Abstract
Laser-induced fluorescence of anisole as tracer of isooctane at an excitation wavelength of 266 nm was investigated for conditions relevant to rapid compression machine studies and for more general application of internal combustion engines regarding temperature, pressure, and ambient gas composition. An optically accessible high pressure and high temperature chamber was operated by using different ambient gases (Ar, N2, CO2, air, and gas mixtures). Fluorescence experiments were investigated at a large range of pressure and temperature (0.2–4 MPa and 473–823 K). Anisole fluorescence quantum yield decreases strongly with temperature for every considered ambient gas, due to efficient radiative mechanisms of intersystem crossing. Concerning the pressure effect, the fluorescence signal decreases with increasing pressure, because increasing the collisional rate leads to more important non-radiative collisional relaxation. The quenching effect is strongly efficient in oxygen, with a fluorescence evolution described by Stern–Volmer relation. The dependence of anisole fluorescence versus thermodynamic parameters suggests the use of this tracer for temperature imaging in specific conditions detailed in this paper. The calibration procedure for temperature measurements is established for the single-excitation wavelength and two-color detection technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A precise knowledge of thermodynamic conditions such as the concentration and the temperature is essential to understand the combustion processes in a rapid compression machine and future application of internal combustion engine (ICE) in the context of homogeneous charge compression ignition combustion mode (HCCI). The initial distribution of temperature inhomogeneities strongly influences the combustion process due to the interaction between heat transfer and internal aerodynamics of the combustion chamber [1].
A widely used technique to better understand the influence of thermodynamic parameters on the combustion process is the laser-induced fluorescence of a tracer added to a nonfluorescing base fuel (e.g., isooctane). In particular, for the investigation of temperature inhomogeneities in a combustion chamber, a well-characterized fluorescence behavior regarding the influence of temperature, pressure, gas composition, and excitation wavelength separately is necessary for the quantitative interpretation of laser-induced fluorescence signals.
For example, aromatics present naturally in gasoline and diesel fuels are currently used in fluorescence measurements: benzene, toluene [2], naphthalene and its derivatives [3], and fluoranthene [4].
As the lower energetic transition for aromatics is π → π* (allowed transition), the absorption cross section is higher than ketones, usual tracers in fluid mechanics experiments, which have typical transition n → π*. The fluorescence quantum yield Φ is so higher for aromatic compounds than ketones: benzene (Φ = 0.04−0.06), toluene (Φ = 0.15), naphtalene (Φ = 0.19−0.21), anthracene (Φ = 0.27−0.30), pyrene (Φ = 0.65), phenanthrene (Φ = 0.13−0.16), fluoranthene (Φ = 0.19−0.22) [3], and acetone (Φ = 0.002).
Toluene is an aromatic tracer used commonly in experimental studies of fluorescence in fluid mechanics and combustion [5–7]. For example, Koban et al. [8] have studied the S 0 → S 1 (π,π*) absorption and fluorescence spectra of toluene in the temperature range of 300–1,130 and 300–930 K, respectively, at atmospheric pressure in nitrogen and for the excitation wavelengths of 248 and 266 nm.
Orain et al. [9] have studied the fluorescence of the S 1 → S 0 transition of naphthalene vapor after excitation at 266 nm in a heated cell. Experiments were carried out for temperatures in the range 350–900 K and for pressures between 0.1 and 3 MPa and for oxygen mole fraction from 0 to 21 %. Under nitrogen atmosphere, naphthalene fluorescence decreases bi-exponentially by an order of magnitude when increasing temperature, whereas it increases by about 10 % with pressure.
Grossmann et al. [10] have investigated the fluorescence signal from the S 1 state of acetone as a function of temperature and pressure using the excitation wavelength of 248 nm. Ambient gases were synthetic air, oxygen, and nitrogen in the pressure range of 0–5 MPa. A strong decrease in the fluorescence signal was observed with temperature increasing in synthetic air in the temperature range of 383–640 K at a constant pressure of 0.1 MPa. Grossmann et al. [10] have shown that acetone can be used as a tracer for a nonintrusive thermometry method, during the compression phase in IC engines.
