evensen and Blevins 1981), soybean (Williams and Sicardi de Mallorca 1982), Lens sp. (Dangar and Basu 1984), Phaseolus aureus (Dangar and Basu 1987), P. vulgaris (Atzorn and others 1988), S. saman (Chattopadhyay and Basu 1989) and Vigna unguiculata (cowpea) (Dobert and others 1992b and c) have all been reported to contain higher levels of GAs than adjacent root tissue, yet to date no direct evidence implies a signalling role for GAs in the regulation of nodule formation.
In 1970, Dullaart and Duba reported in L. luteus that, in addition to having increased GA levels in nodule extracts compared with those of the surrounding root tissue, the application of GA3 to nodule extracts stimulated IAA production from L-tryptophan. These authors speculated that a signalling interaction existed between the two hormones in which GA3 was able to either increase the bioproduction, or decrease the metabolism, of IAA (Figure 1), but the mechanism underlying this interaction has still not been demonstrated. However, the reverse interaction has since been confirmed in stems, where the biosynthesis of GA1 requires the presence of IAA (Ross and others 2000). In addition, the application of PATIs to the stem reduces GA1 levels below the site of PATI application, corresponding with the IAA level at these locations (Ross 1998). PATIs can induce the formation of pseudonodules on the root systems of various species, and as such it will be interesting to investigate what role(s) GAs, and possibly more importantly GA/IAA ratios, play in the formation of these outgrowths. Recently, IAA was shown to promote root growth in Arabidopsis by modulating cellular responses to GAs (Fu and Harberd 2003) and it seems possible that a similar interaction might exist between the two hormones in regulating nodule development.
Nodule GA levels appear to be influenced by the infecting Rhizobium strain in P. lunatus (Tripplett and others 1981; Dobert and others 1992a, c), contrary to a report on P. vulgaris nodules (Atzorn and others 1988). Many reports have demonstrated that various Rhizobium strains are capable of synthesizing GAs in culture (for example, Katznelson and Cole 1965; Rademacher 1994). Recently, putative GA biosynthetic enzymes were identified in Bradyrhizobium japonicum that function anaerobically, including under the symbiotic conditions that bacteroids are subjected to in the symbiosome (Tully and others 1998), suggesting that rhizobia might be capable of regulating GA levels both before and after bacteroid differentiation. However, whether or not the elevated GA levels of P. lunatus nodules stem directly from rhizobial synthesis, or if the bacteria induce the plant to increase GA production, is unknown (Dobert and others 1992c). Dobert and others (1992c) hypothesized that, in addition to the bacterial strain, nitrogen, ABA and even the host plant species may have a role in regulating nodule GA concentrations.
The application of GA3, and to a lesser extent GA4, induced the formation of nodule-like structures on the roots of L. japonicus (Kawaguchi and others 1996). These structures initiated from divisions of the pericycle and could be suppressed with the addition of nitrate. Thus, it appears that an interaction exists in L. japonicus whereby GAs positively regulate the division of pericycle cells necessary for nodule organogenesis and that nitrates modulate this process by acting as signalling elements that suppress these GA-induced divisions.
Nonetheless, it has been argued that an increased concentration of GAs might not be a requirement for nodule formation in some species, such as P. vulgaris (Atzorn and others 1988). If elevated GA levels are not required for nodulation, then based on the previously mentioned work demonstrating that GAs are influenced by IAA, the increased GA levels detected in nodules may be no more than a consequence of the high IAA levels also present there.
As an alternative to having a role in nodule formation, GAs may act as signals for the hydrolysis of nodule starch to provide a substrate for rhizobial respiration requirements. GAs promote the production of α-amylase (for example, Gubler and others 1995), an enzyme involved in the metabolism of starch, and it may be worth investigating whether or not the activities of the hormone and the enzyme are interacting within the nodule. Evidence for a link between GAs and α-amylase in starch hydrolysis exists for various fungal species (reviewed in Rademacher 1994), but to the best of our knowledge, the idea that GAs might have a similar role in nodulation has not been proposed previously. If a correlation is established among GAs, α-amylase and starch in nodulation, it is possible that the bacteria are responsible for regulating nodule GA levels as a means of obtaining nutrients. As we hypothesized for ABA and ethylene, this alludes to multiple roles for GAs in nodulation, including aiding in cell division and elongation and providing the energy requirements for the nitrogen-fixing bacteria. Elevated nodule GA levels have also been correlated with increased internode number and length and increased petiole length in P. lunatus (Tripplett and others 1981; Dobert and others 1992a, c) and cowpea (Dobert and others 1992b, c). Thus, GAs may benefit both symbionts by increasing the plants size, thereby increasing the photosynthetic capability of the plant, resulting in more photosynthates for plant and nodule growth and functioning.
SIGNALLING PEPTIDES
Apart from the classical plant hormones, peptides have recently emerged as potential regulators of nodulation. Compared with animal peptide hormones, only a few plant signalling peptides have been discovered so far. However, this number is likely to rise because more and more receptor kinases are being identified as playing a role in plant development and nodulation, many of which could be activated by peptide ligands. For example, recent discoveries of receptor kinases responsible for early Nod factor perception/signal transduction (“NORK”), (Endre and others 2002; Stracke and others 2002) and for autoregulation of nodulation (“NARK”) (Krusell and others 2002; Nishimura and others 2002a; Searle and others 2003) indicate that peptides or proteins could be ligands for these nodulation-related receptor kinases.
One putative peptide that plays an important role in nodulation is the early nodulin ENOD40. There has been some debate on whether or not ENOD40 is actually translated. Several ORFs have been identified with stable predicted secondary structures, and it was initially suggested that ENOD40 acts in the form of a stable RNA, a so-called “riboregulator” (Asad and others 1994; Crespi and others 1994). However, Sousa and others (2001) found that translation of two small ENOD40 ORFs is necessary for biological function (induction of cortical cell division) and Röhrig and others (2002) reported detection of one of the ENOD40 peptides by immunoprecipitation and Western blotting. Mutational analysis suggests that the translated products might have a role in stabilizing a biologically active ENOD40 mRNA structure (Sousa and others 2001). It is therefore possible that both the peptide and the mRNA are necessary for biological function as a ribonucleoprotein (Sousa and others 2001), although no target or receptor has so far been found.
ENOD40 appears to play an important role in cell cycle control because over-expression (Charon and others 1997) and microtargeting (Sousa and others 2001) of ENOD40 induces cortical cell divisions in alfalfa roots in the absence of rhizobia and causes teratomas in Medicago embryos. In the presence of rhizobia, overexpression of ENOD40 was shown to accelerate nodulation (Charon and others 1999). In contrast, silencing of ENOD40 leads to arrest of callus growth in Medicago (Crespi and others 1994). Recent evidence suggests that ENOD40 might play a role in sucrose partitioning or unloading from the phloem in the nodule (and/or the whole plant), because synthetic ENOD40 peptides bind to nodulin 100, a sucrose synthase (Röhrig and others 2002). A role in sucrose partitioning might be related to ENOD40’s role in promotion of (cortical) cell division because incipient meristems are strong carbohydrate sinks. The expression of ENOD40 in vascular tissue in roots and mature nodules (Kouchi and Hata 1993) supports a role in sucrose unloading.
ENOD40 has been identified in many legumes as well as the non-legume rice (Kouchi and others 1999). In all legumes examined, ENOD40 mRNA has been localized in dividing and meristematic cells (Figure 3; for example, Asad and others 1994; Corich and others 1998; Crespi and others 1994; Fang and Hirsch 1998; Mathesius and others 2000; Yang and others 1993), consistent with the hypothesis that ENOD40 plays a role in cell division. ENOD40 is thought to be involved in the earliest stages of nodule initiation because it is expressed within hours of inoculation with nodulating rhizobia (Corich and others 1998; Fang and Hirsch 1998) and its expression in the pericycle precedes nodule initiation (Figure 3) (Compaan and others 2001). In addition, ENOD40 expression is induced by signal molecules that can initiate cortical cell divisions, including Nod factors (Fang and Hirsch 1998; Minami and others 1996), cytokinins (Fang and Hirsch 1998; Mathesius and others 2000), and auxin transport inhibitors (Fang and Hirsch 1998). ENOD40 is also induced in the nodule primordium by Rhizobium strains that induce cell divisions but do not infect and invade the nodules (Yang and others 1993), which is a further indication that ENOD40 is involved in nodule morphogenesis, rather than the infection process. However, ENOD40 is not specific to the nodulation process, and is also induced during the establishment of lateral root primordia (Mathesius and others 2000) nematode-induced galls (Favery and others 2002; Koltai and others 2001) and mycrorrhizal interactions (Staehelin and others 2001; Sinvany and others 2002).
DEFENCE-RELATED SIGNALLING COMPOUNDS
In addition to its previously mentioned roles in nodulation, ethylene is involved in pathogenic defense as part of a signalling process termed “systemic acquired resistance” (SAR). Other components of SAR include salicylic acid (SA), nitric oxide (NO), reactive oxygen species (ROS), jasmonic acid (JA) and its methyl ester (MeJA) (reviewed in Ryals and others 1996; Rojo and others 2003). Although the mechanism is not fully understood, symbiotic organisms invade the host plant without fully inducing the SAR response. However, Vasse and others (1993) demonstrated that some plant defense compounds do accumulate following the establishment of the first nodule primordia, resulting in increased abortion of infection threads and localized hypersensitivity response (HR), including necrosis. These authors suggested that this response is part of the autoregulatory mechanism used by plants to control the level of nodulation. Despite this and much work involving ethylene (described above), little is known about the signalling involvement of other SAR components regarding nodulation; major findings involving these compounds are addressed in the following section (see also Figure 2).
Salicylic Acid
Pre-soaking seeds with salicylic acid (SA) prior to sowing decreased the nodule number and protein content and root nitrogenase activity of Vigna mungo plants (Ramanujan and others 1998). SA application prior to inoculation with rhizobia or purified Nod factor also decreased the number and dry weight, and delayed the emergence, of alfalfa nodules (Martínez-Abarca and others 1998). van Spronsen and others (2003) found that 0.1 mM SA application completely inhibited indeterminant nodule formation, including the mitogenic effect induced by Nod factors, in vetch, pea (including the hypernodulating mutant P88), alfalfa and white clover but did not affect determinant nodule formation in P. vulgaris, L japonicus and Glycine soya. In contrast to these findings, in soybean, 5 and 1 mM SA did decrease the nodule number and dry weight and suppressed photosynthesis and nitrogen uptake (Lian and others 2000). Also in soybean, Sato and others (2002) found that concentrations of SA as low as 0.1 mM applied 5 days prior to bacterial inoculation decreased the nodule number and dry weight in addition to the level of nitrogen fixation. SA also reduced the nodule number and dry weight in supernodulating soybean mutants, but the decreases were less pronounced than in the wild type. Sato and others (2002) proposed that SA, or SAR induced by SA, might be involved in an autoregulatory signalling pathway of nodulation.
Upon symbiont recognition, the root-SA level of alfalfa did not increase (as occurs upon plant-pathogen recognition), although it did increase in plants inoculated with either an incompatible or a compatible but Nod factor-deficient mutant of Rhizobium (Martínez-Abarca and others 1998; Blilou and others 1999). Thus, it was concluded that a function of Nod factors is to inhibit host SA-mediated defenses. Interestingly, upon inoculation with a compatible rhizobial strain, the root-SA level of the pea sym30 mutant did increase, whereas upon inoculation with plant pathogens, an increase was not detected (Blilou and others 1999). Thus, the gene product appears to function specifically with symbiotic microorganisms leading Blilou and others (1999) to conclude that the product is likely required for symbiosis, as a suppressor of a SA-dependent defense response.
In Rhizobium etli, multi-drug resistance genes have been identified that act as bacterial efflux pumps that confer resistance to the accumulation of toxic compounds. Mutations to two of these genes, termed rmrA and rmrB, enhanced the sensitivity of the bacteria to plant toxins including phytoalexins, flavonoids and SA (González-Pasayo and others 2000). These mutants displayed diminished growth on SA or naringenin, and the rmrA mutant formed 40% fewer nodules on P. vulgaris than its wild type (González-Pasayo and others 2000). It was concluded that by preventing the accumulation of toxic compounds, R. etli have established an advantage that improves their chances of nodulating the host. In addition, SA was found to promote isoflavonoid (for example, genistein) synthesis and secretion from L. luteus roots (Kneer and others 1999). Genistein can function as a phytoalexin due to its slight antimicrobial and fungistatic activity and thus rhizobia containing resistance genes to such a toxin should have an infectious advantage over bacteria lacking the efflux pump.
Nitric Oxide
In nitrogen-fixing rhizobia, heme-based sensors have been detected, such as the oxygen-regulated FixL protein kinase in R. meliloti (Gilles-González and others 1994). When active, the deoxy-FixL protein induces a gene expression cascade required for nitrogen fixation. This process is inhibited by the presence of O2, and possibly also by NO and CO, thus halting nitrogen fixation (Gilles-González and others 1994). Therefore, NO may have a role in regulating gene expression required for nitrogen fixation within the nodule.
