Abstract
While nitric oxide (NO) is being monitored in various fields of application, there is still a lack of available instruments at a sub-ppb level of sensitivity. We report on the first application of Optical Feedback Cavity-Enhanced Absorption Spectroscopy (OF-CEAS) to NO trace gas analysis, with a room-temperature quantum-cascade laser at 5.26 µm (1900.5 cm\(^{-1}\)). A detection limit of 60 ppt is reached in a single measurement performed in 140 ms. The stability of the instrument allows to average for 10 s down to 8.3 ppt, limited by drift of etalon fringes in the spectra. This work opens the path towards new applications notably in breath analysis and environment sciences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nitric oxide (NO) has an important impact in different fields from health to atmospheric chemistry. This molecule, and more generally nitrogen oxides (NOx), are reactive compounds present at trace levels in the atmosphere that play a major role being a precursor of ozone [1, 2]. It is mainly released in the atmosphere by fuel combustion processes [3, 4]. While NO is the majority compound of NOx (about 90%), it is NO\(_2\), the most toxic of the nitrogen oxides, that is monitored and regulated by sanitary authorizations: NO\(_2\) threshold limit is fixed at 100 ppb for hourly exposure. For NO, it is considered that strong damage to human body appears for exposure at the level of tens of ppm. NO monitoring at the ppm level is performed with compact commercial instruments, based on electrochemical processes. Typical NO concentration in ambient air is below the limit of detection (LOD) of these instruments. In urban area it usually ranges from about ten ppb up to nearly one hundred ppb [5]. While miniaturized electrochemical NO sensors is a recent development achieving a LOD around 10 ppb [6, 7], they are not available yet. On the other hand, monitoring NO in the 10–100 ppb range is routinely performed in the field with commercial chemiluminescence instruments.
Chemiluminescence is also considered as the standard technique in medicine for breath analysis: the fraction of exhaled nitric oxide (FeNO) is intensively monitored since decades by this technique. NO was elected as the 1992 molecule of the year [8] and the 1998 Nobel prize in Physiology or Medicine was awarded to the discoveries concerning “nitric oxide as a signaling molecule in the cardiovascular system”. NO is a well-known marker of airway inflammation diseases [9]. Among them is asthma, which diagnostic studies led to typical cutoff values of 10 ppb in exhaled air under which the patient can be considered to be healthy, while the FeNO of asthmatic patients can rise up to a hundred ppb [10, 11]. Even smaller FeNO levels, of about 1 ppb, are to be measured in studies like pulmonary infection or lung transplant procedures where measurements are made on an ex vivo-isolated lung [12].
Chemiluminescence analyzers are based on the conversion of NO in excess ozone to excited NO\(_2\) molecules whose photoluminescence is monitored. This principle leads to interferences with other nitrogen compounds and ozone concentration in the gas sample [13]. A high-performance commercial chemiluminescence instrument is now available from ThermoFischer Scientific with a 50 ppt LOD in 120 s (42i-TL analyzer). To be operated at this level, it needs to be carefully and regularly calibrated. While its response time is compatible with most atmospheric measurements it is not sufficient, for example, to resolve the respiratory cycles for real-time breath analysis [14].
In atmospheric chemistry, there is a real need to measure NO concentration at the sub-ppb level. For example, this is required for the study of air–snow interactions, necessary for a deep understanding of the current atmospheric processes as well as for the investigation of past climate [15]. NOx measurement campaigns in Greenland [16] and at South Pole [17, 18] reported NO measurements ranging from only a few ppt to hundreds of ppt. These measurements were performed with research-grade chemiluminescence instruments featuring a LOD of about 5 ppt over a 30 s averaging time, still requiring careful periodic calibrations. During other measurement campaigns, the lack of ppt level NO analyzers forced the use of NO concentrations derived from computation models [19].
To face the various needs in trace gas analysis, NO analyzers are being developed based on different laser spectroscopy techniques. While not being a widespread approach, indirect measurements were successfully exploited by Brown et al. [20]. By the same chemical reaction of chemiluminescence instruments, NO is converted into NO\(_2\) that is detected by cavity ring-down spectroscopy (CRDS) with a blue laser diode (404 nm). The difference of two parallel measurements of NO\(_2\) with and without NO conversion yields a 140 ppt LOD for NO in 1 s during airborne measurements that can be averaged down to 20 ppt in 60 s for ground-based measurements [20, 21]. On the other hand, a main advantage of laser spectroscopy is that it allows for direct NO measurements. To reach the best sensitivity, the fundamental absorption band of NO in the mid-infrared region (MIR) around 5.3 µm is chosen. Here cross sections are two orders of magnitude higher than in the near-infrared (NIR) rovibrational band around 2.7 µm. The development of trace gas laser spectrometers in the MIR for field measurements was triggered by relatively recent developments of thermoelectrically cooled continuous wave-distributed feedback quantum-cascade lasers (QCL) [22, 23]. With these lasers, instruments with a LOD of a few ppb in 1 s integration time were reported by quartz-enhanced photo-acoustic spectroscopy (4.9 ppb [24]) or Faraday rotation spectroscopy (5 ppb [25]). Sub-ppb levels in 1 s are reached by integrated cavity output spectroscopy (ICOS, 0.7 ppb [26]) or by wavelength modulation spectroscopy in a multipass cell (0.5 ppb [27] and 0.6 ppb [28]). Some laboratory developments led to commercially available instruments such as the “NO/CO\(_2\) Analyzer” offered by Los Gatos, based on ICOS, with a LOD of 100 ppt in 10 s [29]. This instrument fits in a 4U 19”rack (68 kg). The “Dual Laser Trace Gas Monitor” of Aerodyne Research, based on direct absorption in a Herriot multipass cell, reaches a LOD of 20 ppt in 100 s [30]. This level of sensitivity requires careful and regular calibration together with a thermoelectric liquid cooling of the optical module. The whole instrument, including the vacuum pump, weights 175 kg.