In this work, anisole is selected as tracer for its satisfactory spectroscopy properties [11], and its thermodynamic properties are close to those of isooctane (C8H18), the traced fuel (cf. Table 1). Anisole is a methyl oxide-ether of phenol C7H8O, which is under liquid form at ambient temperature. It is an extremely volatile compound, its saturate vapor pressure is 1,360 Pa at 293 K, and its boiling point is equal to 428 K [12] that enables a good vaporization in our experimental conditions. To our knowledge, anisole was used as tracer in a flame [13], but not for a spectroscopic analysis in a large range of pressure and temperature. Pasquier et al. [13] investigated the propagation of a propane/air flame through a spatially stratified mixture and turbulent flow. Simultaneous particle image velocimetry (PIV) and laser-induced fluorescence (LIF) measurements on anisole were performed to study flame propagation in order to provide instantaneous velocity and equivalence ratio fields in the region ahead of the flame front. Quantitative equivalence ratio measurements were obtained from anisole fluorescence by a method based on oxygen quenching of the excited state (FARLIF, fuel air ratio LIF).
The aim of the present work is to study anisole as a fluorescence tracer in LIF thermometry for IC engines application. For 266 nm excitation wavelength, the dependence of the anisole fluorescence signal with parameters such as pressure, temperature, and gas composition is characterized from experiments performed in a high pressure and high temperature (HP–HT) constant volume vessel. The parameters influencing the anisole fluorescence are studied to define the most appropriate LIF configuration technique for temperature measurements depending on experimental conditions constraints.
2 Experimental setup
Fluorescence of anisole blend with isooctane in vapor phase was investigated in an optical accessible HP–HT chamber in temperature and pressure ranges, respectively, of 473–823 K and 0.2–4 MPa at the excitation wavelength of 266 nm.
The setup used for experiments is represented in Fig. 1 and described in details in Ref [4]. The HP–HT chamber is a cylinder (height H = 200 mm, external diameter Øext = 170 mm, internal diameter Øint = 70 mm, inner volume V in = 7.6969 × 10−4 m3), heated and maintained at constant temperature by eight electrical resistances of 230 W. A system composed of three thermocouples (precision of ±2.3 K) located inside the cell enables to regulate it and ensures homogeneity of the cell temperature flowfield. The maximum operating pressure of the HP–HT chamber is 5 MPa and 873 K. The liquid fuel/tracer mixture is injected via a syringe of a 1 mL volume (precision of ±1 %) and a capillary tube into the chamber and then vaporized by reaching the tracer vapor pressure according to the temperature through a vacuum pump (anisole vapor pressure of 1,360 Pa at 293 K). The experimental chamber is pressurized with different bath gases (Ar, N2, CO2, air, and gas mixtures) via the admission circuit composed of pressure sensors (precision of ±1 %) and valves.
The optical system is composed of a frequency-quadrupled and pulsed Nd:Yag laser (Spectra Physics Quanta-Ray, 266 nm, 90 mJ/pulse at 10 Hz) for the fluorescence measurements and of a deuterium lamp (model D2000, Ocean optics, large emission spectrum from 215 to 400 nm, total light power is 25 W in U.V) coupled to an optical fiber (Ocean Optics, 600 μm diameter, 25 cm length, and a transmission coefficient of more 85 % from 200 to 1,000 nm) for the absorption spectrum measurements. The laser beam is not focused and measures 10-mm diameter with a pulse duration of 9 ns. The laser pulse width fluctuation is 3–4 ns with an energy stability of ±8 %. The laser beam passes through the cell, and it is recovered by a beam trap and a flux-meter (Ophir Optronics AN/2) that enables to know the temporal laser fluctuations of energy and the laser intensity. The fluorescence signal is collected at 90° from the incident beam by a high sensitivity spectrometer (Acton SpectraPro 2500i, 500 mm of focal length, entrance slit fixed at 500 μm, diffracting grating of 150 grooves per mm used with a resolution of 350 nm), supplied with an ICCD camera (Pimax 1300, 1,024 × 256 pixels2, 16 bytes of dynamic range, 1 kHz of framing rate, intensified by Pimax 1024-25-GenII, an exposition time of 100 ns) and an UV lens (Pentax, B7838-UV, 78 mm of focal length, a aperture ratio of 3.8, and a spectrum resolution from 230 to 800 nm). The filters are placed in front of measurement devices for the calibration of the temperature measurement by LIF.