NO has been identified as an inhibitor of bacteroid nitrogenase (for example, Trinchant and Rigaud 1982). Maskell and others (1977) illustrated that NO tightly binds to leghemoglobin (Lb) in soybean and cowpea nodules forming nitrosyleghemoglobin complexes (NO-Lb) and suggested that Lb may actually have a higher affinity for NO than it does for O2. Thus, the NO-Lb complex may act as a protective mechanism used by the nodule to prevent the inhibiting NO from reaching the NO-sensitive nitrogenase of the bacteroid. Alternatively, the accumulation of NO-Lb may result in the inhibition of nitrogenase activity (Kanayama and Yamamoto 1990) as the binding of NO to Lb may competitively inhibit the binding of oxygen, subsequently diminishing the oxygen supply available to bacteroids, thereby reducing nitrogen fixation (Mathieu and others 1998).
Soybean nodules on roots exposed to high concentrations of nitrate mainly contained NO-Lb (Kanayama and Yamamoto 1990) and declined in nitrogen fixation rates paralleled by the increase in NO-Lb in these nodules. Thus, the plant may induce NO synthase (NOS) in response to excess exogenous nitrate as a means of regulating nitrogen fixation activity. However, Mathieu and others (1998) found that even in the absence of applied nitrate, some NO-Lb exists in soybean nodules. These authors found that the amount of NO-Lb was highest in young nodules, decreased with nodule age, and was nearly absent in senescent or H2O2-treated nodules. Moreover, in soybean plants grown in controlled environmental conditions, NO-Lb was shown to comprise almost a third of the total nodule Lb content (Maskell and others 1977), but to date, no definitive evidence exists to explain this occurrence.
NOS activity has been detected in nodules of Lupinus albus (Cueto and others 1996). Two putative NOS sites were detected: one in the vascular bundles and the other in the inner cells of the infected zone (Cueto and others 1996). In contrast to root preparations, the synthesis of nodule NO was found to be Ca2+ independent and the authors speculated that nodule NOS could possibly be induced by compounds such as lipopolysaccharides of compatible Rhizobia sp.
Reactive Oxygen Species
To prevent pathogen invasion, reactive oxygen species (ROS) or active oxygen species (AOS), including hydrogen peroxide (H2O2), superoxide radicals O2and the hydroxide radical (•OH) are upregulated in the plant upon pathogen recognition. Together, these compounds reinforce plant cell walls and trigger a localized hypersensitive response (HR) involving defense gene expression, the induction of SAR and programmed cell death (reviewed in Ryals and others 1996). ROS are also induced in host plants upon inoculation with Rhizobium (for example, Bueno and others 2001; Santos and others 2001) and thus it is imperative that the bacteria compensate for these defense molecules in order to achieve nodule organogenesis. Both plant and bacterial compounds exist that help protect against the harmful effects of ROS, including peroxidases, catalases and superoxide dismutase (SOD) among others, and Sinorhizobium meliloti genes induced upon host infection include those that protect against ROS (Oke and Long 1999). However, aside from having negative effects, ROS can also positively regulate the nodulation process.
Peroxidase activity increases shortly after inoculation at the site of root hair deformation (Salzwedel and Dazzo 1993). The activity appears to have a role in oxidative cross-linking of cell wall polymers at the site of rhizobial penetration, resulting in a hardening of the cell wall structure. H2O2 can act as a substrate for peroxidase in this process, thus illustrating a potential role for low levels of certain ROS during nodulation. Salzwedel and Dazzo (1993) speculated that for successful infection to occur, the rhizobia must first suppress root hair peroxidase activity, therefore allowing the bacteria to penetrate the cell wall of the host. The authors suggested that a rapid and transient decrease in peroxidase activity could be evoked by rhizobial exopolysaccharides (EPS) which rapidly bind to root hairs, increase infection frequency and may aid the bacteria in avoiding the elucidation of SAR during invasion. Following penetration, highly localized peroxidase activity might be required to repair the eroded root hair cell wall at the site of rhizobial entry and infection thread initiation. Salzwedel and Dazzo (1993) also speculated that the plants might resist non-host bacteria and pathogens by rapidly increasing localized peroxidase levels to harden the root cell walls and prevent their invasion.
Prior to rhizobial infection of M. truncatula, Nod factors trigger a rapid and localized expression of the putative peroxidase-encoding RIP1 early nodulin gene (Cook and others 1995), as does ethylene (Olroyd and others 2001). As a peroxidase, RIP1 could have a role in metabolizing H2O2 and/or in peroxidase-mediated cross-linking of cell wall polymers. The RIP1 transcript was localized to epidermal cells that subsequently were infected by Rhizobium and were expressed for the duration of pre-infection (Cook and others 1995), suggesting a possible involvement in cell wall repair at the site of infection. Recently, Ramu and others (2002) demonstrated that RIP1 transcripts and ROS share a similar pattern of localization in M. truncatula and that Nod factor application elicits a rapid induction of each. Neither ROS nor RIP1 expression was detected using a Nod factor-deficient mutant of Sinorhizobium meliloti or a mutant of M. truncatula impaired in Nod factor signal transduction. Moreover, Ramu and others (2002) found that H2O2 specifically induced RIP1 expression, leading the authors to speculate that Nod factor perception by the plant induces H2O2 production, which then mediates the Nod factor-induced expression of RIP1. This finding seems logical because H2O2 can act as a substrate for peroxidases, such as the putative RIP1.
In pea, Wisnewski and others (2000) found that the insolubilization of matrix glycoproteins creates a barrier inhibiting the continued ingress of invading bacteria. These authors speculated that diamine oxidase activity could locally produce H2O2 that can be used by peroxidase to induce the insolubilization of the glycoproteins thereby modulating cell wall plasticity. Within the infection thread, the matrix glycoproteins are found to be insoluble at the tip and hardened elsewhere (Wisnewski and others 2000). This allows invading rhizobia to progress towards the infection zone of the nodule in the infection thread as long as the peroxidase level at the tip remains at a low enough level to avoid hardening of the infection thread tip walls.
In addition, actin monoubiquitylation is induced in developing nodules of P. vulgaris (Dantán-González and others 2001). These actin modifications are likely part of a defense response against invading organisms and appear to provide microfilament stability against proteolytic degradation. This response can be mimicked in suspension cell culture by H2O2 application (Dantán-González and others 2001), thus further suggesting that H2O2 has a role in modifying cell wall structures.
Salzar and others (1999) demonstrated that H2O2 accumulates in M. truncatula cortical cells in the region occupied by arbuscular mycorrhiza. More specifically, H2O2 was concentrated around hyphal tips attempting to penetrate a host cell, similar to phenomenon described by Salzwedel and Dazzo (1993) following root hair penetration and infection thread formation by rhizobia. This was suggested to be indicative of an oxidative burst involved in the control of intracellular colonization of the host (Salzar and others 1999).
In agreement with the above findings, Santos and others (2001) detected an oxidative burst of H2O2 and O -2 in the curled region of the root hair immediately following inoculation of M. truncatula. Interestingly, these elevated levels of ROS were also found in infected cells suggesting that this burst is prolonged and could have a role in regulating the infection process (Santos and others 2001). van Spronsen and others (2003) suggested that an oxidative burst could be prolonged by SA, which could bind to, and therefore inactivate, peroxidases such as RIP1.
In addition to modulating cell wall repair and plasticity, ROS can be detrimental to nodulation as they can damage and degenerate the proteins, DNA and lipids of both symbionts, and their levels are often elevated in senescent nodule tissue. ROS such as O -2 and •OH inhibit nitrogen fixation and it has been suggested that the inhibition by O -2 may be due to its breakdown into the highly reactive and damaging •OH (Puppo and Halliwell 1988). To compensate for the stress of ROS, rhizobia are equipped with enzymes such as SOD, which detoxifies O -2 . M. truncatula inoculated with Sinorhizobia meliloti, defective in SOD, nodulate poorly and display abnormal infection (Santos and others 2000). In addition, most of the bacteria failed to differentiate into nitrogen fixing bacteroids and senesced rapidly. This led Santos and others (2000) to speculate that oxidative stress interferes at numerous stages of the symbiosis and not simply at the level of nitrogen fixation. Thus, rhizobial SOD is a requirement for nodule development as well as functioning.
As mentioned, in addition to rhizobial SOD, plants contain antioxidant defense enzymes that also can break down ROS. In leaves of Zea mays, treatment with 10–100 µM ABA induced the production of O -2 and H2O2 followed by increases in the activities of antioxidant enzymes at levels sufficient enough to scavenge the elevated levels of O -2 and H2O2 (Figure 1; Jiang and Zhang 2001). The authors of this report concluded that ROS have a dual role in plants depending on their quantity: acting as toxins inducing oxidative stress when abundant or as triggers eliciting the upregulation of antioxidant enzymes when elevated only slightly. It seems plausible that the invading Rhizobium could positively regulate the plants antioxidant enzymes, possibly via elevated ABA levels, to avoid the damaging ROS and thereby promoting nodulation.
Like ABA, Bueno and others (2001) showed that inoculation of alfalfa plants with Rhizobium elevates both antioxidant enzyme activities and H2O2 generation. These elevated levels of scavenging antioxidant enzymes likely have a role in controlling the oxidative burst. Interestingly, among the enzymes elevated is LOX, which was earlier described as being influenced by ABA (Figure 2). Taken together with the previous paragraph, the complexity of signalling in nodulation becomes increasingly apparent.
Jasmonic Acid
Jasmonic acid (JA) both induces LOX mRNA accumulation (Figure 2) (Porta and others 1999) and is produced by the action of LOX upon polyunsaturated fatty acids (Gundlach and others 1992). In addition, methyl jasmonate (MeJA) induces the transcription of PAL (Gundlach and others 1992), an enzyme that catalyzes the first step in SA biosynthesis, and in L. luteus roots, its application promotes the synthesis and rhizosecretion of the isoflavonoid genistein (Kneer and others 1999).
JA also appears to promote the colonization and development of mycorrhizal structures in Allium sativum (Regvar and others 1996) and mycorrhizal colonization has been reported to elevate JA biosynthesis in Hordeum vulgare (barley) (Hause and others 2002). It is possible that JA has similar roles in nodule formation and mutants impaired in JA synthesis or response would greatly aid in understanding this signalling molecule in nodulation.
OTHER SIGNALLING COMPOUNDS
Brassinosteroids
Foliar application of epibrassinolide to Arachis hypogaea (groundnut) substantially increased the number and weight of nodules and promoted root nitrogenase activity (Vardhini and Rao 1999). In contrast, application of epibrassinolide to the roots of soybean (Hunter 2001) decreased the number of nodules and amount of nitrogen fixation. These differences between studies may be attributed to variation in methods or species used.
Endogenous brassinosteriods (BRs) also appear to influence nodule formation as preliminary evidence shows that BR-deficient mutants of pea form significantly fewer nodules than their wild type ( B.J. Ferguson, J. Reid and J. Ross unpublished). However, precise roles of BRs in nodulation are unclear and no molecular evidence or signalling interactions pertaining to the roles of BRs in nodule organogenesis exist to date.
Flavonoids
Flavonoids have multiple roles in plant development, defense and nodulation (reviewed in Dakora 1995; Spaink 1999); they constitute a large class of compounds of the phenylpropanoid pathway, and their exact structure is important for their varied functions, including concomitantly inducing the chemotaxis of the Rhizobium to the root and elevating the production of Nod factors (for example, Redmond and others 1986; Stafford 1997). Flavonoid production is also induced by rhizobia in roots and nodules (for example, see Cooper and Rao 1992; Recourt and others 1992) and different flavonoids are synthesized in response to rhizobia that up- and down-regulate Nod factor production, both before and during infection (for example, see Zuanazzi and others 1998).
Flavonoids are distributed in a strictly tissue-specific pattern in many species. In particular, flavonoids are often located in dividing and meristematic tissues, including dividing cortical cells of nodules (Mathesius and others 1998b). It is possible that flavonoids merely protect dividing cells from oxidative damage because of their activity as antioxidants (Rice-Evans 2001). However, as discussed above, it could also be possible that flavonoids affect cell division either by regulating auxin transport or turnover (Figure 1), thereby regulating auxin accumulation (Figure 3), or by directly regulating cell cycle regulators. In animals, much evidence has been found that flavonoids regulate cell cycle activity, but in plants this evidence has so far been very tentative (for example, see Logemann and others 1995; Jinsart and others 1991). The existence of a flavonoid-deficient mutant in Arabidopsis has shown that flavonoids are not essential for plant survival, although interestingly the mutant showed alterations in lateral root formation, root growth and plant height, which could be the result of increased auxin transport due to the absence of flavonoids acting as PATI (Brown and others 2001). At this stage, flavonoid-deficient mutants have not been isolated in legumes.
Uridine
The position of a nodule is not only determined by the initiation of cell divisions in either the inner or the outer cortex of indeterminate and determinate legumes, respectively, but also in respect to the protoxylem poles (Figure 3). In most legume species, the majority of nodule primordia are initiated in front of one of the protoxylem poles and it has been suggested that a signal (the “stele factor”) diffuses out of the xylem and acts together with auxin and cytokinins to induce cell divisions comprising the nodule primordia (Libbenga and Harkes 1973).
The stele factor has been identified as uridine (Smit and others 1995). In the presence of very low uridine concentrations, cell divisions can be induced in every cortical cell by cytokinins in pea (Libbenga and Harkes 1973) and in inner cortical cells by chitin oligosaccharides following ballistic micro-targeting in vetch (Schlaman and others 1997). Differences between the concentrations of uridine in front of xylem versus phloem poles could explain the preference for nodules to initiate opposite xylem poles. The fact that nodules are initiated in the outer cortex in determinate legumes and in the inner cortex in indeterminate ones could be explained by the fact that determinate and indeterminate species have different sensitivities for uridine, although definitive evidence is lacking.