This paper reports on the first NO measurements performed by optical feedback cavity-enhanced absorption spectroscopy (OF-CEAS). This technique was introduced and patented by Morville et al. in 2005 [31]. It allows selective and quantitative trace gas analysis and isotopic measurements with robust and compact instruments for field applications. It delivers real-time absolute measurements with a smallest detectable absorption coefficient in the range of a few 10\(^{-10}\)/cm for 1 s acquisition time [32], it does not require periodic calibration with certified gas mixtures [33], its sampling volume is small (\(\simeq\)20 cm\(^3\)) allowing for response times well below 1 s (still with a modest sample flow, smaller than 1 L/min). Exploiting optical feedback (OF) allows OF-CEAS to solve one of the most pertinent problems of other implementations of a resonant optical cavity like conventional cavity-enhanced absorption spectroscopy and cavity ring-down spectroscopy, the difficulty of injecting a sufficient amount of laser light into a high-finesse optical cavity, and provides a better spectral resolution and higher signal-to-noise ratio [34]. This potentially offsets the relatively poor performance of optical detectors in the MIR as compared to the NIR and is a major advantage of OF-CEAS compared to other highly sensitive spectroscopic techniques previously reported. A private company (AP2E, Aix-en-Provence, France) presently exploits the OF-CEAS patent to realize commercial NIR instruments (namely ProCEAS) in the domains of industrial and air quality monitoring.
While OF-CEAS was previously introduced in the NIR with distributed feedback diode lasers, recently a few OF-CEAS prototype setups in the MIR were reported, demonstrating that QCL sensitivity to optical feedback is adequate [35,36,37]. In this paper, we report on OF-CEAS measurements of trace NO with a QCL at 5.26 µm (1900.5 cm\(^{-1}\)). We will present the instrument we developed insisting on the difference with respect to previously reported NIR OF-CEAS analyzers. This setup achieves a sensitivity of 60 ppt for a single measurement performed in only 140 ms. The stability of the instrument allows to average for 10 s to below 10 ppt.
All values reported in this paper are mole fractions (expressed in ppb or ppt) but are called concentration as commonly done by the community.
2 Materials and methods
2.1 Optical feedback cavity-enhanced absorption spectroscopy
OF-CEAS has been described in detail in different publications [31, 33, 34, 38]. Here we will recall its basics. As other cavity-enhanced spectroscopy techniques, OF-CEAS achieves its high sensitivity by realizing a very long optical absorption path length by the use of a resonant optical cavity as the sample cell. Photons trapped between the highly reflective mirrors (R\(\,>\,\)99.99\(\%\)) of the optical cavity will have a long lifetime in the absence of intra-cavity absorption corresponding to an effective absorption path length of several kilometers, with a very compact setup: the cavity length is 1 m, folded to a base length of just 50 cm. The originality of OF-CEAS is that the optical cavity is made of three mirrors placed in a “V-shaped” configuration (Fig. 1). In this way, a fraction of the light trapped inside the optical cavity and, therefore, frequency selected by a cavity resonance can be returned to the laser. The non-linear response of the laser forces it to lase on the exact frequency of the excited cavity mode. This optical feedback effect is also responsible for a narrowing of the laser emission line width and an increase of the cavity transmission to a level that is orders of magnitude larger than in competing techniques [34]. OF-CEAS absorption spectra are acquired in a small spectral region by scanning the laser frequency over 90 cavity modes, at a fast acquisition rate (7 Hz in Fig. 2).
Importantly, OF-CEAS provides selective quantitative absorption measurements in real-time without the need for periodic calibration (using certified gas mixtures). A normalization procedure of the absorbance scale is applied continuously based on cavity optical loss measurements performed by CRDS once per laser scan (Fig. 3) without the need for “empty cavity” measurements [33]. Successful inter-comparison of an OF-CEAS analyzer against a self-calibrating gas chromatograph has been reported for CH\(_{4}\) and CO [39, 40], as well as a water isotope OF-CEAS instrument against an isotope ratio mass spectrometer [41].