The laser intensity is set at 90 mJ/pulse at 10 Hz in order to avoid the saturation level and to stay in the fluorescence linearity regime. The fluorescence tracer concentration is set at 360 μl of 1 % anisole in isooctane solution, which represents 2.58 × 1016 molecule.cm−3 of anisole. This allows to avoiding reabsorption and self-quenching phenomena.
For a given experimental condition, the fluorescence signals are averaged from 900 emission spectra. Every experiment is reproduced three times. The normal standard deviations are between 1.1 and 3.7 % depending on temperature and pressure for the different bath gases.
In a global and macroscopic balance formulation, the fluorescence signal depends, for a weak laser excitation, on thermodynamics parameters according to the following equation [1]:
with E the laser fluence (J m−2), (hc/λ) the energy of a photon (J) at the excitation wavelength λ (nm), η opt the overall efficiency of the collection optics, dV c the collection volume (cm3), σ the molecular absorption cross section of the tracer (cm2), Φ the fluorescence quantum yield, X the mole fraction, P the total pressure (Pa), k the Boltzmann constant (J K−1), and T the temperature (K).
Estimation of the measurement incertitudes was calculated from the incertitude contribution of each terms of the fluorescence macroscopic equation:
The absorption cross section, the mole fraction, and the pressure represent the most important contribution to the incertitude with \(6\% \le \frac{\Updelta \sigma }{\sigma } \le 8\%\), \(2.1\% \le \frac{\Updelta X}{X} \le 2.2\%\), \(5\% \le \frac{\Updelta P}{P} \le 10\%\).
Finally, measurement incertitude is as follows:
3 Results and discussion
3.1 Selection of the tracer
The selection of the fluorescent molecule is an essential step in the presented methodology. The technique used in this work is based on the dependence of the fluorescence signal upon thermodynamic parameters (pressure, temperature, ambient gas composition). Some spectroscopic and physical criteria are proposed in order to choose the most adapted fluorescence tracer for the temperature measurement in vapor phase confined flows.
First, the fluorescence spectral emission compared to the excitation wavelength must be large enough to be able to single out the fluorescence signal from Rayleigh elastic scattering and wall reflections (stray light). Additionally, in order to collect a satisfactory amount of fluorescence emission, the fluorescence quantum yield must be high, with a significant dependence upon temperature. Furthermore, the tracer must have an appropriate absorption cross section in tested ambient gases to neglect quenching effect but leads to comfortable fluorescence signals. Finally, physical aspects are considered for the selection of the tracer. Tracer must take into the miscibility with the used fuel (isooctane), the stability with temperature, and the vapor phase condensation properties at high pressure.
Two molecules of PAHs family, which have a higher fluorescence quantum yield than ketones (e.g., acetone), were selected for their spectroscopic properties: anisole (C7H8O) and fluoranthene (C16H12).
Figure 2 represents the absorption cross section of studied molecules in nitrogen for a pressure of 0.1 MPa and a temperature of 573 K. Fluoranthene presents a higher absorption cross section at 266 nm (σ = 9.353 × 10−19 cm2 molecule−1) than anisole (σ = 7.013 × 10−18 cm2 molecule−1). Both molecules can be excited easily at 266 nm.
Figure 3 compares the fluorescence spectra for the studied molecules excited at 266 nm. The maximum fluorescence intensity is larger for anisole (I max = 6.3 × 104 au) when compared to fluoranthene (I max = 4.25 × 104 au), whereas the spectral integration of fluoranthene signal is stronger than anisole. The fluorescence emission of both molecules is shifted enough from the Rayleigh elastic scattering at 266 nm. In the point of view of spectroscopy results, fluoranthene has better characteristics than anisole. But fluoranthene is initially in solid phase, and its manipulation even dissolved in isooctane is not obvious as at high pressure; the fluoranthene vapor phase could recondensate in solid phase. For the latter reason, fluoranthene was not selected as tracer to perform temperature measurement.