Nitrate
Nitrate interacts with plant hormones to regulate nodule formation (Figure 1). The presence of nitrate in the soil at concentrations above 1–5 mM suppresses nodulation locally at several levels, including infection, nodule primordium initiation and nitrogen fixation (reviewed by Streeter 1988). How nitrate inhibits nodulation is not exactly known, although its purpose may be to limit the formation of nodules under conditions that provide sufficient nitrate.
The existence of mutants that hypernodulate even in the presence of nitrate shows that nitrate is not the inhibiting factor itself, but that it leads to secondary signals that suppress nodulation (Carroll and others 1985). According to the auxin burst hypothesis (Gresshoff 1993), high auxin levels inhibit nodule formation, and it is hypothesized that nitrate increases the sensitivity of the root to auxin, thus reducing nodule formation. In the supernodulation nts mutants, the auxin burst control is altered and therefore these mutants can still nodulate in the presence of nitrate because not as much auxin is available in the root to suppress further nodule initiation. In support of that hypothesis, Caba and others (2000) found that nitrate decreased auxin levels in inoculated and uninoculated roots of wild type and nts mutants, whereas root growth was not altered. The authors hypothesized that this represented an increased sensitivity to auxin in the presence of nitrate, which would be consistent with the auxin burst hypothesis; however, auxin sensitivity will need to be assessed by more direct means. An effect of nitrate on the auxin response pathway has been found in Arabidopsis (Zhang and others 1999) and it is possible that, in legumes, at least some of the effects of nitrate are also mediated by auxin.
Nitrate’s regulation of nodulation could be imposed via an effect on flavonoid accumulation in the root, which can alter auxin transport or Nod gene activity (Coronado and other 1995). There is also evidence for the involvement of ethylene in mediating the inhibitory effect of nitrate. The findings that inhibitors of ethylene synthesis or action (for example, AVG and Ag+, respectively) restore nodulation in the presence of nitrate suggest that nitrate induces the production of ethylene which then inhibits nodulation (Caba and others 1998; Ligero and others 1991). Because ethylene can regulate auxin transport (Burg and Burg 1966; Suttle 1988) and turnover (Ke and Saltveit 1988), nitrate’s effect via alterations in auxin levels could be mediated by nitrate-induced ethylene. Caba and others (1999) found that the tolerance of the nts mutant to nitrate with respect to nodulation is paralleled by a tolerance for ethylene, which supports an involvement of ethylene in nitrate regulation. Unlike the nts mutants, in L. japonicus, the nodulation phenotype of the recently characterized early- and hyper-nodulating mutant astray displayed normal sensitivity to ethylene and nitrate as its nodule number declined in the presence of both (Nishimura and others 2002c). Interestingly, the mutated gene of astray was found to be the homologue of the Arabidopsis HY5 gene (Nishimura and others 2002b), which is involved in photomorphogenesis.
Nitrate also inhibits ENOD40 induction by rhizobia, but not by cytokinins (Mathesius and others 2000), suggesting two possibilities for the action of nitrate (see Figure 1): (1) if rhizobia induce ENOD40 independently of cytokinins, nitrate would act between Nod factor perception and ENOD40 induction, or (2) if rhizobia change cytokinin levels, which subsequently stimulate ENOD40, nitrate would inhibit the cytokinin changes induced by rhizobia.
Mutants are valuable to test the interactions between nitrate and hormone signalling. For example, the nitrate reductase-deficient mutant ANR1 and the auxin response mutant axr4 were used in Arabidopsis to establish a role for the auxin response pathways during nitrate regulation of lateral root development (Zhang and others 1999). Assuming that lateral root and nodule development share aspects of their regulation by nitrate, it is possible that nitrate also acts via the auxin response pathway during nodulation and that the effects of nitrate on cytokinin, ENOD40 expression and ethylene could indirectly be caused by changes in auxin response.
Nod Factors and Other Chitin Derivatives
Nod factors are Rhizobium-produced lipochitin oligosaccharides and represent the major morphogenic molecule regulating nodule organogenesis, bringing us back to the start of the story. In addition to determining host specificity, Nod factors elicit root hair curling and deformation and cortical cell divisions in alfalfa (Truchet and others 1991). There has been some debate about whether Nod factors are hormone-like signals per se or act indirectly, for example, via changing the plant hormone balance as discussed above. Although specific Nod factor action during nodulation has been extensively reviewed elsewhere (for example, Cullimore and others 2001; D’Haeze and Holsters 2002; Miklashevichs and others 2001), we focus here on the hormone-like roles of chitin oligosaccharides in general.
Whereas Nod factors are specific in their morphogenetic effect for certain host plants, Nod factor-related molecules have been suggested to play a more general role in plant development (Spaink and others 1993; van der Holst and others 2001). Structurally related chitin oligosaccharides play a role in animal development and have been detected in plants (Benhamou and Asselin 1989; Spaink and other 1993). They can be recognized by receptors for chitin oligosaccharides (Stacey and Shibuya 1997), and are substrates for chitinases, which have been shown to play a role in different aspects of plant development (Collinge and others 1993). Expression of a chitinase was shown to rescue an embryonic mutant of carrot (de Jong and others 1992) and modifying chitin structures by expression of the bacterial nodA and nodB genes, which modify Nod factors in rhizobia, led to changes in plant development (Schmidt and others 1993).
Directed microtargeting of chitin oligosaccharides induced cortical cell divisions in vetch roots (Schlaman and others 1997). Dyachok and others (2000) found that Nod factors could stimulate embryogenesis in cell cultures of Norway spruce, a non-nodulating plant, and more recently isolated a lipochitin oligosaccharide-like compound from these cultures which stimulated embryogenesis (Dyachok and others 2002). Collectively, these experiments suggest that chitin perception could be widespread in both plants and animals and that chitin-related molecules play a role in development. However, the mode of action of chitin derivatives remains elusive and identification of receptors and downstream response elements will be necessary to establish whether chitin oligosaccharides act via classical hormones or directly on target genes.
CONCLUSIONS AND OUTLOOK
This review demonstrates the manifold effects of classical plant hormones and other compounds on nodule initiation, differentiation and numbers. Additional factors, such as soil nutrients, light, polyunsaturated fatty acids, CO2, Ca2+, phenylalanine ammonia lyase, chalcone synthase, Rhizobium , exopolysaccharides, lipopolysaccharides, and so on are all probably required for proper nodule development and functioning, but could not be fully discussed here.
Reports on classical plant hormones in nodulation are often ambiguous and contradictory because (1) nodulation is a fine balance between induction and repression of new nodule formation; (2) hormone requirements change with the varying stages of nodulation; (3) hormone levels and requirements change in different places in the shoot, root and nodule; (4) hormones interact with each other, leading to complex negative and positive feedback loops; (5) hormone requirements differ in different legume species, and (6) nodulation is regulated by both local and long distance signalling interactions involving varying actions of the same hormone in each regulatory pathway.
The search for homologues for many of the recently discovered Arabidopsis hormone response genes in legumes and their silencing or overexpression should help pinpoint the action of hormones during nodulation. For example, it should be tested whether Rhizobium directly affect cytokinin levels or whether cytokinin-related responses are the result of changing the auxin:cytokinin ratio due to changes in auxin transport or levels (see Figure 1). This could be tested in an inducible mutant for cytokinin synthesis. Inducible or temperature-sensitive mutants in polar auxin transport could be used to test whether auxin transport inhibition is necessary for nodule induction and whether changes in auxin occur in the absence of PATI, for example, via flavonoid-regulated changes in peroxidase activity, as indicated in Figure 1. Accordingly, it could be tested whether auxin transport inhibition is a result of changes in ethylene induction in an ethylene synthesis-deficient mutant. A mutant in ABA synthesis would also be useful for testing the functional relationships indicated in Figure 2. If elevated ABA levels are necessary for changes in phytoalexins, LOX, ROS and therefore indirectly for changes in peroxidase levels, JA and regulation of defense responses, these responses should be reduced in the mutant.
There are challenging questions to address in future research. First, how does Nod factor perception lead to downstream events that could affect the plant hormone balance? Not much is known about how the early events in the root hair are linked to the events in the cortex, but the analysis of nodulation mutants is beginning to address that problem (Kistner and Parniske 2002). Secondly, there is a need for more large-scale experiments to discover the broad response pathways for plant hormones during nodulation, because each hormone usually has many targets and interacts with other hormones, which also have multiple effects. The use of mutants with hormone insensitivity, overproduction, or underproduction, the use of accurate reporters for different hormones, concentrating on model species for different types of analyses, as well as keeping an open mind about possible interactions should help to unravel the complex interactions of hormone-regulated signalling during nodulation. In addition, the recent identification of ESTs in M. truncatula has opened the door for expression analyses on the transcript (Fedorova and others 2002) and proteome level (Mathesius and others 2001). Thirdly, it is almost certain that new signalling compounds will be discovered apart from those presently known. Among them will be peptide hormones that might regulate receptor kinase activity. But other long-range signals are also likely to be discovered, including the autoregulatory signal from the shoot (Searle and others 2003). The molecular and physiological characterization of these novel compounds should help further the understanding of the intricate nodulation process that we are just beginning to understand.
References
N Arora F Skoog ON Alien (1959) ArticleTitleKinetin-induced pseudonodules on tobacco roots. Am J Bot 46 610–613 Occurrence Handle1:CAS:528:DyaF3cXivFGitA%3D%3D