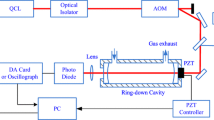
Scheme of the experimental setup. The QCL is coupled to the V-cavity made of three highly reflective mirrors (\(M_1\), \(M_2\) and \(M_3\)). The two cavity arms are equal and measure 49.5 cm. A \(\lambda /2\) plate and a ZnSe wedged window allow to adjust the OF rate while maintaining an s-polarized light at the input of the cavity. For clarity, the mirrors used to match the distance between \(PD_{\rm signal}\) and \(M_2\) to the cavity length (99 cm) are not represented, nor the mirrors used to equalize the free air propagation length before both photodiodes. The optical setup fits in a 50 cm × 65 cm breadboard.
2.2 Optical setup
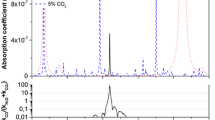
Single OF-CEAS spectrum (140 ms scan) of ambient air at 100 mbar, after baseline substraction. The laser frequency is scanned around the NO resonance at 5.262 µm (1900.5 cm\(^{-1}\)). The HITRAN simulation [42] and the fit residuals are offset for clarity.
The optical setup of an OF-CEAS instrument is simple as schematized in Fig. 1. Here, the laser is a water-cooled QCL (AdTechOptics) that emits about 10 mW at 5.26 µm when it is temperature stabilized around 15 °C by a Peltier connected to a homemade close-loop water chiller. The laser is collimated by a lens mounted inside the sealed HLL package whose window is anti-reflection coated. The emitted light is focused with another lens (\(L_1\)) into the high-finesse optical cavity made of three mirrors of 1 m radius of curvature. At 5.26 µm the ring-down time measured with dry N\(_2\) flowing through the V-cavity is \(\tau_{{\rm RD}}\)=6.6 µs, corresponding to a cavity finesse of 6200 (equivalent absorption path length of 4 km). The phase of the optical feedback beam [31, 34, 43] is finely adjusted by setting the laser to cavity distance equal to the length of one arm of the V-shaped cavity (49.5 cm) with a mirror mounted in an actively controlled piezolelectric transducer (PZT). The OF induces intense and broad-looking resonant modes observed on the cavity-transmitted light when the laser frequency is scanned (Fig. 3). These modes are uniformly spaced by the cavity-free spectral range (FSR = 151 MHz). The optical power transmitted by the cavity is sufficient to be detected with high signal-to-noise ratio with a photodiode (\({\rm PD}_{{\rm signal}}\)) operating at ambient temperature (Vigo SA). A second photodiode (\({\rm PD}_{{\rm ref}}\)) is used to normalize the incident laser power.
The light is focused on the photodiodes to limit backscattering that creates fringes in the absorption spectra [31, 34]. Despite the collimating lens and a tilt of the photodiode, optical fringes are observed arising from scattered light from \({\rm PD}_{{\rm signal}}\) coupled back to the cavity. The induced etalon effect on OF-CEAS spectra was canceled by adjusting the photodiode to cavity output distance to exactly match the cavity length (99 cm). In this way, the etalon effect induces a power modulation at a frequency that matches the FSR [44]: all the cavity modes experience the same effect that adds to the other optical losses continuously monitored with the CRDS normalization. In practice, the photodiode to cavity distance has to be finely adjusted with a translation stage on \({\rm PD}_{{\rm signal}}\). On the other hand, the position of the reference photodiode is adjusted to cancel ambient air absorption by equalizing both air propagation lengths before the photodiodes.
In an OF-CEAS setup, the feedback rate, defined as the ratio of feedback power to the laser output power, has to be adjusted while the polarization of the incident light should match one polarization eigenstate of the cavity modes [31, 34, 45, 46]. Indeed, s- and p-polarization modes are not spectrally degenerate inducing different cavity losses associated with each family of polarization modes. In our previous OF-CEAS setups operating in the NIR, the polarization is fixed with a polarizer placed at the cavity entrance while the feedback rate is adjusted by rotating the distributed feedback diode laser which is linearly polarized (this avoids the use of a half-wave plate). While during recent years polarization optics in the MIR have been improved, there is still a lack of low losses and compact polarizers. Furthermore, the grid polarizers that we tested induced significant etalon fringes that could not be removed. In this context, to maintain the polarization at the input of the cavity, we use another configuration based on the s-polarized reflection at Brewster’s angle (\(\theta _{\rm B}\)) on a ZnSe window. For practical reasons, instead of rotating the water-cooled QCL, the feedback rate is adjusted with a \(\lambda\)/2 plate (Alphalas) placed before the Brewster window (Fig. 1). The attenuation of the reflected beam can then be adjusted from a total extinction when the incident beam is p-polarized, up to a maximum reflectivity, \(R_{{\rm s}}(\theta_{{\rm B}})\), at Brewster’s angle in s-polarization:
where \(\theta\) is the refraction angle fixed by the Snell’s law. The higher the window refractive index (n) the higher is the reflection coefficient obtained. For ZnSe at 5.3 µm (n = 2.43), one obtains \(R_{\rm{s}}(\theta _{\rm B})\)=50.4% at \(\theta _{\rm B}\)=67.6°. As previously reported, a significative difference in the use of a QCL in the MIR for an OF-CEAS instrument as compared to a DFB laser in the NIR [31] or an interband cascade laser (ICL) in the MIR [45] is that the feedback rate required is higher [36, 37]. As a result the \(\lambda\)/2 plate is adjusted to get nearly the maximum reflected power (47%).