From this preliminary study, the molecule of anisole has been retained due to its satisfactory photo-physical properties. Moreover, anisole is stable, even at high temperature. Neither pyrolysis nor photophysical decomposition was observed from measurements over long time lapse for the studied temperature range.
3.2 Anisole absorption and fluorescence
The absorption cross section is taken into account to calculate the fluorescence signal which varies with the temperature and the excitation wavelength. At a constant excitation wavelength, the absorption cross section σ depends only on the temperature. It is deduced from Beer’s-Lambert law:
with I 0 the incident intensity (J), I the transmitted intensity (J), l the path length (cm), and C the anisole concentration (molecule cm−3).
Figure 4 presents the absorption cross section of anisole which has three peaks located around at 266, 272, and 278 nm. This absorption spectrum is similar to the one of Robertson et al. [14]. With increasing temperature, the absorption spectrum becomes a broadband continuum at high temperature from 773 K.
Koban et al. [15] also observed with toluene, a losing of fines structures of absorption spectrum for temperature higher than 600 K. They explained this phenomenon by the thermal population of vibrationally excited ground state levels, and absorption on the red side of the (0,0)-band becomes energetically possible and broadens the absorption features. Whereas in anisole case, the absorption cross section decreases with increasing temperature for different ambient gases (Ar, CO2, N2) as presented in Fig. 5. As anisole is a mono-substitution of benzene, the absorption cross section of benzene can be compared. Fally et al. [16] measured the evolution of benzene absorption cross section over a temperature range from 253 to 293 K for different wavelengths. The absorption cross section at 266 nm decreases with increasing temperature. However, it is difficult to explain the temperature effect on such absorption broadbands which are compounded of numerous individual rovibrational transition lines. Each ones have a particular dependence with temperature as described by Boltzmann’s law. The maximum absorption peak is also shifted with temperature from 270 to 273 nm over the studied temperature range.
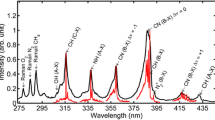
The anisole fluorescence spectrum in isooctane is between 250 and 600 nm, and the peak is centered at 296 nm, as presented in Fig. 6 and underlined in Ref. [14]. In Fig. 6, each spectrum is normalized by the maximum value of the fluorescence spectrum recorded at 473 K for a pressure of 1.2 MPa. The ambient gas is CO2. Fluorescence intensity strongly decreases by a factor of 50 when the temperature increases from 473 to 823 K, due to a high non-radiative decay rate of excited state at high temperatures. This observation underlines the interest of anisole as tracer for the measurement of temperature by LIF.
However, for such a measurement, it is also necessary to check the influence of the temperature and pressure on the redshift of the fluorescence spectrum. As shown in Fig. 7, a redshift of 2.5 nm per 100 K can be observed around the area centered at the fluorescence peak. Each spectrum is normalized by the maximum value of the fluorescence at 1.2 MPa in CO2. This redshift is due to the excitation of additional vibrational and rotational levels with higher energy as the temperature increases. Same trends have been noted by Orain et al. [17] with 1,2,4- trimethylbenzene for a pressure of 0.1 MPa, a temperature range from 350 to 900 K, and an excitation wavelength of 266 nm. They have observed a redshift of 2 nm per 100 K and a redshift of 5 nm per 100 K for naphthalene [9]. Likewise, Koban et al. [15] have noted with increasing temperature a slight shift of toluene fluorescence peak of 2 nm per 100 K. Kaiser et al. [3] have found a spectral shift of methyl-naphthalene of 16.4 and 21.1 nm per 100 K in air and in nitrogen, respectively.