S Asad Y Fang KL Wykoff AM Hirsch (1994) ArticleTitleIsolation and characterisation of cDNA and genomic clones of MsENOD40: transcripts are detected in meristematic cells of alfalfa. Protoplasma 183 10–23 Occurrence Handle1:CAS:528:DyaK2MXks1SmsLs%3D
R Atzorn A Crozier CT Wheeler G Sandberg (1988) ArticleTitleProduction of gibberellins and indole-3-acetic acid by Rhizobium phaseoli in relation to nodulation of Phaseolus vulgaris roots. Planta 175 532–538 Occurrence Handle1:CAS:528:DyaL1cXmt1KntL8%3D
J Badenoch-Jones CW Parker DS Letham (1987) ArticleTitlePhytohormones, Rhizobium mutants, and nodulation in legumes. VII. Identification and quantification of cytokinins in effective and ineffective pea root nodules using radioimmunoassay. J Plant Growth Regul 6 97–111 Occurrence Handle1:CAS:528:DyaL2sXltlSqtL8%3D
J Badenoch-Jones RE Summons BG Rolfe DS Letham (1984) ArticleTitlePhytohormones, Rhizobium mutants, and nodulation in legumes. IV. Auxin metabolites in pea root nodules. J Plant Growth Regul 3 23–39 Occurrence Handle1:CAS:528:DyaL2cXlsVOqsrs%3D
A Bano JE Harper RM Auge DS Neuman (2002) ArticleTitleChanges in phytohormone levels following inoculation of two soybean lines differing in nodulation. Funct Plant Biol 29 965–974 Occurrence Handle1:CAS:528:DC%2BD38XntFyqtLg%3D
A Bano JE Harper (2002) ArticleTitlePlant growth regulators and phloem exudates modulate root nodulation of soybean. Funct Plant Biol 29 1299–1307 Occurrence Handle1:CAS:528:DC%2BD38Xps1ygtL8%3D
A Bano JR Hillman (1986) ArticleTitleEffect of abscisic acid on nodule morphology, nitrogenase activity and H2 evolution in Faba vulgaris. Ann Bot 58 281–283 Occurrence Handle1:CAS:528:DyaL28XltlSrsrw%3D
P Bauer P Ratet MD Crespi M Schultze A Kondorosi (1996) ArticleTitleNod-factors and cytokinins induce similar cortical cell divisions, amyloplast deposition and MsENOD12A expression patterns in alfalfa roots. Plant J 10 91–105 Occurrence Handle1:CAS:528:DyaK28XltVamtrw%3D
N Benhamou A Asselin (1989) ArticleTitleAttempted localisation for a substrate for chitinases in plant cells reveals abundant N-acetyl-D-glucosamine residues in secondary walls. Biol Cell 67 341–350 Occurrence Handle1:CAS:528:DyaK3cXhtlKisb8%3D
RP Bhalerao J Eklof K Ljung A Marchant M Bennett G Sandberg (2002) ArticleTitleShoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29 325–332 Occurrence Handle1:CAS:528:DC%2BD38XhvFOitL8%3D Occurrence Handle11844109
DC Billington BT Golding SB Primrose (1979) ArticleTitleBiosynthesis of ethylene from methionine. Isolation of the putative intermediate 4-methylthio-2-oxobutanoate from culture fluids of bacteria and fungi. Biochem J 182 827–836 Occurrence Handle1:CAS:528:DyaL3cXhtVOjsLY%3D Occurrence Handle42392
I Blilou JA Ocampo JM Garcia-Garrido (1999) ArticleTitleResistance of pea roots to endomycorrhizal fungus or Rhizobium correlates with enhanced levels of endogenous salicylic acid. J Exp Bot 50 1663–1668 Occurrence Handle1:CAS:528:DyaK1MXntlyhu7o%3D
KJM Boot AAN van Brussel T Tak HP Spaink JW Kijne (1999) ArticleTitleLipochitin oligosaccharides from Rhizobium leguminosarum bv. viclae reduce auxin transport capacity in Vicia sativa subsp. nigra roots. Mol Plant-Microbe Interact 12 839–844 Occurrence Handle1:CAS:528:DyaK1MXmtl2gsr4%3D
DE Brown AM Rashotte AS Murphy J Normanly BW Tague WA Peer L Taiz GK Muday (2001) ArticleTitleFlavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126 524–535 Occurrence Handle1:CAS:528:DC%2BD3MXks1Gnu7s%3D Occurrence Handle11402184
P Bueno MJ Soto MP Rodríguez-Rosales J Sanjuan J Olivares JP Donaire (2001) ArticleTitleTime-course of lipoxygenase, antioxidant enzyme activities and H2O2 accumulation during the early stages of Rhizobium-legume symbiosis. New Phytol 152 91–96 Occurrence Handle1:CAS:528:DC%2BD3MXnvVyls70%3D
SP Burg EA Burg (1966) ArticleTitleThe interaction between auxin and ethylene and its role in plant growth. Proc Natl Acad Sci USA 55 262–269 Occurrence Handle1:CAS:528:DyaF28XoslOlsQ%3D%3D Occurrence Handle5220945
JM Caba ML Centeno B Fernandez PM Gresshoff F Ligero (2000) ArticleTitleInoculation and nitrate alter phytohormone levels in soybean roots: differences between a supernodulating mutant and the wild type. Planta 211 98–104 Occurrence Handle1:CAS:528:DC%2BD3cXjvVyhsLk%3D Occurrence Handle10923709
JM Caba L Poveda PM Gresshoff F Ligero (1999) ArticleTitleDifferential sensitivity of nodulation to ethylene in soybean cv. Bragg and a supernodulating mutant. New Phytol 142 233–242 Occurrence Handle1:CAS:528:DyaK1MXkslOmsr0%3D
JM Caba L Recalde F Ligero (1998) ArticleTitleNitrate-induced ethylene biosynthesis and the control of nodulation in alfalfa. Plant Cell Env 21 87–93 Occurrence Handle1:CAS:528:DyaK1cXitlCgsLY%3D
A Camas L Cardenas C Quinto M Lara (2002) ArticleTitleExpression of different calmodulin genes in bean (Phaseolus vulgaris L.): role of Nod factor on calmodulin gene regulation. Mol Plant-Microbe Interact 15 428–436 Occurrence Handle1:CAS:528:DC%2BD38XjslSit7o%3D Occurrence Handle12036273
BJ Carroll DL McNeil PM Gresshoff (1985) ArticleTitleIsolation and properties of soybean (Glycine max) mutants that nodulate in the presence of high nitrate concentrations. Proc Natl Acad Sci USA 82 4164–4166
GA Charbonneau W Newcomb (1985) ArticleTitleGrowth regulators in developing effective root nodules of the garden pea (Pisum sativum L.). Biochem Physiol Pflanzen 180 667–681 Occurrence Handle1:CAS:528:DyaL2MXlsFOms7k%3D
C Charon C Johansson E Kondorosi A Kondorosi M Crespi (1997) ArticleTitle enod40 induces dedifferentiation and division of root cortical cells in legumes. Proc Natl Acad Sci USA 94 8901–8906 Occurrence Handle1:CAS:528:DyaK2sXltlWhtLo%3D Occurrence Handle11038563
C Charon C Sousa M Crespi A Kondorosi (1999) ArticleTitleAlteration of enod40 expression modifies Medicago truncatula root nodule development induced by Sinorhizobium meliloti. Plant Cell 11 1953–1965 Occurrence Handle1:CAS:528:DyaK1MXntFCgtL0%3D Occurrence Handle10521525
KK Chattopadhyay PS Basu (1989) ArticleTitleStudies on root nodules of leguminous trees: II. The bioproduction of different phytohormones in root nodules of Samanea saman (Jacq.) Merril and by its rhizobial symbiont. Biochem Physiol Pflanzen 184 387–394 Occurrence Handle1:CAS:528:DyaL1MXit1yltb0%3D
MJ Cho JE Harper (1993) ArticleTitleEffect of abscisic acid application on root isoflavonoid concentration and nodulation of wild type and nodulation-mutant soybean plants. Plant Soil 152 145–149
DB Collinge KM Kragh JD Mikkelsen KK Nielsen U Rasmussen K Vad (1993) ArticleTitlePlant chitinases. Plant J 3 31–40 Occurrence Handle1:CAS:528:DyaK3sXksVGitbc%3D Occurrence Handle8401605
B Compaan WC Yang T Bisseling H Franssen (2001) ArticleTitle ENOD40 expression in the pericycle precedes cortical cell division in Rhizobium-legume interaction and the highly conserved internal region of the gene does not encode a peptide. Plant Soil 230 1–8 Occurrence Handle1:CAS:528:DC%2BD3MXivFKjt70%3D
D Cook D Dreyer D Bonnet M Howell E Nony K VandenBosch (1995) ArticleTitleTransient induction of a peroxidase gene in Medicago truncatula precedes infection by Rhizobium meliloti. Plant Cell 7 43–55 Occurrence Handle1:CAS:528:DyaK2MXjsVyjsbw%3D Occurrence Handle7696879
JB Cooper SR Long (1994) ArticleTitleMorphogenetic rescue of Rhizobium meliloti nodulation mutants by trans-zeatin secretion. Plant Cell 6 215–225 Occurrence Handle1:CAS:528:DyaK2cXlslWlsrY%3D Occurrence Handle12244237
JE Cooper R Rao (1992) ArticleTitleLocalised changes in flavonoid biosynthesis in roots of Lotus pendiculatus after infection by Rhizobium loti. Plant Physiol 100 444–450 Occurrence Handle1:CAS:528:DyaK38XmtlShsrY%3D Occurrence Handle16652981
V Corich S Goormachtig S Lievens M Van Montagu M Holsters (1998) ArticleTitlePatterns of ENOD40 gene expression in stem-borne nodules of Sesbania rostrata. Plant Mol Biol 37 67–76 Occurrence Handle1:CAS:528:DyaK1cXjtlajtLk%3D Occurrence Handle9620265
C Coronado JAS Zuanazzi C Sallaud JC Quirion R Esnault HP Husson A Kondorosi P Ratet (1995) ArticleTitleAlfalfa root flavonoid production is nitrogen regulated. Plant Physiol 108 533–542 Occurrence Handle1:CAS:528:DyaK2MXmtlaru7s%3D Occurrence Handle12228491
A Costacurta J Vanderleyden (1995) ArticleTitleSynthesis of phytohormones by plant-associated bacteria. Crit Rev Microbiol 21 1–18 Occurrence Handle7576148
M Crespi S Galvez (2000) ArticleTitleMolecular mechanisms in root nodule development. J Plant Growth Regul 19 155–166 Occurrence Handle1:CAS:528:DC%2BD3cXnvF2hurc%3D Occurrence Handle11038225
MD Crespi E Jurkevitch M Poiret Y d’Aubenton-Carafa G Petrovics E Kondorosi A Kondorosi (1994) ArticleTitle enod40, a gene expressed during nodule organogenesis, codes for a non-translatable RNA involved in plant growth. EMBO J 13 5099–5112 Occurrence Handle1:CAS:528:DyaK2MXit1ektb8%3D Occurrence Handle7957074
M Cueto O Hernández-Perera R Martín ML Bentura J Rodrigo S Lamas MP Golvano (1996) ArticleTitlePresence of nitric oxide synthase activity in roots and root nodules of Lupinus albus. FEBS Lett 398 159–164 Occurrence Handle1:CAS:528:DyaK28Xnt1Kqsb4%3D Occurrence Handle8977098
JV Cullimore R Ranjeva JJ Bono (2001) ArticleTitlePerception of lipo-chitooligosaccharidic Nod factors in legumes. Trends Plant Sci 6 24–30 Occurrence Handle1:CAS:528:DC%2BD3MXlsFyrsbo%3D Occurrence Handle11164374
IB D’Agostino JJ Kieber (1999) ArticleTitleMolecular mechanisms of cytokinin action. Curr Opin Plant Biol 2 359–364 Occurrence Handle10508753
FD Dakora (1995) ArticleTitlePlant flavonoids - biological molecules for useful exploitation. Aust J Plant Physiol 22 87–99 Occurrence Handle1:CAS:528:DyaK2MXls1Kltbg%3D
TK Dangar PS Basu (1984) ArticleTitleSeasonal changes and metabolism of plant hormones in root nodules of Lens sp. Biol Plant 26 253–259 Occurrence Handle1:CAS:528:DyaL2cXmtVOlu78%3D
TK Dangar PS Basu (1987) ArticleTitleStudies on plant growth substances, IAA metabolism and nitrogenase activity in root nodules of Phaseolus aureus Roxb. var. mungo. Biol Plant 29 350–354 Occurrence Handle1:CAS:528:DyaL1cXhvFehtw%3D%3D
TK Dangar PS Basu (1991) ArticleTitleAbscisic acid production in culture by some rhizobium spp. of leguminous trees and pulses. Folia Microbiol 36 527–532 Occurrence Handle1:CAS:528:DyaK38XitFSrur4%3D
E Dantán-González Y Rosenstein C Quinto F Sánchez (2001) ArticleTitleActin monoubiquitylation is induced in plants in response to pathogens and symbionts. Mol Plant-Microbe Interac 14 1267–1273
J Davies J Zhang (1991) ArticleTitleRoot signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42 55–76 Occurrence Handle1:CAS:528:DyaK3MXltFSmsr8%3D
F de Billy C Grosjean S May M Bennett JV Cullimore (2001) ArticleTitleExpression studies on AUX1-like gens in Medicago truncatula suggest that auxin is required at two steps in early nodule development. Mol Plant-Microbe Interact 14 267–277 Occurrence Handle1:CAS:528:DC%2BD3MXhsVKgsLw%3D Occurrence Handle11277424
C Dehio FJ de Bruijn (1992) ArticleTitleThe early nodulin gene SrEnod2 from Sesbania rostrata is 10 inducible by cytokinin. Plant J 2 117–128 Occurrence Handle1:CAS:528:DyaK3sXktVCquro%3D Occurrence Handle1303791
AJ DeJong J Cordewener F Lo Schiavo M Terzi J Vanderkerckove A van Kammen S de Vries (1992) ArticleTitleA carrot somatic embryo mutant is rescued by chitinase. Plant Cell 4 425–433 Occurrence Handle1:CAS:528:DyaK38XktVCgtrg%3D