Another difference observed with QCL-based OF-CEAS is the change in the optical laser power induced by the optical feedback. As shown in Fig. 3 in the reference photodiode signal, the OF produces here a power increase that changes from 1 to 4% during the laser frequency scan. Different values were reported before with QCL: about 1% in [36] and much larger increase of about 25% in [35]. With ICL in the MIR [45, 47] and DFB in the NIR [31] typical values of 1% are obtained. These observations on the response of a QCL to resonant OF would require further investigation.
In red is the signal from the light transmitted by the high-finesse cavity during one laser frequency scan recorded with ambient air flowing in the cavity at 100 mbar. Absorption lines of NO, CO\(_2\) and H\(_2\)O are observed. Two consecutive modes are separated by the cavity FSR, this is used to calibrate the frequency scale. The OF is adjusted to broaden the transmitted profile of the resonant modes to nearly 1 FSR as shown in the zoom (a). On the last mode, the laser is switched off to record the ring-down event shown in zoom (b). In blue is the signal that monitors the change in the laser power: an increase of a few % is observed in correspondence with OF from cavity modes
2.3 QCL electronics
The QCL is a thermoelectrically cooled continuous wave laser tuned to 5.26 µm by temperature stabilization at 14 °C with a homemade electronic board allowing regulation at the mK level. The laser current threshold is 350 mA, about ten times higher than for DFB lasers in the NIR. This large threshold imposes thermal dissipation ensured by a Peltier element cooled by a close-loop water flow at ambient temperature. The temperature and current tuning coefficients are 0.45 nm/K and 0.07 nm/mA, respectively. Again, these values are significantly higher than in the NIR (typically 0.2 nm/K and 0.02 nm/mA for a DFB Nanoplus laser).
These specifications put very tough constraints on the noise characteristics of the laser current controller requiring significant modification relative to the electronics of our NIR OF-CEAS analyzers. Here, the current is supplied by a low-noise commercial driver (QCL1000 from Wavelength Electronics). Its setpoint is controlled by a homemade electronic board for the generation of the laser current scan as well as the laser interruption for CRDS measurements. The laser current noise is estimated to be 0.5 µA rms noise. This estimation is deduced from the OF-CEAS photodiode signal (Fig. 3) by comparison of the noise on the raising edge of one mode to the noise at the top where the laser frequency is nearly insensitive to the current supplied, being optically locked to the cavity resonance. Indeed, the noise at the top of one mode corresponds to the photodiode noise measured when the laser is off (0.6 mV rms). The noise measurement bandwidth is limited to 25 kHz by the cavity response bandpass (1/2\(\, \pi \,\tau _{\rm RD}\)). It gives a noise power density of 3 nA/\(\sqrt{\text {Hz}}\), slightly higher than the driver specification. At this electronic noise level, OF-CEAS absorption spectra (Fig. 2) are now limited by optical fringes effect.
2.4 Gas handling
Measurements are performed with the gas sample continuously flowing in the internal volume of the V-shaped cavity (Fig. 1) where the pressure is fixed to 100 mbar by a downstream pressure regulator (Bronkhorst). The flux is adjusted to typically 0.1 L/min with a manual valve placed at the inlet of the cavity and measured by a flux meter (Honeywell). The tubes are made of PFA and no memory effect of the gas handling system was observed during NO concentration measurements. The V-cavity is designed to offer a small sample volume with minimal dead space. It is made out of a stainless steel piece where a channel was drilled and shaped by electroerosion to go from a round profile at the input to an oval profile at the two-mirror outputs. As compared to cavities used in the NIR, the internal holes diameter of the V-cavity were increased from 5 to 6 mm, not to induce diffraction losses that would degrade the cavity finesse. Indeed, as the waist of the resonant cavity modes scales as the square root of the wavelength, the beam diameter on the mirrors increases from 1.3 to 2 mm (1/e\(^2\) beam diameter) going from a wavelength of 2.3 to 5.3 µm. The internal volume in the cell is 27 cm\(^3\), which gives a transit time of the gas inside the cavity of 1.6 s in our flux and pressure configuration. It corresponds to the response time of the analyzer, which could be lowered closer to the data acquisition rate (7 Hz) using higher flux and a stronger vacuum pump.