3.3 Pressure dependence of anisole fluorescence
To study the pressure influence on the fluorescence quantum yield Φ, Eq. (1) is normalized to the molecular density, and thus, the obtained fluorescence signal is noted S f*. For given excitation wavelength and temperature, the fluorescence signal S f* is directly proportional to the fluorescence quantum yield as shown below:
Fluorescence signal decreases continuously with increasing pressure from 0.1 to 4 MPa by a factor of 2.8 as shown in Fig. 8 for different temperatures in carbon dioxide. This is due to the collisional rate increase leading to a more important collisional non-radiative relaxation. As shown by Matsumoto et al. [18], this relaxation is important for anisole molecule and is mainly due to the inter-system crossing ISC, the energetic gap from S 1 to T 1 (8,300 cm−1) and from S 2 to T 2 (about 1,000 cm−1) is very weak. By contrast with ketones and diones, another evolution is observed: The fluorescence signal increases with the pressure, the vibrational relaxation mechanisms are favored, and the low energy levels with high fluorescence quantum yield quickly reached.
This decrease in fluorescence intensity with pressure is also observed in the case of 1,2,4-trimethylbenzene excited at 266 nm at 450 K in nitrogen by Orain et al. [17]. They have noticed a decrease in intensity with a factor larger than two for a pressure increase from 0.1 to 3 MPa.
Moreover, the redshift has not been observed when the pressure varies. Consequently, the pressure has no influence on the population density in the excited energy state S 1, which may suggest a fast energy transfer between the vibrational states.
3.4 Temperature dependence of anisole fluorescence
The study of the temperature influence on the anisole fluorescence quantum yield is performed at 266 nm for the pressure range from 0.2 to 4 MPa.
The direct determination of the fluorescence quantum yield evolution with temperature is obtained by normalizing the signal S f by molecular density and the absorption cross section, the obtained fluorescence signal is noted S f** as given below:
Figure 9 presents the influence of temperature on anisole fluorescence quantum yield excited at 266 nm, in carbon dioxide for different pressures. An important decrease in S **f is observed with increasing of temperature for all pressure range.
The influence of temperature on the evolution of fluorescence quantum yield is explained by the competition between the radiative relaxation mechanisms (fluorescence, phosphorescence) and the non-radiative relaxation mechanisms (inter-system crossing, internal conversion). In the case of anisole, the increase in the temperature may lead to the increase in inter-system crossing relaxation rate from excited state S 1 to triplet states T 1 which are less energetic. In fact, energetic gaps are very low between these states. This process of energy transfer by ISC may be possible at high temperature, because the anisole molecule reaches to electronic level whose vibrational energy level are higher than states T1 (S 1, υ > 0). The relaxation by inter-system crossing increases also with the temperature due to the thermal activation of the ISC. Consequently, non-radiative relaxation rate becomes faster, with increasing temperature. Orain et al. [17] have observed a similar decrease in 1,2,4- trimethylbenzene fluorescence signal with temperature between 350 and 750 K.
3.5 Influence of ambient gases and quenching by oxygen on anisole fluorescence
The influence of thermodynamic parameters is studied for different ambient gases (nitrogen, argon, carbon dioxide, and oxygen) in order to characterize eventual effects of quenching on the fluorescence quantum yield of anisole.
The decrease of S f** with temperature for different pressures is similar in nitrogen, argon, and carbon dioxide (cf. Fig. 10). There is no apparent influence of the nature of ambient gas on the evolution of anisole fluorescence quantum yield over the temperature range 473–823 K for the considered pressures.
The quenching effect on the fluorescence signal has been studied for oxygen mole fraction between 0 and 20 % in nitrogen at different temperatures. The influence of the oxygen mole fraction on anisole fluorescence spectra normalized to the maximum value obtained in pure nitrogen (0 % oxygen) is represented in Fig. 11 for a temperature of 573 K and a pressure of 2 MPa.