AC Delves A Mathews DA Day AS Carter BJ Carroll PM Gresshoff (1986) ArticleTitleRegulation of soybean-Rhizobium nodule symbiosis by shoot and root factors. Plant Physiol 82 588–590 Occurrence Handle1:STN:280:DC%2BC3cnhslagtQ%3D%3D Occurrence Handle16665072
W D’Haeze M Holsters (2002) ArticleTitleNod factor structures, responses, and perception during initiation of nodule development. Glycobiol 12 79R–105R
MA Djordjevic U Mathesius T Arioli JJ Weinman E Gärtner (1997) ArticleTitleChalcone synthase gene expression in transgenic subterranean clover correlates with localised accumulation of flavonoids. Aust J Plant Physiol 24 119–132 Occurrence Handle1:CAS:528:DyaK2sXjs1Wrurs%3D
RC Dobert SB Rood DG Blevins (1992a) ArticleTitleGibberellins and the legume-Rhizobium symbiosis I. Endogenous gibberellins of Lima Bean (Phaseolus lunatus L.) stems and nodules. Plant Physiol 98 221–224 Occurrence Handle1:CAS:528:DyaK38Xht1CqurY%3D
RC Dobert SB Rood DG Blevins (1992b) ArticleTitleRhizobial-induced increase in internode length and identification of endogenous GAs of cowpea (Vigna unguiculata [L.] Walp) stems and nodules. J Plant Growth Regul 11 155–164 Occurrence Handle1:CAS:528:DyaK3sXjs1SgtA%3D%3D
RC Dobert SB Rood K Zanewich DG Blevins (1992c) ArticleTitleGibberellins and the legume-Rhizobium symbiosis III. Quantification of gibberellins from stems and nodules of Lima Bean and cowpea. Plant Physiol 100 1994–2001 Occurrence Handle1:CAS:528:DyaK3sXls1ejug%3D%3D
P Doerner JE Jorgensen R You J Steppuhn C Lamb (1996) ArticleTitleRoot growth and cyclin control. Trends Plant Sci 1 211–212
DSH Drennan C Norton (1972) ArticleTitleThe effect of ethrel on nodulation in Pisum sativum L. Plant Soil 36 53–57 Occurrence Handle1:CAS:528:DyaE38XhtlKktrY%3D
J Dullaart LI Duba (1970) ArticleTitlePresence of gibberellin-like substances and their possible role in auxin bioproduction in root nodules and roots of Lupinus luteus L. Acta Bot Neerl 19 877–883 Occurrence Handle1:CAS:528:DyaE3MXht1Wgur4%3D
JV Dyachok AE Tobin NPJ Price S von Arnold (2000) ArticleTitleRhizobial Nod factors stimulate somatic embryo development in Picea abies. Plant Cell Rep 19 290–297 Occurrence Handle1:CAS:528:DC%2BD3cXjsVeitb4%3D
JV Dyachok M Wiweger L Kenne S von Arnold (2002) ArticleTitleEndogenous nod-factor-like signal molecules promote early somatic embryo development in Norway spruce. Plant Physiol 128 523–533 Occurrence Handle1:CAS:528:DC%2BD38XhsVSru7k%3D Occurrence Handle11842156
G Endre A Kereszt Z Kevei S Mihacea P Kalo GB Kiss (2002) ArticleTitleA receptor kinase gene regulating symbiotic nodule development. Nature 417 962–966 Occurrence Handle1:CAS:528:DC%2BD38XkvVagsbk%3D Occurrence Handle12087406
EM Estabrook C Sengupta-Gopalan (1991) ArticleTitleDifferential expression of phenylalanin ammonia-lyase and chalcone synthase during soybean nodule development. Plant Cell 3 299–308 Occurrence Handle1:CAS:528:DyaK3MXks1Grsbg%3D Occurrence Handle1840912
KB Evensen DG Blevins (1981) ArticleTitleDifferences in endogenous levels of gibberellin-like substances in nodules of Phaseolus lunatus L. plants inoculated with two Rhizobial strains. Plant Physiol 65 195–198
Y Fang AM Hirsch (1998) ArticleTitleStudying early nodulin gene ENOD40 expression and induction by nodulation factor and cytokinin in alfalfa. Plant Physiol 116 53–68 Occurrence Handle1:CAS:528:DyaK1cXkslCkug%3D%3D Occurrence Handle9449836
B Favery A Complainville JM Vinardell P Lecomte D Vaubert P Mergaert A Kondorosi E Kondorosi M Crespi P Abad (2002) ArticleTitleThe endosymbiosis-induced genes ENOD40 and CCS52a are involved in endoparasitic-nematode interactions in Medicago truncatula. Mol Plant-Microbe Interact 15 1008–1013 Occurrence Handle1:CAS:528:DC%2BD38XnvFGjtL4%3D Occurrence Handle12437298
JC Fearn TA LaRue (1991) ArticleTitleEthylene inhibitors restore nodulation to sym5 mutants of Pisum sativum L. cv Sparkle. Plant Physiol 96 239–244 Occurrence Handle1:CAS:528:DyaK3MXkt1Kju70%3D Occurrence Handle16668158
EE Fedorova ZK Al’zhapparova GY Zhiznevskaya EN Artemenko SF Izmailov (1992) ArticleTitlePhytohormones in soybean root nodules. Sov Plant Physiol 39 135–139
EE Fedorova GY Zhiznevskaya ZV Kalibernaya EN Artemenko SF Izmailov AV Gus’kov (2000) ArticleTitleIAA metabolism during development of symbiosis between Phaseolus vulgaris and Rhizobium phaseoli. Russ J Plant Physiol 47 203–206 Occurrence Handle1:CAS:528:DC%2BD3cXisVSit7k%3D
M Fedorova J van der Mortel PA Matsumoto J Cho CD Town K VandenBosch S Gnatt CP Vance (2002) ArticleTitleGenome-wide identification of nodule-specific transcripts in the model legume Medicago truncatula. Plant Physiol 130 519–537 Occurrence Handle1:CAS:528:DC%2BD38XotVKntrs%3D Occurrence Handle12376622
M Fernández-López S Goormachtig M Goa W D’Haeze M van Montagu M Holsters (1998) ArticleTitleEthylene-mediated phenotypic plasticity in root nodule development on Sesbania rostrate. Proc Natl Acad Sci USA 95 12724–12728 Occurrence Handle9770553
WW Fletcher JWS Alcorn JC Raymond (1959) ArticleTitleGibberellic acid and nodulation of legumes. Nature 184 1576 Occurrence Handle1:CAS:528:DyaF3cXmsVCrug%3D%3D
F Foucher E Kondorosi (2000) ArticleTitleCell cycle regulation in the course of nodule organogenesis in Medicago. Plant Mol Biol 43 773–786 Occurrence Handle1:CAS:528:DC%2BD3cXosVGksbo%3D Occurrence Handle11089876
X Fu NP Harberd (2003) ArticleTitleAuxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421 740–743 Occurrence Handle1:CAS:528:DC%2BD3sXhsV2rtr8%3D Occurrence Handle12610625
AW Galston (1959) ArticleTitleGibberellins and nodulation. Nature 183 545 Occurrence Handle1:STN:280:DyaG1M%2Fmt1erug%3D%3D Occurrence Handle13632787
MA Gilles-González G González MF Perutz L Kiger MC Marden C Poyart (1994) ArticleTitleHeme-based sensors, exemplified by the kinase FixL, are a new class of heme protein with distinctive ligand binding and autoxidation. Biochem 33 8067–8073
N Goicoechea MC Antolín M Sánchez-Díaz (1997) ArticleTitleGas exchange is related to the hormone balance in mycorrhizal or nitrogen-fixing alfalfa subjected to drought. Physiol Plant 100 989–997 Occurrence Handle1:CAS:528:DyaK2sXlvFCiurk%3D
EM González L Gálvez C Arrese-Igor (2001a) ArticleTitleAbscisic acid induces a decline in nitrogen fixation that involves leghaemoglobin, but is independent of sucrose synthase activity. J Exp Bot 52 285–293
EM González L Gálvez M Royuela PM Aparicio-Tejo C Arrese-Igor (2001b) ArticleTitleInsights into the regulation of nitrogen fixation in pea nodules: lessons from drought, abscisic acid and increased photoassimilate availability. Agronomic 21 607–613
R González-Pasayo E Martinez-Romero (2000) ArticleTitleMultiresistance genes of Rhizobium etli CFN42. Mol Plant-Microbe Interact 13 572–577 Occurrence Handle10796024
G Goodlass KA Smith (1979) ArticleTitleEffects of ethylene on root extension and nodulation of pea (Pisum sativum L.) and white clover (Trifolium repens L.). Plant Soil 51 387–395 Occurrence Handle1:CAS:528:DyaE1MXltVGhtb8%3D
S Goormachtig M Alves-Ferreira M van Montague G Engler M Holsters (1997) ArticleTitleExpression of cell cycle genes during Sesbania rostrata stem nodule development. Mol Plant-Microbe Interact 10 316–325 Occurrence Handle1:CAS:528:DyaK2sXitlOhurg%3D Occurrence Handle9100377
A Goverse H Overmars J Engelbertink A Schots J Bakker J Helder (2000) ArticleTitleBoth induction and morphogenesis of cyst nematode feeding cells are mediated by auxin. Mol Plant-Microbe Interact 13 1121–1129 Occurrence Handle1:CAS:528:DC%2BD3cXntVarsrY%3D Occurrence Handle11043473
PM Gresshoff A Mathews A Krotzky JE Olsson BJ Carroll AC Delves R Kosslak ER Applebaum DA Day (1988) Supernodulating and non-nodulating mutants of soybean. R Palacio DPS Verma (Eds) Molecular Genetics Of Plant-Microbe Interactions. APS Press St Paul MN 364–369
PM Gresshoff (1993) ArticleTitleMolecular-genetic analysis of nodulation genes in soybean. Plant Breed Rev 11 275–318
N Grobbelaar B Clarke MC Hough (1971) ArticleTitleThe nodulation and nitrogen fixation of isolated roots of Phaseolus vulgaris L. III. The effect of carbon dioxide and ethylene. Plant Soil (Spec Vol) 215–223
F Gubler R Kalla JK Roberts JV Jacobsen (1995) ArticleTitleGibberellin-regulated expression of a myb gene in barley aleurone cells: evidence for Myb transactions of a high-pl α-amylase gene promoter. Plant Cell 7 1879–1891 Occurrence Handle1:CAS:528:DyaK2MXpslOnsLw%3D Occurrence Handle8535141
FC Guinel RD Geil (2002) ArticleTitleA model for the development of the rhizobial and arbuscular mycorrhizal symbioses in legumes and its use to understand the roles of ethylene in the establishment in these two symbioses. Can J Bot 80 695–720 Occurrence Handle1:CAS:528:DC%2BD38XntlSrsrY%3D
FC Guinel TA LaRue (1992) ArticleTitleEthylene inhibitors partly restore nodulation to pea mutant E107 (brz). Plant Physiol 99 515–518 Occurrence Handle1:CAS:528:DyaK38XkvVKqtr0%3D Occurrence Handle16668916
FC Guinel LL Sloetjes (2000) ArticleTitleEthylene is involved in the nodulation phenotype of Pisum sativum R50 (sym16), a pleiotropic mutant that nodulates poorly and has pale green leaves. J Exp Bot 51 885–894 Occurrence Handle1:CAS:528:DC%2BD3cXjsl2mt7o%3D Occurrence Handle10948214
H Gundlach MJ Müller TM Kutchan MH Zenk (1992) ArticleTitleJasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA 89 2389–2393 Occurrence Handle1:CAS:528:DyaK38XisFagsb8%3D Occurrence Handle11607285
B Hause W Maier O Miersch R Kramell D Strack (2002) ArticleTitleInduction of jasmonate biosynthesis in arbuscular mycorrhizal barley roots. Plant Physiol 130 1213–1220 Occurrence Handle1:CAS:528:DC%2BD38XovVOmsrg%3D Occurrence Handle12427988
R Heidstra YC Yang Y Yalcin S Peck AM Emons A van Kammen T Bisseling (1997) ArticleTitleEthylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium legume interaction. Development 124 1781–1787 Occurrence Handle1:CAS:528:DyaK2sXjs1Kls7o%3D Occurrence Handle9165125
IE Henson CT Wheeler (1976) ArticleTitleHormones in plants bearing nitrogen-fixing root nodules: the distribution of cytokinins in Vicia faba L. New Phytol 76 433–439 Occurrence Handle1:CAS:528:DyaE28XktFOitLs%3D
IE Henson CT Wheeler (1977) ArticleTitleHormones in plants bearing nitrogen-fixing root nodules: gibberellin-like substances in Alnus glutinosa (L.) gaertn. New Phytol 78 373–381 Occurrence Handle1:CAS:528:DyaE2sXhsFaksL4%3D
K Himanen E Boucheron S Vanneste JD Engler D Inzé T Beeckman (2002) ArticleTitleAuxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14 2339–2351 Occurrence Handle1:CAS:528:DC%2BD38XotFels70%3D Occurrence Handle12368490
AM Hirsch TV Bhuvaneswari JG Torrey T Bisseling (1989) ArticleTitleEarly nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc Natl Acad Sci USA 86 1244–1248 Occurrence Handle1:CAS:528:DyaL1MXhvV2mtrs%3D Occurrence Handle16594017
AM Hirsch Y Fang S Asad Y Kapulnik (1997) ArticleTitleThe role of phytohormones in plant-microbe symbioses. Plant Soil 194 171–184 Occurrence Handle1:CAS:528:DyaK2sXnt1Wns7g%3D
AM Hirsch Y Fang (1994) ArticleTitlePlant hormones and nodulation: What’s the connection? Plant Mol Biol 26 5–9 Occurrence Handle1:CAS:528:DyaK2MXitVKnt7w%3D Occurrence Handle7948898
AM Hirsch (1992) ArticleTitleDevelopmental biology of legume nodulation. New Phytol 122 211–237
WJ Hunter (2001) ArticleTitleInfluence of root-applied epibrassinolide and carbenoxolone on the nodulation and growth of soybean (Glycine max L.) seedlings. J Agron Crop Sci 186 217–221 Occurrence Handle1:CAS:528:DC%2BD3MXnvVCru70%3D