3 Results and discussion
The laser is tuned around the NO absorption line at 5.262 µm sufficiently well isolated from CO\(_2\) and H\(_2\)O lines, these two species being the major interferents in this spectral region. During data acquisition, the laser frequency scan is reduced to a spectral range of 13 GHz, performed at a rate of 7 Hz. A real-time data processing allows to first convert the PD signals into absolute absorption values with CRDS measurements as mentioned earlier (Fig. 2). Second, a non-linear fit [37] enables the selective determination of the concentration of all molecules that possess absorption lines in the spectral window: NO, CO\(_2\) and H\(_2\)O. The baseline corresponds to cavity losses (mirror transmission and losses) as well as absorption by the wings of lines present around the spectral window. It is fitted by a second-order polynomial. Despite special care to reduce etalonning effect, the baseline is affected by two fringes that are stable enough to be fitted with sine and cosine functions at two different fixed periods. The obtained periods allow us to attribute one fringe to a backreflection to the laser chip from the window of the sealed laser package, and the other one to the cavity mirrors thickness. The latest fringe could be removed with wedged mirrors (AR coating is not sufficient).
The line intensities resulting from the fit procedure are then converted to concentration values using a fixed conversion factor for each molecule that is derived from HITRAN 2012 simulation [42] in the temperature and pressure operation conditions. For a better accuracy, conversion factors will be later deduced from OF-CEAS measurements of certified gas mixtures [40]. The conversion factors need to be carefully determined only once and then no calibration is further required as the CRDS measurement performed at the end of each scan allows for absolute absorption measurements. For field applications, this is an important advantage of direct optical absorption-based measurements compared to traditional analytical methods (chromatography, mass spectrometry, chemiluminescence...).
The noise-equivalent absorption level reached in a single spectrum is estimated from the standard deviation of the residuals that is typically \(2\times 10^{-9}\) cm\(^{-1}\), as shown in the lower part of Fig. 2, where the effect of fringes is removed by the fitting procedure. This value obtained in the MIR is at the level of the first results obtained in the NIR [31] that has been improved in a decade by more than one order of magnitude [32] thanks to advances in high-reflectivity coatings.
Top two series of NO measurements with \(\simeq\) 6000 data points. Each point corresponds to a single scan acquired in 140 ms. Blue squares NO strongly diluted in dry nitrogen. Red circles zero NO sample (0.2% of CO\(_2\) in dry N\(_2\)). Bottom the corresponding Allan standard deviations of NO concentration values. The \(\sqrt{t}\) slopes expected from white noise are indicated in dashed lines
Two examples of NO concentration measurements are shown in the upper part of Fig. 4. A first set of measurement (in blue) is obtained for NO diluted to 32.6 ppb in dry nitrogen, while CO\(_2\) and water concentrations were below the LOD for these molecules (<5 ppm for CO\(_2\) and <200 ppm for H\(_2\)O). The second set (in red) corresponds to the analysis of a zero NO gas sample (0.2% of CO\(_2\) in dry nitrogen) to estimate the LOD of the NO analyzer. A small offset of about 40 ppt can be attributed to NO at trace level in the gas sample. To analyze the stability and the detection limit of the instrument, Allan standard deviations [48] are plotted in the lower part of Fig. 4. Measurements on the zero NO sample allows to demonstrate a sensitivity of 60 ppt for a single scan acquired in 140 ms that can be averaged down to 8.3 ppt in 10 s. The averaging time in the zero NO sample seems to be limited by the stability of the fringe pattern while in the other measurement it is limited by the concentration drifts of the gas sample. The LOD obtained in this work is more than one order of magnitude smaller than the best previously reported LOD of laser-based NO spectrometers that operate in the same spectral region with a thermoelectrically cooled laser [26,27,28,29,30]. Furthermore, the fast acquisition rate allows to average faster to a smaller LOD than the best chemiluminescent available instrument (20 ppt in 100 s from Aerodyne instrument) but without the need of calibration and with nearly no interference by other molecules.
These results on the LOD, very promising for field measurements, need to be completed with an accuracy evaluation of the instrument. Preliminary calibration tests have shown a clear signature of saturation of the NO optical transition observed by varying the intra-cavity laser power or the gas sample pressure. Optical saturation is expected to induce a decrease in the measured absorption coefficient at the line center, \(\alpha\), according to [49] :
where \(\alpha _0\) is the absorption expected without saturation effect and \(s=I/I_{\rm sat}\) is the saturation parameter defined as the ratio of the intra-cavity laser intensity, I, to the saturation intensity, \(I_{\rm sat}\). We have estimated [50] \(I_{\rm sat}=12\,\) W/cm\(^2\) (at 100 mbar) from Einstein coefficient and degeneracy factor of the excited state found in HITRAN database, and considering a transit time of the molecules limited by collisions. With a laser intensity of \(I\simeq\) 4 W/cm\(^2\), this gives a saturation parameter of s = 0.33 that corresponds to an expected underestimation of the absorption by 15% (Eq. 2). In practice, the saturation effect seems to induce a stronger decrease of 40% (that would correspond to a s value five times higher). This disagreement remains after having carefully tested the calibration and the linearity of the photodiodes measured the transmission of the cavity mirrors and confirmed that sample concentration is not affected by the tubing used for sample handling (PFA). A detailed study to model and calibrate properly this effect is in progress. Nevertheless, it is already clear that it can be minimized by reducing the intra-cavity laser power and/or by increasing the pressure of the gas sample.