With the increase of oxygen percent in nitrogen pressure, the fluorescence spectrum intensity decreases. In fact, oxygen is a paramagnetic molecule in which the fundamental state T 0 is a triplet state, favoring inter-system crossing mechanisms and thus decreasing fluorescence signal. The efficiency of the quenching by O2 on PAHs’ fluorescence emission is predominant in de-excitation mechanisms. Redshift has not been observed with oxygen percent increase in the present experiments. Koban et al. [15] also have not observed any redshift on the toluene fluorescence spectrum (266 nm excitation) with the increase in the oxygen mole fraction, as opposed with an excitation wavelength of 248 nm, where a redshift of 8 nm has been observed. Orain et al. [17] have found a redshift of only 1 nm for 1,2,4- trimethylbenzene spectrum excited at 266 nm at 450 K and 0.1 MPa with the increase in oxygen mole fraction from 0 to 21 %. With naphthalene, Kaiser et al. [3] have observed a redshift of about 4 nm with the increase in oxygen mole fraction by using an excitation wavelength of 266 nm. Due to the quenching of fluorescence by oxygen, the signal intensity is divided by 12.5 in the explored domain. However, due to the short lifetime of anisole excited electronic state S 1 (~20 ns) [18], the relaxation rate from collisions with oxygen molecules remains low. The quenching for anisole is relatively limited compared to some molecules which have a longer excited state lifetime, such as PAH molecules with two benzene rings.
As observed in Fig. 12, the quenching behavior is described closely by Stern–Volmer relation, with a linear evolution of the fluorescence intensity versus the oxygen percentage in nitrogen:
where S 0 and S are the fluorescence intensities in the absence, and in the presence of oxygen, respectively, kSV represents the Stern–Volmer constant and is the product of the quenching rate constant and the fluorescence lifetime; [O2] is the concentration of the quencher molecule.
The kSV is equal to 2.8, 0.53, and 0.38 s−1, respectively, for 473, 523, and 573 K.
3.6 Single-excitation two-color detection technique for the temperature measurement
Three techniques for temperature measurement by LIF exist and are based on Eq. (1): the single-excitation, single-color detection technique for a homogeneous distribution of tracer in the studied volume, the single-excitation, two-color detection for an inhomogeneous distribution of tracer, and the dual-excitation wavelength technique for an inhomogeneous distribution of the tracer partial pressure and concentration. The selection of one of them is dictated by thermodynamic quantities to study, the selected tracer, thus the experimental conditions.
From the previous results, in the case of the one excitation wavelength technique and one integration band, the temperature can be deduced from the following expression, obtained for anisole excited at 266 nm between 473 and 823 K at 0.2 MPa in nitrogen:
The temperature is then obtained from the fluorescence quantum yield:
This experimental correlation is compared with those of the literature in Table 2. These calibration fits could be used for the temperature measurement from the single-color detection technique, valid in homogeneously seeded systems. Similar correlations of temperature versus fluorescence quantum yield are observed. However, anisole presents an important sensitivity with temperature compared to other fluorescence molecules.
The single-excitation, two-color detection thermometry is usually used in combustion field. For example, Luong et al. [19, 20] applied this technique with the excitation wavelength of 248 nm and toluene as tracer in air over a temperature range of 300–675 K to measure in-cylinder temperature distribution in shock tube and heated flow-cell. Kaiser et al. [3] have also used this technique with the excitation wavelength of 266 nm and naphthalene as tracer in nitrogen and oxygen over a temperature range of 400–928 K at 0.1 MPa for equivalent ratio and temperature imaging.
The single-excitation, two-color detection technique is selected in this work, because it enables to measure fluorescence signal in inhomogeneously seeded flow, conditions commonly encountered in experimental configurations.
The detected fluorescence signal ratio of spectral bands Δλ 1 and Δλ 2 is presented below:
The fluorescence signal ratio depends only on the temperature.
The sensitivity of the fluorescence signal ratio depends on the chosen spectral bands. It is necessary to study the sensitivity of the technique for different combinations of integration spectral bands in order to find the best spectral band couple regarding the highest fluorescence signal ratio and best signal-to-noise ratio.
Figure 13 presents the evolution of anisole fluorescence signal ratio with the temperature for different spectral bands in nitrogen at 1.2 MPa and an excitation wavelength of 266 nm. The values are normalized to the ratio value at 473 K. The integration spectral band couple, Δλ 2 = 300–325 nm and Δλ 1 = 399–424 nm, seems to correspond to the best fluorescence signal ratio. Indeed, over a range from 473 to 823 K, this ratio increases by about a factor of twelve; however, the signal-to-noise ratio of the integration spectral band Δλ 1 is only 1.32. For the spectral couple, Δλ 2 = 300–325 nm and Δλ 1 = 374–399 nm, the fluorescence ratio increases by a factor of seven over the considered temperature range and the signal-to-noise ratio of the integration spectral band Δλ 1 is 2.33, which is sufficient. This configuration will be chosen for the temperature measurement.