P Hutangura U Mathesius BG Rolfe MEK Jones (1999) ArticleTitleAuxin induction is a trigger for root gall formation caused by root-knot nematodes in white clover and is associated with the activation of the flavonoid pathway. Aust J Plant Physiol 26 221–231 Occurrence Handle1:CAS:528:DyaK1MXktlKht7c%3D
M Jacobs PH Rubery (1988) ArticleTitleNaturally occurring auxin transport regulators. Science 241 346–349 Occurrence Handle1:CAS:528:DyaL1cXkvV2rs7s%3D Occurrence Handle17734864
V Jaiswal SJH Rizvi D Mukerji SN Mature (1981) ArticleTitleCytokinins in root nodules of Phaseolus mungo. Ann Bot 48 301–305 Occurrence Handle1:CAS:528:DyaL38XkvV2gtg%3D%3D
J Jelenska J Deckert E Kondorosi AB Legocki (2000) ArticleTitleMitotic B-type cyclins are differentially regulated by phytohormones and during yellow lupine nodule development. Plant Sci 150 29–39 Occurrence Handle1:CAS:528:DC%2BD3cXlsFWmsw%3D%3D
M Jiang J Zhang (2001) ArticleTitleEffect of abscisic acid and active oxygen species, antioxidant defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42 1265–1273 Occurrence Handle1:CAS:528:DC%2BD3MXos1Oms74%3D Occurrence Handle11726712
JI Jimenez-Zurdo F Frugier MD Crespi A Kondorosi (2000) ArticleTitleExpression profiles of 22 novel molecular markers for organogenetic pathways acting in alfalfa nodule development. Mol Plant-Microbe Interact 13 96–106 Occurrence Handle1:CAS:528:DC%2BD3cXislSgtw%3D%3D Occurrence Handle10656590
W Jinsart B Ternai GM Polya (1991) ArticleTitleInhibition of wheat embryo calcium-dependent protein kinase and avian light chanin kinase by flavonoids and related compounds. Biol Chem Hoppe-Seyler 372 819–827 Occurrence Handle1:CAS:528:DyaK38Xjs1Omug%3D%3D Occurrence Handle1772594
PCL John K Zhang C Cong L Diederich F Wightman (1993) ArticleTitlep34cdc2 related proteins in control of cell cycle progression, the switch between division and differentiation in tissue development, and stimulation of division by auxin and cytokinin. Aust J Plant Physiol 20 503–526 Occurrence Handle1:CAS:528:DyaK2cXhvVOiur8%3D
Y Kanayama Y Yamamoto (1990) ArticleTitleInhibition of nitrogen fixation in soybean plants supplied with nitrate II. Accumulation and properties of nitrosylleghemoglobin in nodules. Plant Cell Physiol 31 207–214 Occurrence Handle1:CAS:528:DyaK3cXhvFGgtr8%3D
H Katznelson SE Cole (1965) ArticleTitleProduction of gibberellin-like substances by bacteria and actinomycetes. Can J Microbiol 11 733–741 Occurrence Handle1:CAS:528:DyaF2MXksFKntLk%3D Occurrence Handle5861292
M Kawaguchi H Imaizumi-Anraku S Fukai K Syono (1996) ArticleTitleUnusual branching in the seedlings of Lotus japonicus - gibberellins reveal the nitrogen-sensitive cell divisions within the pericycle on roots. Plant Cell Physiol 37 461–470 Occurrence Handle1:CAS:528:DyaK28Xjs1yisLY%3D
D Ke ME Saltveit (1988) ArticleTitlePlant hormone interaction and phenolic metabolism in the regulation of russet spotting in iceberg lettuce. Plant Physiol 88 1136–1140 Occurrence Handle1:CAS:528:DyaL1MXnsVWjtQ%3D%3D Occurrence Handle16666434
C Kistner M Parniske (2002) ArticleTitleEvolution of signal transduction in intracellular symbiosis. Trends Plant Sci 7 511–518 Occurrence Handle1:CAS:528:DC%2BD38Xotlyltbs%3D Occurrence Handle12417152
R Kneer AA Poulev A Olesinski I Raskin (1999) ArticleTitleCharacterization of the elicitor-induced biosynthesis and secretion of genestein from roots of Lupinus luteus L. J Exp Bot 50 1553–1559 Occurrence Handle1:CAS:528:DyaK1MXms1Ogt74%3D
H Koltai M Dhandaydham C Opperman J Thomas D Bird (2001) ArticleTitleOverlapping plant signal transduction pathways induced by a parasitic nematode and a rhizobial endosymbiont. Mol Plant-Microbe Interact 14 1168–1177 Occurrence Handle1:CAS:528:DC%2BD3MXnsVeltLs%3D Occurrence Handle11605956
E Kondorosi B Hoffmann G Endre L Börge C Koncz D Dudits J Szecsi G Kiss A Kondorosi (1993) Involvement of hormones in nodule initiation: auxin sensitivity and hormone balance affect nodulation of Medicago. R Palacios J Mora E Newton (Eds) New Horizons in Nitrogen Fixation Kluwer Academic Publishers Dordrecht, The Netherlands 357
H Kouchi S Hata (1993) ArticleTitleIsolation and characterisation of novel nodulin cDNAs representing genes expressed at early stages of soybean nodule development. Mol Gen Gent 238 106–119 Occurrence Handle1:CAS:528:DyaK3sXlslOltLw%3D
H Kouchi K Takane RB So JK Ladha PM Reddy (1999) ArticleTitleRice ENOD40: isolation and expression analysis in rice and transgenic soybean root nodules. Plant J 18 121–129 Occurrence Handle1:CAS:528:DyaK1MXjs1ansbY%3D Occurrence Handle10363365
L Krusell LH Madsen S Sato G Aubert A Genua K Szczyglowski G Due T Kaneko S Tabata F de Bruijn E Pajuelo N Sandals J Stougaard (2002) ArticleTitleShoot control of root development and nodulation is mediated by a receptor kinase. Nature 420 422–426 Occurrence Handle1:CAS:528:DC%2BD38XptFCis7w%3D Occurrence Handle12442170
CGR Lawson BG Rolfe MA Djordjevic (1996) ArticleTitle Rhizobium inoculation induces condition-dependent changes in the flavonoid composition of root exudates from Trifolium subterraneum. Aust J Plant Physiol 23 93–101 Occurrence Handle1:CAS:528:DyaK28XhslanurY%3D
KH Lee TA LaRue (1992a) ArticleTitlePleiotropic effects of sym-17: a mutation in Pisum sativum L. cv Sparkle causes decreased nodulation, altered root and shoot growth, and increased ethylene production. Plant Physiol 100 1326–1333 Occurrence Handle1:CAS:528:DyaK3sXhvFCruw%3D%3D
KH Lee TA LaRue (1992b) ArticleTitleEthylene as a possible mediator of light- and nitrate-induced inhibition of nodulation of Pisum sativum L. cv Sparkle. Plant Physiol 100 1334–1338 Occurrence Handle1:CAS:528:DyaK3sXhvFCqsg%3D%3D
KH Lee TA LaRue (1992c) ArticleTitleExogenous ethylene inhibits nodulation of Pisum sativum L. cv 7 Sparkle. Plant Physiol 100 1759–1763 Occurrence Handle1:CAS:528:DyaK3sXnslWqsQ%3D%3D
TT Lee (1971) ArticleTitleCytokinin-controlled indoleacetic acid oxidase isoenzymes in tobacco callus cultures. Plant Physiol 47 181–185 Occurrence Handle1:CAS:528:DyaE3MXos1Kntg%3D%3D Occurrence Handle16657590
FGP Lhuissier NCA De Ruijter BJ Sieberer JJ Esseling AMC Emons (2001) ArticleTitleTime course of cell biological events evoked in legume root hairs by Rhizobium Nod factors: state of the art. Ann Bot 87 289–302 Occurrence Handle1:CAS:528:DC%2BD3MXht1artbk%3D
B Lian X Zhou M Miransari DL Smith (2000) ArticleTitleEffects of salicylic acid on the development and root nodulation of soybean seedlings. J Agron Crop Sci 185 187–192 Occurrence Handle1:CAS:528:DC%2BD3cXns1Gku78%3D
KR Libbenga PAA Harkes (1973) ArticleTitleInitial proliferation of cortical cells in the formation of root nodules in Pisum sativum L. Planta 114 17–28
KR Libbenga F van Iren RJ Bogers MF Schraag-Lamers (1973) ArticleTitleThe role of hormones and gradients in the initiation of cortex proliferation and nodule formation in Pisum sativum L. Planta 114 29–39 Occurrence Handle1:CAS:528:DyaE2cXnvFSnsw%3D%3D
F Ligero JM Caba C Lluch J Olivares (1991) ArticleTitleNitrate inhibition of nodulation can be overcome in the presence of the ethylene inhibitor, aminoethoxyvinylglycine. Plant Physiol 97 1221–1225 Occurrence Handle1:CAS:528:DyaK38XhvVGjsA%3D%3D Occurrence Handle16668512
F Ligero C Lluch J Olivares (1986) ArticleTitleEvolution of ethylene from roots of Medicago sativa plants inoculated with Rhizobium meliloti. J Plant Physiol 125 361–365 Occurrence Handle1:CAS:528:DyaL2sXhsFOlsg%3D%3D
E Logemann SC Wu J Schroder E Schmelzer IE Somssich K Hahlbrock (1995) ArticleTitleGene activation by UV light, fungal elicitor or fungal infection in Petroselinum crispum is correlated with repression of cell cycle-related genes. Plant J 8 865–876 Occurrence Handle1:CAS:528:DyaK28XosFahsA%3D%3D Occurrence Handle8580959
M-A Lorteau BJ Ferguson FC Guinel (2001) ArticleTitleEffects of cytokinin on ethylene production and nodulation in pea (Pisum sativum) cv. Sparkle. Physiol Plant 112 421–428 Occurrence Handle1:CAS:528:DC%2BD3MXlt1Kru70%3D Occurrence Handle11473700
CM Markwei TA LaRue (1997) ArticleTitlePhenotypic characterization of sym 21, a gene conditioning shoot-controlled inhibition of nodulation in Pisum sativum cv Sparkle. Physiol Plant 100 927–932 Occurrence Handle1:CAS:528:DyaK2sXlvFCitbc%3D
F Martinez-Abarca JA Herrera-Cervara P Bueno J Sanjuan T Bisseling J Olivares (1998) ArticleTitleInvolvement of salicylic acid in the establishment of the Rhizobium meliloti-alfalfa symbiosis. Mol Plant-Microbe Interact 11 153–155 Occurrence Handle1:CAS:528:DyaK1cXmsFWhsQ%3D%3D
CS Maskell JF Gibson PJ Dart (1977) ArticleTitleElectron-paramagnetic-resonance studies of leghaemoglobins from soya-beans and cowpea root nodules. Biochem J 167 435–445
U Mathesius (2001) ArticleTitleFlavonoids induced in cells undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. J Exp Bot 52 419–426 Occurrence Handle1:CAS:528:DC%2BD3MXjs1ajsr4%3D Occurrence Handle11326048
U Mathesius C Bayliss JJ Weinman HRM Schlaman HP Spaink BG Rolfe ME McCully MA Djordjevic (1998b) ArticleTitleFlavonoids synthesised in cortical cells during nodule initiation are early developmental markers in white clover. Mol Plant-Microbe Interac 11 1223–1232 Occurrence Handle1:CAS:528:DyaK1cXnslyltbw%3D
U Mathesius C Charon BG Rolfe A Kondorosi M Crespi (2000) ArticleTitleTemporal and spatial order of events during the induction of cortical cell divisions in white clover by Rhizobium leguminosarum bv. trifolii inoculation or localized cytokinin addition. Mol Plant-Microbe Interact 13 617–628 Occurrence Handle1:CAS:528:DC%2BD3cXjsFensbc%3D Occurrence Handle10830261
U Mathesius G Keijzers SHA Natera JJ Weinman MA Djordjevic BG Rolfe (2001) ArticleTitleEstablishment of a root proteome reference map for the model legume Medicago truncatula using the EST database for peptide mass fingerprinting. Proteomics 1 1424–1440 Occurrence Handle1:CAS:528:DC%2BD3MXpt1Gisro%3D Occurrence Handle11922602
U Mathesius HRM Schlaman HP Spaink C Sautter BG Rolfe MA Djordjevic (1998a) ArticleTitleAuxin transport inhibition precedes nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J 14 23–34 Occurrence Handle1:CAS:528:DyaK1cXjtV2is7o%3D
C Mathieu S Moreau P Frendo A Puppo MJ Davies (1998) ArticleTitleDirect detection of radicals in intact soybean nodules: presence of nitric oxide-leghemoglobin complexes. Free Radic Biol Med 24 1242–1249 Occurrence Handle1:CAS:528:DyaK1cXjsFWhtrY%3D Occurrence Handle9626580
HI McKhann F Frugier G Petrovics T Coba de la Peña E Jurkevitch S Brown E Kondorosi A Kondorosi M Crespi (1997) ArticleTitleCloning of a WD-repeat-containing gene from alfalfa (Medicago saliva): a role in hormone-mediated cell division? Plant Mol Biol 34 771–780 Occurrence Handle1:CAS:528:DyaK2sXlvFyrsbg%3D Occurrence Handle9278167
RB Mellor DB Collinge (1995) ArticleTitleA simple model based on known plant defence reactions is sufficient to explain most aspects of nodulation. J Exp Bot 46 1–18 Occurrence Handle1:CAS:528:DyaK2MXjslKiurk%3D
MG Mes (1959) ArticleTitleInfluence of gibberellic acid and photoperiod on the growth, flowering nodulation and nitrogen assimilation of Vicia villosa. Nature 184 2035–2036 Occurrence Handle1:CAS:528:DyaF3cXmvFSntg%3D%3D
E Miklashevichs H Rohrig J Schell J Schmidt (2001) ArticleTitlePerception and signal transduction of rhizobial NOD factors. Crit Rev Plant Sci 20 373–394 Occurrence Handle1:CAS:528:DC%2BD3MXmsVOnuro%3D