4 Conclusion and perspectives
The OF-CEAS technique has been used to measure for the first time NO trace concentration that requires laser spectroscopy in the MIR. The instrument is based on a room-temperature QCL. The rms noise-equivalent absorption is 2\(\times 10^{-9}\) cm\(^{-1}\) for one acquired spectrum (140 ms) with a cavity of finesse 6200. A real-time fit enables the conversion of absolute absorption spectra to NO concentration values, using a normalization coefficient based on HITRAN-simulated spectra in the 1900.5 cm\(^{-1}\) spectral range. Allan variance analysis allows to demonstrate a LOD of 8.3 ppt over 10 s averaging time. This optimum time averaging of 10 s is limited by drift of parasitic fringes whose amplitude stays basically constant with time. Phase variations of the fringes only induce a bias on the fitted intensity of an absorption line which is bounded. Therefore, the Allan variance is not expected to increase arbitrarily at long times. The dynamic range of the OF-CEAS instrument [34] would allow to monitor concentrations with a linear response and a constant signal-to-noise level up to about 300 ppb.
The 8.3 ppt LOD is today the best value reported for NO detection in the literature by laser spectroscopy [26,27,28,29,30]. It is very close to the sensitivity reached by research-grade chemiluminescence instruments (\(\simeq\)5 ppt over 30 s [17, 18]). Compared to chemiluminescence, OF-CEAS directly and selectively measures a NO absorption line with high spectral resolution, allowing to handle interferences by neighboring CO\(_2\) and H\(_2\)O lines, the only atmospheric interfering species in this spectral region. The achieved sensitivity is well adapted to monitor NO in most demanding applications. For air monitoring, to strongly decrease interferences by CO\(_2\), the laser emission will be tuned to the NO doublet absorption lines lying close-by, at 1900.1 cm\(^{-1}\). This spectral range is reached by decreasing the laser temperature. Here, the closest CO\(_2\) line is two orders of magnitude weaker than the strong CO\(_2\) line in the 1900.5 cm\(^{-1}\) spectral region used here (Fig. 2), while the NO doublet lines are slightly more intense (50%). For NO atmospheric monitoring, where CO\(_2\) concentrations remain below 0.1%, HITRAN-based simulations show that CO\(_2\) lines remain spectrally well separated from the NO doublet lines, and should not affect the achieved sensitivity allowing NO monitoring at the 10 ppt level. In breath analysis, direct CO\(_2\) measurements will allow to normalize NO measurements [51]. But as CO\(_2\) is rising up to several %, NO and CO\(_2\) lines will not remain spectrally separated at sub-ppb NO concentrations, even in the 1900.1 cm\(^{-1}\) spectral region. Sub-ppb level remains at the smallest NO concentration reported in the literature for isolated ex vivo lung [12]. Nonetheless, if further sensitivity is required, lowering the pressure sample cell will allow for narrowing of spectral lines towards the Doppler regime, thus leading to a better separation of CO\(_2\) and NO lines (with a compromise of an acceptable reduction in sensitivity related to the line intensities decrease).
The accuracy of the instrument is currently limited by saturation effects found to be more important than expected. It can be minimized by increasing the pressure and decreasing the laser power. An in-depth study is now in progress. Furthermore, interband cascade lasers have recently become commercially available at the wavelength range of interest. ICLs have already proven to be effective for use with OF-CEAS [45, 46]. These lasers would allow to reduce saturation effects thanks to their 10 times lower emission power compared to QCLs [45]. Moreover, ICLs have specifications very close to those of NIR DFB lasers and are better adapted for field instruments (no need of water cooling).