E Minami H Kouchi JR Cohn T Ogawa G Stacey (1996) ArticleTitleExpression of the early nodulin, ENOD40, in soybean roots in response to various lipo-chitin signal molecules. Plant J 10 23–32 Occurrence Handle1:CAS:528:DyaK28XltVamsbg%3D Occurrence Handle8758977
GK Muday A DeLong (2001) ArticleTitlePolar auxin transport: controlling where and how much. Trends Plant Sci 6 535–542 Occurrence Handle1:CAS:528:DC%2BD3MXovFGksLc%3D Occurrence Handle11701382
Y Murakami-Mizukami Y Yamamoto S Yamaki (1991) ArticleTitleAnalysis of indole acetic acid and abscisic acid contents in nodules of soybean plants bearing VA Mycorrhizas. Soil Sci Plant Nutr 37 291–298 Occurrence Handle1:CAS:528:DyaK3MXmt1ygt78%3D
A Murphy WA Peer L Taiz (2000) ArticleTitleRegulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211 315–324 Occurrence Handle1:CAS:528:DC%2BD3cXlsVWqs7o%3D Occurrence Handle10987549
W Newcomb K Syono JG Torrey (1976) ArticleTitleDevelopment of an ineffective pea root nodule: morphogenesis, fine structure, and cytokinin biosynthesis. Can J Bot 55 1891–1907
R Nishimura M Hayashi GJ Wu H Kouchi H Imaizumi-Anraku Y Murakami S Kawasaki S Akao M Ohmori N Nagasawa K Harada M Kawaguchi (2002a) ArticleTitleHAR1 mediates systemic regulation of symbiotic organ development. Nature 420 426–429 Occurrence Handle1:CAS:528:DC%2BD38XptFCis7k%3D
R Nishimura M Ohmori H Fujita M Kawaguchi (2002b) ArticleTitleA lotus basic leucine zipper protein with a RING-finger motif negatively regulates the developmental program of nodulation. Proc Natl Acad Sci USA 99 15206–15210 Occurrence Handle1:CAS:528:DC%2BD38Xpt1yqtLY%3D
R Nishimura M Ohmori M Kawaguchi (2002c) ArticleTitleThe novel symbiotic phenotype of enhanced-nodulating mutant of Lotus japonicus: astray mutant is an early nodulating mutant with wider nodulation zone. Plant Cell Physiol 43 853–859 Occurrence Handle1:CAS:528:DC%2BD38XmsFOktbY%3D
N Nukui H Ezura K-I Yuhashi T Yasuta K Minamisawa (2000) ArticleTitleEffects of ethylene precursor and inhibitors for ethylene biosynthesis and perception on nodulation in Lotus japonicus and Macroptilium atropurpureum. Plant Cell Physiol 41 893–897 Occurrence Handle1:CAS:528:DC%2BD3cXlt1WlurY%3D Occurrence Handle10965947
PS Nutman (1952) ArticleTitleStudies on the physiology of nodule formation III. Experiments on the excision of root-tips and nodules. Ann Bot 16 79–101 Occurrence Handle1:CAS:528:DyaG38XitlOksA%3D%3D
V Oke SR Long (1999) ArticleTitleBacterial genes induced within the nodule during the Rhizobium-legume symbiosis. Mol Microbiol 32 837–849 Occurrence Handle1:CAS:528:DyaK1MXjs1ent7k%3D Occurrence Handle10361286
GED Oldroyd EM Engstrom SR Long (2001) ArticleTitleEthylene inhibits the Nod factor signal transduction pathway of Medicago truncatula. Plant Cell 13 1835–1849 Occurrence Handle1:CAS:528:DC%2BD3MXmsFKiu70%3D Occurrence Handle11487696
RV Penmetsa DR Cook (1997) ArticleTitleA legume ethylene-insensitive mutant hyperinfected by its Rhizobium symbiont. Science 275 527–530 Occurrence Handle1:CAS:528:DyaK2sXnvVekuw%3D%3D Occurrence Handle8999796
NK Peters DK Chris-Estes (1989) ArticleTitleNodule formation is stimulated by the ethylene inhibitor, aminoethoxyvinylglycine. Plant Physiol 91 690–693 Occurrence Handle1:CAS:528:DyaK3cXkslem Occurrence Handle16667088
DA Phillips JG Torrey (1970) ArticleTitleCytokinin production by Rhizobium japonicum. Physiol Plant 23 1057–1063 Occurrence Handle1:CAS:528:DyaE3MXivFWnsA%3D%3D
DA Phillips JG Torrey (1972) ArticleTitleStudies on cytokinin production by Rhizobium. Plant Physiol 49 11–15 Occurrence Handle1:CAS:528:DyaE38XktlOltg%3D%3D Occurrence Handle16657888
DA Phillips (1971) ArticleTitleAbscisic acid inhibition of root nodule initiation in Pisum sativum. Planta 100 181–190 Occurrence Handle1:CAS:528:DyaE38XitFyksw%3D%3D
H Porta P Rueda-Benitez F Campos JM Colmenaro-Flores JM Colorado MJ Carmona AA Covarrubias M Rocha-Sosa (1999) ArticleTitleAnalysis of lipoxygenase mRNA accumulation in the common bean (Phaseolus vulgaris L.) during development and under stress conditions. Plant Cell Physiol 40 850–858 Occurrence Handle1:CAS:528:DyaK1MXlsVOhsbw%3D Occurrence Handle10555305
A Puppo B Halliwell (1988) ArticleTitleGeneration of hydroxyl radicals by soybean nodule leghaemoglobin. Planta 173 405–410 Occurrence Handle1:CAS:528:DyaL1cXht1Ojtbc%3D
W Rademacher (1994) ArticleTitleGibberellin formation in microorganisms. Plant Growth Regul 15 303–314 Occurrence Handle1:CAS:528:DyaK2MXis1Cgurw%3D
M Radley (1961) ArticleTitleGibberellin-like substances in plants. Nature 191 684–685 Occurrence Handle1:CAS:528:DyaF38Xpt1yq Occurrence Handle13738974
SK Ramu HM Peng DR Cook (2002) ArticleTitleNod factor induction of reactive oxygen species production is correlated with expression of the early nodulin gene rip1 in Medicago truncatula. Mol Plant-Microbe Interac 15 522–528 Occurrence Handle1:CAS:528:DC%2BD38XktlSntr0%3D
MP Ramanujam V Abdul Jaleel G Kumaravelu (1998) ArticleTitleEffect of salicylic acid on nodulation, nitrogenous compounds and related enzymes of Vigna mungo. Biol Plant 41 307–311 Occurrence Handle1:CAS:528:DyaK1cXotVGksbY%3D
K Recourt AJ van Tunen LA Mur AAN van Brussel BJJ Lugtenberg JW Kijne (1992) ArticleTitleActivation of flavonoid biosynthesis in roots of Vicia sativa subsp. nigra plants by inoculation with Rhizobium leguminosarum biovar viciae. Plant Mol Biol 19 211–220
JR Redmond M Batley MA Djordjevic RW Innes PL Keumpel BG Rolfe (1986) ArticleTitleFlavones induce expression of nod genes in Rhizobium. Nature 323 632–635 Occurrence Handle1:CAS:528:DyaL2sXivVehtw%3D%3D
M Regvar N Gogala P Zalar (1996) ArticleTitleEffects of jasmonic acid on mycorrhizal Allium sativum. New Phytol 134 703–707 Occurrence Handle1:CAS:528:DyaK2sXls1yitA%3D%3D
B Relic X Perret MT Estrada-Garcia J Kopcinska W Golinowski H Krishnan SG Pueppke WJ Broughton (1994) ArticleTitleNod factors of Rhizobium are the key to the legume door. Mol Microbiol 13 171–178 Occurrence Handle1:CAS:528:DyaK2cXltVeqtL0%3D Occurrence Handle7984092
C Rice-Evans (2001) ArticleTitleFlavonoid antioxidants. Curr Med Chem 8 797–807 Occurrence Handle1:CAS:528:DC%2BD3MXjsVChtbc%3D Occurrence Handle11375750
RW Ridge GL Bender BG Rolfe (1992) ArticleTitleNodule-like structures induced on roots of wheat seedlings by addition of the synthetic auxin 2,4-dichlorophenoxyacetic acid and the effects of microorganisms. Aust J Plant Physiol 19 481–492 Occurrence Handle1:CAS:528:DyaK3sXjs1Sitg%3D%3D
LF Roddam WR Lewis-Henderson MA Djordjevic (2002) ArticleTitleTwo novel chromosomal loci influence cultivar-specific nodulation failure in the interaction between strain ANU794 and subterranean clover cv. Woogenellup. Funct Plant Biol 29 473–483 Occurrence Handle1:CAS:528:DC%2BD38XktF2ht7c%3D
C Rodrigez-Barrueco F Bermudez de Castro (1973) ArticleTitleCytokinin-induced pseudonodules on Alnus glutinosa. Physiol Plant 29 277–280
C Rodriguez-Barrueco C Miguel LMS Palni (1979) ArticleTitleCytokinins in root nodules of the nitrogen-fixing non-legume Myrica gale L. Z Pflanzenphysiol 95 275–278 Occurrence Handle1:CAS:528:DyaE1MXmtVOntrY%3D
H Röhrig J Schmidt E Miklashevichs J Schell M John (2002) ArticleTitleSoybean ENOD40 encodes two peptides that bind to sucrose synthase. Proc Natl Acad Sci USA 99 1915–1920 Occurrence Handle11842184
E Rojo R Solano JJ Sánchez-Serrano (2003) ArticleTitleInteractions between signalling compounds involved in plant defence. J Plant Growth Regul 22 22–22
JJ Ross DP O’Neil JJ Smith HJ Kerckhoffs RC Elliot (2000) ArticleTitleEvidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant J 21 547–552 Occurrence Handle1:CAS:528:DC%2BD3cXjsVeru7g%3D Occurrence Handle10758505
JJ Ross (1998) ArticleTitleEffects of auxin transport inhibitors on gibberellin in pea. J Plant Growth Regul 17 141–146 Occurrence Handle1:CAS:528:DyaK1cXms1Ght74%3D
JA Ryals UH Neuenschwander MG Willits A Molina H-Y Steiner MD Hunt (1996) ArticleTitleSystemic acquired resistance. Plant Cell 8 1809–1819 Occurrence Handle1:CAS:528:DyaK28Xmsl2murw%3D Occurrence Handle12239363
P Salzer H Corbiere T Boller (1999) ArticleTitleHydrogen peroxide accumulation in Medicago truncatula roots colonized by the arbuscular mycorrhiza-forming fungus Glomus intraradicies. Planta 208 319–325 Occurrence Handle1:CAS:528:DyaK1MXjtlOgtbk%3D
JL Salzwedel FB Dazzo (1993) ArticleTitlepSym nod gene influence on elicitation of peroxidase activity from white clover and pea roots by rhizobia and their cell-free supernatants. Mol Plant-Microbe Interect 6 127–134 Occurrence Handle1:CAS:528:DyaK3sXitlSjtLo%3D
R Santos D Hérouart A Puppo D Touati (2000) ArticleTitleCritical protective role of bacterial superoxide dismutase in Rhizobium-legume symbiosis. Mol Microbiol 38 750–759 Occurrence Handle1:CAS:528:DC%2BD3MXitFOr Occurrence Handle11115110
R Santos D Hérouart S Sigaud D Touati A Puppo (2001) ArticleTitleOxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol Plant-Microbe Interact 14 86–89 Occurrence Handle1:CAS:528:DC%2BD3MXot1Og Occurrence Handle11194876
T Sato H Fujikaki N Ohtake K Sueyoshi T Takahashi A Sato T Ohyama (2002) ArticleTitleEffect of exogenous salicylic acid on nodule formation of hypernodulating mutant and wild type of soybean. Soil Sci Plant Nutr 48 413–420 Occurrence Handle1:CAS:528:DC%2BD38XltFOrt7o%3D
B Scheres HI McKhann A Zalensky M Lobler T Bisseling AM Hirsch (1992) ArticleTitleThe PsENOD12 gene is expressed at two different sites in Afghanistan pea pseudonodules induced by auxin transport inhibitors. Plant Physiol 100 1649–1655 Occurrence Handle1:CAS:528:DyaK3sXnslWntA%3D%3D Occurrence Handle16653180
HRM Schlaman AA Gisel NEM Quaedvlieg GV Bloemberg BJJ Lugtenberg JW Kijne I Potrykus HP Spaink C Sautter (1997) ArticleTitleChitin oligosaccharides can induce cortical cell division in roots of Vicia sativa when delivered by ballistic microtargeting. Development 124 4887–4895 Occurrence Handle1:CAS:528:DyaK1cXit1Kisw%3D%3D Occurrence Handle9428425
J Schmid H Röhrig M John U Wienecke G Stacey C Koncz J Schell (1993) ArticleTitleAlteration of plant growth and development by Rhizobium nodA and nodB genes involved in the synthesis of oligosaccharide signal molecules. Plant J 4 651–658
JS Schmidt JE Harper TK Hoffman AF Bent (1999) ArticleTitleRegulation of soybean nodulation independent of ethylene signalling. Plant Physiol 119 951–959 Occurrence Handle1:CAS:528:DyaK1MXhvFymtbc%3D Occurrence Handle10069833
PE Schmidt WJ Broughton D Werner (1994) ArticleTitleNod-factors of Bradyrhizobium japonicum and Rhizobium sp. NGR234 induce flavonoid accumulation in soybean root exudate. Mol Plant-Microbe Interact 7 384–390 Occurrence Handle1:CAS:528:DyaK2cXlsFaqu70%3D
IR Searle AE Men T Laniya DM Buzas I Iturbe-Ormaetxe BJ Carroll PM Gresshoff (2003) ArticleTitleLong-distance signalling in nodulation directed by a CLAVATA1-like receptor kinase. Science 299 109–112 Occurrence Handle1:CAS:528:DC%2BD38XpvVensLo%3D Occurrence Handle12411574
G Sinvany Y Kapulnik S Wininger H Badani E Jurkevitch (2002) ArticleTitleThe early nodulin enod40 is induced by, and also promotes arbuscular mycorrhizal root colonization. Physiol Mol Plant Pathol 60 103–109 Occurrence Handle1:CAS:528:DC%2BD38XktVyjsbo%3D
G Smit CC de Koster J Schripsema HP Spaink AA van Brussel JW Kijne (1995) ArticleTitleUridine, a 5 cell division factor in pea roots. Plant Mol Biol 29 869–873 Occurrence Handle1:CAS:528:DyaK28XitF2lsw%3D%3D Occurrence Handle8541512