References
I.B. Pollack, T.B. Ryerson, M. Trainer, J.A. Neuman, J.M. Roberts, D.D. Parrish, J. Geophys. Res. Atmos. 118, 5893–5911 (2013)
D. Fowler, J.A. Pyle, J.A. Raven, M.A. Sutton, Philos. Trans. R. Soc. B 368, 20130120–20130120 (2013)
M. Amann, Z. Klimont, F. Wagner, Annu. Rev. Environ. Resour. 38, 31–55 (2013)
S.W. Kim, B.C. McDonald, S. Baidar, S.S. Brown, B. Dube, R.A. Ferrare, G.J. Frost, R.A. Harley, J.S. Holloway, H.J. Lee, S.A. McKeen, J.A. Neuman, J.B. Nowak, H. Oetjen, I. Ortega, I.B. Pollack, J.M. Roberts, T.B. Ryerson, A.J. Scarino, C.J. Senff, R. Thalman, M. Trainer, R. Volkamer, N. Wagner, R.A. Washenfelder, E. Waxman, C.J. Young, J. Geophys. Res. 121, 1340–1360 (2016)
A. Bigi, R.M. Harrison, Atmos. Environ. 44, 2004–2012 (2010)
S.-H. Wang, C.-Y. Shen, H.-M. Huang, Y.-C. Shih, Sens. Actuators A 216, 237–242 (2014)
H.K. Gatty, S. Leijonmarck, M. Antelius, G. Stemme, N. Roxhed, Sens. Actuators B 209, 639–644 (2015)
D.E. Koshland. Science 258, 1861 (1992)
P.R. Boshier, G.B. Hanna, N. Marczin, J. Breath Res. 7, 017118 (2013)
A. Schneider, L. Tilemann, T. Schermer, L. Gindner, G. Laux, J. Szecsenyi, F.J. Meyer, Respir. Res. 10, 15 (2009)
M. Gashouta, C. Merlo, M. Pipeling, J. McDyer, J. Hayanga, J. Orens, R.E. Girgis, J. Heart Lung Transplant. 34, 557–562 (2015)
N. Marczin, B. Riedel, J. Gal, J. Polak, M. Yacoub, The Lancet 350, 1681–1682 (1997)
E.J. Dunlea, S.C. Herndon, D.D. Nelson, R.M. Volkamer, F. San Martini, P.M. Sheehy, M.S. Zahniser, J.H. Shorter, J.C. Wormhoudt, B.K. Lamb, E.J. Allwine, J.S. Gaffney, N.A. Marley, M. Grutter, C. Marquez, S. Blanco, B. Cardenas, A. Retama, C.R. Ramos Villegas, C.E. Kolb, L.T. Molina, M.J. Molina. Atmos. Chem. Phys. 7, 2691–2704 (2007)
I. Ventrillard-Courtillot, T. Gonthiez, C. Clerici, D. Romanini, J. Biomed. Opt. 14, 064026 (2009)
F. Dominé, P.B. Shepson, Science 297, 1506–1510 (2002)
R.E. Honrath, Y. Lu, M.C. Peterson, J.E. Dibb, M.A. Arsenault, N.J. Cullen, K. Steffen, Atmos. Environ. 36, 2629–2640 (2002)
D. Davis, J.B. Nowak, G. Chen, M. Buhr, R. Arimoto, A. Hogan, F. Eisele, L. Mauldin, D. Tanner, R. Shetter, B. Lefer, P. McMurry, Geophys. Res. Lett. 28, 3625–3628 (2001)
S. Oncley, M. Buhr, D. Lenschow, D. Davis, S. Semmer, Atmos. Environ. 38, 5389–5398 (2004)
R. Grilli, M. Legrand, A. Kukui, G. Méjean, S. Preunkert, D. Romanini, Geophys. Res. Lett. 40, 791–796 (2013)
H. Fuchs, W.P. Dubé, B.M. Lerner, N.L. Wagner, E.J. Williams, S.S. Brown, Environ. Sci. Technol. 43, 7831–7836 (2009)
N.L. Wagner, W.P. Dube, R.A. Washenfelder, C.J. Young, I.B. Pollack, T.B. Ryerson, S.S. Brown, Atmos. Meas. Tech. 4, 1227–1240 (2011)
C. Gmachl, F. Capasso, D.L. Sivco, A.Y. Cho, Rep. Prog. Phys. 64, 1533–1601 (2001)
A. Kosterev, G. Wysocki, Y. Bakhirkin, S. So, R. Lewicki, M. Fraser, F. Tittel, R. Curl, Appl. Phys. B 90, 165–176 (2008)
L. Dong, V. Spagnolo, R. Lewicki, F.K. Tittel, Opt. Express 19, 24037 (2011)
R. Lewicki, J.H. Doty, R.F. Curl, F.K. Tittel, G. Wysocki, Proc. Natl. Acad. Sci. USA 106, 12587–12592 (2009)
D. Marchenko, J. Mandon, S.M. Cristescu, P.J.F.M. Merkus, F.J.M. Harren, Appl. Phys. B 111, 359–365 (2013)
J. Mandon, M. Hogman, P.J.F.M. Merkus, J. van Amsterdam, F.J.M. Harren, S.M. Cristescu, J. Biomed. Opt. 17, 017003 (2012)
M. Köhring, S. Huang, M. Jahjah, W. Jiang, W. Ren, U. Willer, C. Caneba, L. Yang, D. Nagrath, W. Schade, F.K. Tittel, Appl. Phys. B 117, 445–451 (2014)
M.L. Silva, D.M. Sonnenfroh, D.I. Rosen, M.G. Allen, A. O'Keefe, Appl. Phys. B 81, 705–710 (2005)