C Sousa C Johansson C Charon H Manyani C Sautter A Kondorosi M Crespi (2001) ArticleTitleTranslational and structural requirements of the early nodulin gene enod40, a short-open reading frame-containing RNA, for elicitation of a cell-specific growth response in the alfalfa root cortex. Mol Cell Biol 21 354–366 Occurrence Handle1:CAS:528:DC%2BD3MXptVSk Occurrence Handle11113209
HP Spaink AHM Wijfjes TB Vanvliet JW Kijne BJJ Lugtenberg (1993) ArticleTitleRhizobial lipo-oligosaccharide signals and their role in plant morphogenesis. Are analogous lipophilic chitin derivatives produced by the plant? Aust J Plant Physiol 20 381–392 Occurrence Handle1:CAS:528:DyaK2cXhsFyktro%3D
HP Spaink (1999) Flavonoids as regulators of plant development: new insights from studies of plant-rhizobia interactions. R Verpoorte K Downum J Romeo (Eds) Recent Advances in Phytochemistry Interactions of Plants and Microorganisms vol. 32 167–178
G Stacey N Shibuya (1997) ArticleTitleChitin recognition in rice and legumes. Plant Soil 194 161–169 Occurrence Handle1:CAS:528:DyaK2sXnt1Wns78%3D
C Staehelin C Charon T Boller M Crespi A Kondorosi (2001) ArticleTitle Medicago truncatula plants overexpressing the early nodulin gene enod40 exhibit accelerated mycorrhizal colonization and enhanced formation of arbuscules. Proc Natl Acad Sci USA 98 15366–15371 Occurrence Handle1:CAS:528:DC%2BD38XpsVGm Occurrence Handle11752473
C Staehelin J Granado J Muller A Wiemken RB Mellor G Felix M Regenaas WJ Broughton T Boller (1994) ArticleTitlePerception of Rhizobium nodulation factors by tomato cells and inactivation by root chitinases. Proc Natl Acad Sci USA 91 2196–2200 Occurrence Handle1:CAS:528:DyaK2cXitFalur0%3D Occurrence Handle8134372
HA Stafford (1997) ArticleTitleRoles of flavonoids in symbiotic and defence functions in legume roots. Bot Rev 63 27–39
G Stenlid (1976) ArticleTitleEffects of flavonoids on the polar transport of auxins. Physiol Plant 38 262–266 Occurrence Handle1:CAS:528:DyaE2sXosVKhuw%3D%3D
LC Stephenson TW Bunker WE Dubbs HD Grimes (1998) ArticleTitleSpecific soybean lipoxygenases localize to discrete subcellular compartments and their mRNAs are differentially regulated by source-sink status. Plant Physiol 116 923–933 Occurrence Handle1:CAS:528:DyaK1cXitVKqu78%3D Occurrence Handle9501125
J Stougaard (2001) ArticleTitleGenetics and genomics of root symbiosis. Curr Opin Plant Biol 4 328–335 Occurrence Handle1:CAS:528:DC%2BD3MXksVyktL0%3D Occurrence Handle11418343
S Stracke C Kistner S Yoshida L Mulder S Sato T Kaneko S Tabata N Sandal J Stougaard K Szczyglowski M Parniske (2002) ArticleTitleA plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 417 959–962 Occurrence Handle1:CAS:528:DC%2BD38XkvVagsbg%3D Occurrence Handle12087405
JG Streeter (1988) ArticleTitleInhibition of legume nodule formation and N2 fixation by nitrate. Crit Rev Plant Sci 7 1–23 Occurrence Handle1:CAS:528:DyaL1MXnt1CgtQ%3D%3D
N Suganuma H Yamauchi K Yamamoto (1995) ArticleTitleEnhanced production of ethylene by soybean roots after inoculation with Bradyrhizobium japonicum. Plant Sci 111 163–168 Occurrence Handle1:CAS:528:DyaK2MXptVemur4%3D
JC Suttle (1988) ArticleTitleEffect of ethylene treatment on polar IAA transport, net IAA uptake and specific binding of N-1-naphthylphthalamic acid in tissues and microsomes isolated from etiolated pea epicotyls. Plant Physiol 88 795–799 Occurrence Handle1:CAS:528:DyaL1MXkslOm Occurrence Handle16666386
K Syono W Newcomb JG Torrey (1976) ArticleTitleCytokinin production in relation to the development of pea root nodules. Can J Bot 54 2155–2162 Occurrence Handle1:CAS:528:DyaE28XlsFKlsbo%3D
KV Thimann (1936) ArticleTitleOn the physiology of the formation of nodules on legumes roots. Proc Natl Acad Sci USA 22 511–513 Occurrence Handle1:CAS:528:DyaA2sXjvVektw%3D%3D Occurrence Handle16577735
GA Thurber JR Douglas AW Galston (1958) ArticleTitleInhibitory effects of gibberellins on nodulation in dwarf beans, Phaseolous vulgaris. Nature 181 1082–1083 Occurrence Handle1:CAS:528:DyaG1cXpvFyjtA%3D%3D
JC Trinchant J Rigaud (1982) ArticleTitleNitrite and nitric oxide as inhibitors of nitrogenase from soybean bacteroids. Appl Environ Microbiol 44 1385–1388 Occurrence Handle1:CAS:528:DyaL3sXjtFajtg%3D%3D Occurrence Handle16346155
EW Triplett JJ Heitholt KB Evensen DG Blevins (1981) ArticleTitleIncrease in internode length in Paseolus lunatus L. caused by inoculation with a nitrate reductase-deficient strain of Rhizobium sp. Plant Physiol 67 1–4 Occurrence Handle1:CAS:528:DyaL3MXotVemtA%3D%3D Occurrence Handle16661605
G Truchet DG Barker S Camut F de Billy J Vasse T Huguet (1989) ArticleTitleAlfalfa nodulation in the absence of Rhizobium. Mol Gen Genet 219 65–68 Occurrence Handle1:CAS:528:DyaK3cXlt1CjtQ%3D%3D
G Truchet P Roche P Lerouge J Vasse S Camut F de Billy J-C Promé J Dénarié (1991) ArticleTitleSulphated lipo-oligosaccharide signals of Rhizobium meliloti elicit root nodule organogenesis in alfalfa. Nature 351 670–673 Occurrence Handle1:CAS:528:DyaK3MXltlWmsbo%3D
RE Tully P van Berkum KW Lovins DL Keister (1998) ArticleTitleIdentification and sequencing of a cytochrome P450 gene cluster from Bradyrhizobium japonicum. Biochim Biophys Acta 1398 243–255 Occurrence Handle1:CAS:528:DyaK1cXkt1yiurc%3D Occurrence Handle9655913
AAN van Brussel T Tak KJM Boot JW Kijne (2002) ArticleTitleAutoregulation of root nodule formation: signals of both symbiotic partners studied in a split-root system of Vicia sativa subsp. nigra. Mol Plant-Microbe Interact 15 341–349 Occurrence Handle1:CAS:528:DC%2BD38XivV2mt7o%3D Occurrence Handle12026172
PPG van der Holst HRM Schlaman HP Spaink (2001) ArticleTitleProteins involved in the production and perception of oligosaccharides in relation to plant and animal development. Curr Opin Struct Biol 11 608–616 Occurrence Handle1:CAS:528:DC%2BD3MXntlyisLs%3D Occurrence Handle11785763
PC van Spronsen AAN van Brussel JW Kijne (1995) ArticleTitleNod-factors produced by Rhizobium leguminosarum biovar. viciae induce ethylene-related changes in root cortical cells of Vicia sativa subsp. nigra. Eur J Cell Biol 68 463–469 Occurrence Handle1:STN:280:DyaK283ksFyjtw%3D%3D Occurrence Handle8690027
PC van Spronsen T Tak AMM Rood AAN van Brussel JW Kijne KJM Boot (2003) ArticleTitleSalicylic acid inhibits indeterminant-type nodulation but not determinant-type nodulation. Mol Plant Microbe Interact 16 83–91 Occurrence Handle1:CAS:528:DC%2BD38XpvVWgs7c%3D Occurrence Handle12580285
WAT van Workum AAN van Brussel T Tak CA Wijffelman JW Kijne (1995) ArticleTitleEthylene prevents nodulation of Vicia sativa ssp. nigra by exopolysaccharide-deficient mutants of Rhizobium leguminosarum bv. viciae. Mol Plant Microbe Interact 8 278–285 Occurrence Handle1:CAS:528:DyaK2MXls1Okurc%3D
BV Vardhini SSR Rao (1999) ArticleTitleEffect of brassinosteroids on nodulation and nitrogenase activity in groundnut (Arachis hypogaea L.). Plant Growth Regul 28 165–167 Occurrence Handle1:CAS:528:DyaK1MXmsFWht7g%3D
J Vasse F de Billy G Truchet (1993) ArticleTitleAbortion of infection during the Rhizobium meliloti-alfalfa symbiotic interaction is accompanied by a hypersensitive reaction. Plant J 4 555–566
KL-C Wang H Li JR Ecker (2002) ArticleTitleEthylene biosynthesis and signalling networks. Plant Cell S131 S131–S151
SH Watts CT Wheeler JR Hillman AMM Berrie A Crozier VB Math (1983) ArticleTitleAbscisic acid in the nodulated root system of Alnus glutinosa. New Phytol 95 203–208 Occurrence Handle1:CAS:528:DyaL2cXjsFGm
CT Wheeler IE Henson ME McLaughlin (1979) ArticleTitleHormones in plants bearing actinomycete root nodules. Bot Gaz 140S 52–57
PM Williams M Sicardi De Mallorca (1982) ArticleTitleAbscisic acid and gibberellin-like substances in roots and root nodules of Glycine max. Plant Soil 65 19–26 Occurrence Handle1:CAS:528:DyaL38Xkt1Sktb0%3D
JP Wisniewski CD Gardner NJ Brewin (1999) ArticleTitleIsolation of lipoxygenase cDNA clones from pea nodule mRNA. Plant Mol Biol 39 775–783 Occurrence Handle1:CAS:528:DyaK1MXjtFSqs7g%3D Occurrence Handle10350091
JP Wisniewski EA Rathbun JP Knox NJ Brewin (2000) ArticleTitleInvolvement of diamine oxidase and peroxidase in insolubilization of the extracellular matrix: implications for pea nodule initiation by Rhizobium leguminosarum. Mol Plant Microbe Interact 13 413–420 Occurrence Handle1:CAS:528:DC%2BD3cXitFOksb4%3D Occurrence Handle10755304
J Wopereis E Pajuelo FB Dazzo Q Jiang PM Gresshoff FJ de Bruijn J Stougaard K Szczyglowski (2000) ArticleTitleShort root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J 23 97–114 Occurrence Handle1:CAS:528:DC%2BD3cXlvFWnsrg%3D Occurrence Handle10929105
C Wu R Dickstein AJ Cary JH Norris (1996) ArticleTitleThe auxin transport inhibitor N-(1-naphthyl)phthalamic acid elicits pseudonodules on non-nodulating mutants of white sweetclover. Plant Physiol 110 501–510 Occurrence Handle1:CAS:528:DyaK28XhtF2msr8%3D Occurrence Handle12226200
WC Yang C de Blank I Maskiene H Hirt J Bakker A van Kammen H Franssen T Bisseling (1994) ArticleTitle Rhizobium Nod factors reactivate the cell cycle during infection and nodule primordium formation, but the cell cycle is only completed in primordium formation. Plant Cell 6 1415–1426 Occurrence Handle1:CAS:528:DyaK2MXhvV2is78%3D Occurrence Handle7994175
WC Yang P Katinakis P Hendriks A Smolders F de Vries J Spee A van Kammen T Bisseling H Franssen (1993) ArticleTitleCharacterisation of GmENOD40, a gene showing novel patterns of cell-specific expression during soybean nodule development. Plant J 3 573–585 Occurrence Handle1:CAS:528:DyaK3sXltFSgtL4%3D Occurrence Handle8220464
K-I Yuhashi N Ichikawa H Ezura S Akao Y Minakawa N Nukui T Yasuta K Minamisawa (2000) ArticleTitleRhizobitoxine production by Bradyrhizobium elkanii enhances nodulation and competitiveness on Macroptilium atropurpureum. Appl Environ Microbiol 66 2658–2663 Occurrence Handle1:CAS:528:DC%2BD3cXjvFWnsbg%3D Occurrence Handle10831453
SAJ Zaat AAN van Brussel T Tak BJJ Lugtenberg JW Kijne (1989) ArticleTitleThe ethylene-inhibitor aminoethoxyvinylglycine restores normal nodulation by Rhizobium leguminosarum biovar. viciae on Vicia saliva subsp. nigra by suppressing the ‘Thick and short roots’ phenotype. Planta 177 141–150 Occurrence Handle1:CAS:528:DyaL1MXhvV2mtb4%3D
H Zhang A Jennings PW Barlow BG Forde (1999) ArticleTitleDual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA 96 6529–6534 Occurrence Handle1:CAS:528:DyaK1MXksFKktbc%3D Occurrence Handle10339622
JAS Zuanazzi PH Clergeot J-C Quirion HP Husson A Kondorosi P Ratet (1998) ArticleTitleProduction of Sinorhizobium meliloti nod gene activation and repressor flavonoids from Medicago sativa roots. Mol Plant Microbe Interact 11 784–794 Occurrence Handle1:CAS:528:DyaK1cXkslWht7o%3D
Acknowledgements
Due to the vast size of the topic, the authors have undoubtedly omitted some researchers’ work, for which we apologize. Financial support was provided to BJF by the Tasmanian International Research Scholarship and the Thomas Crawford Memorial Research Scholarship, both of the University of Tasmania, and to UM by the Australian Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferguson, B., Mathesius, U. Signaling Interactions During Nodule Development . J Plant Growth Regul 22, 47–72 (2003). https://doi.org/10.1007/s00344-003-0032-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-003-0032-9