B. Tuzson, K. Zeyer, M. Steinbacher, J.B. McManus, D.D. Nelson, M.S. Zahniser, L. Emmenegger, Atmos. Meas. Tech. 6, 927–936 (2013)
J. Morville, S. Kassi, M. Chenevier, D. Romanini, Appl. Phys. B 80, 1027–1038 (2005)
J. Landsberg, D. Romanini, E. Kerstel, Opt. Lett. 39, 1795–1798 (2014)
E. Kerstel, R. Iannone, M. Chenevier, S. Kassi, H.-J. Jost, D. Romanini, Appl. Phys. B 85, 397–406 (2006)
J. Morville, D. Romanini, E. Kerstel. in Cavity-Enhanced Spectroscopy and Sensing, eds. by G. Gagliardi, H.-P. Loock (Springer, Berlin), pp. 163–207
G. Maisons, P. Gorrotxategi-Carbajo, M. Carras, D. Romanini, Opt. Lett. 35, 3607–3609 (2010)
D.J. Hamilton, A.J. Orr-Ewing, Appl. Phys. B 102, 879–890 (2011)
P. Gorrotxategi-Carbajo, E. Fasci, I. Ventrillard, M. Carras, G. Maisons, D. Romanini, Appl. Phys. B 110, 309–314 (2013)
S. Kassi, M. Chenevier, L. Gianfrani, A. Salhi, Y. Rouillard, A. Ouvrard, D. Romanini, Opt. Express 14, 11442–11452 (2006)
D. Romanini, M. Chenevier, S. Kassi, M. Schmidt, C. Valant, M. Ramonet, J. Lopez, H.-J. Jost, Appl. Phys. B 83, 659–667 (2006)
I. Ventrillard, I. Xueref-Remy, M. Schmidt, C.Y. Kwok, X. Faïn, D. Romanini, Atmos. Meas. Tech. 20, 1803–1812 (2017)
R.Q. Iannone, D. Romanini, S. Kassi, H.A. Meijer, E.R.T. Kerstel, J. Atmos. Ocean. Technol. 26, 1275–1288 (2009)
L. Rothman, I. Gordon, Y. Babikov, A. Barbe, D.C. Benner, P. Bernath, M. Birk, L. Bizzocchi, V. Boudon, L. Brown, A. Campargue, K. Chance, E. Cohen, L. Coudert, V. Devi, B. Drouin, A. Fayt, J.-M. Flaud, R. Gamache, J. Harrison, J.-M. Hartmann, C. Hill, J. Hodges, D. Jacquemart, A. Jolly, J. Lamouroux, R. Le Roy, G. Li, D. Long, O. Lyulin, C. Mackie, S. Massie, S. Mikhailenko, H. Müller, O. Naumenko, A. Nikitin, J. Orphal, V. Perevalov, A. Perrin, E. Polovtseva, C. Richard, M. Smith, E. Starikova, K. Sung, S. Tashkun, J. Tennyson, G. Toon, V. Tyuterev, G. Wagner. J. Quant. Spectros. Radiat. Transf. 130, 4–50 (2013)
D.J. Hamilton, M.G.D. Nix, S.G. Baran, G. Hancock, A.J. Orr-Ewing, Appl. Phys. B 100, 233–242 (2009)
T.J.A. Butler, D. Mellon, J. Kim, J. Litman, A.J. Orr-Ewing, J. Phys. Chem. A 113, 3963–3972 (2009)
L. Richard, I. Ventrillard, G. Chau, K. Jaulin, E. Kerstel, D. Romanini, Appl. Phys. B 2, 247 (2016)
K.M. Manfred, K.M. Hunter, L. Ciaffoni, G.A.D. Ritchie, Anal. Chem. 89, 902–909 (2016)
K.M. Manfred, G.A.D. Ritchie, N. Lang, J. Röpcke, J.H. van Helden, Appl. Phys. Lett. 106, 221106 (2015)
P. Werle, Appl. Phys. B 102, 13–329 (2011)
G. Giusfredi, S. Bartalini, S. Borri, P. Cancio, I. Galli, D. Mazzotti, P. De Natale, Phys. Rev. Lett. 104, 110801 (2010)
M.S. Taubman, B.D. Cannon, T.L. Myers, C.A. Bonebrake, P.M. Aker, J.F. Schultz. Report from pacific northwest national laboratory, 14484 (2003)
T.H. Risby, J. Breath Res. 2, 030302 (2008)
Acknowledgements
We thank Jean-Luc Martin and Thibault Desbois for their technical support. This work was partly supported by a Grant from the french LEFE-CHAT (CHimie ATmosphrique) program of Institut National des Sciences de l’Univers and the LabexOSUG@2020 program (ANR10 LABX56).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the topical collection “Field Laser Applications in Industry and Research” guest edited by Francesco D’Amato, Erik Kerstel, and Alan Fried.
Rights and permissions
About this article
Cite this article
Ventrillard, I., Gorrotxategi-Carbajo, P. & Romanini, D. Part per trillion nitric oxide measurement by optical feedback cavity-enhanced absorption spectroscopy in the mid-infrared. Appl. Phys. B 123, 180 (2017). https://doi.org/10.1007/s00340-017-6750-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-017-6750-7