This integration spectral band couple approaches to commercial filters: The first filter with spectral band centered at 292 nm with a spectral width of 27 nm is a BrightLine FF01-292/27-50, and the second filter fixed at the fluorescence spectrum end, centered at 386 nm and with a spectral width of 23 nm, is a BrightLine FF01-386/23-50. The transmittance factor is, respectively, of 75 and 90 % for these filters.
The measurement of fluorescence signal ratio is presented in Fig. 14. The anisole emission spectrum is integrated over the two spectral bands corresponding to the chosen filters. The ratio of these signals is normalized to the reference value at 473 K for each pressure.
The fluorescence signal ratio increases with temperature in the studied range, by a factor of 8 and 11 at 0.4 and 4 MPa, respectively.
Luong et al. [20] have shown the same trends with toluene fluorescence filtered over two spectral domains in nitrogen and for an excitation wavelength of 248 nm. Over a temperature range from 300 to 900 K, the fluorescence signal ratio increases by a factor of 5 with the couple of long-pass filters (WG280 and WG320), whereas with a combination of filters (BP280, band pass centered at 280 ± 5 nm and WG320), the fluorescence signal ratio increases by a factor of 20.
From experiments investigated in the HP–HT cell, the evolution of fluorescence signal ratio of anisole filtered at 386 and 292 nm with temperature is known between 473 and 823 K, 0.2 and 4 MPa, for different ambient gases. Experimental points from the previous calibration can be now included and represented in a 2D plot (cf. Fig. 15). Indeed, this two dimension (temperature, pressure) enables to calculate by interpolating the ratio value on each mesh node in a planar LIF image obtained in RCM for instance over a temperature range from 473 to 1,000 K and a pressure range from 0.2 to 4 MPa for different ambient gases (nitrogen, carbon dioxide, argon, and three gas compositions).
4 Conclusions
The influence of the pressure, temperature, and gas composition on anisole fluorescence has been characterized in a high pressure and high temperature facility from an excitation wavelength of 266 nm. This parametric study gives a whole overview of the trends for different ambient gases, pressure, and temperature range. This study gives data for a necessary calibration step to aim a quantitative temperature measurement.
From these experimental results, temperature can be inferred from the fluorescence ratio according to the excitation wavelength and the detection band. The single-color detection technique for homogeneously seeded flows can be used thanks to the experimental correlation deduced from the evolution of fluorescence signal with temperature. Finally, the single-excitation, two-color detection technique for inhomogeneously seeded flows is chosen, and 2D calibration map is proposed in a wide pressure, large temperature, and ambient gases domain in conditions close to those encountered in IC engines.
References
P. Guibert, A. Keromnes, G. Legros, Flow Turbul. Combust. 84, 79 (2010)
F. Zimmermann, W. Koban, C. Roth, D. Herten, C. Schulz, Chem. Phys. Lett. 426, 248 (2006)
S. Kaiser, M. Long, Proc. Combust. Inst. 30, 1555 (2005)
M. Kühni, C. Morin, P. Guibert, Appl. Phys. B 102, 659 (2011)
C. Schulz, V. Sick, Prog. Energy Combust. Sci. 3, 75 (2005)
S. Einecke, C. Schulz, V. Sick, Appl. Phys. B 71, 717 (2000)
H. Zhao, N. Ladommatos, Prog. Energy Combust. Sci. 24, 297 (1998)
W. Koban, J. Koch, R. Hanson, C. Schulz, Appl. Phys. B 80, 147 (2005)
M. Orain, B. Baranger, B. Rossow, F. Grisch, Appl. Phys. B 102, 163 (2011)
F. Grossmann, P. Monkhouse, M. Ridder, V. Sick, J. Wolfrum, Appl. Phys. B 62, 249 (1996)
M. Pasquini, N. Schiccheri, M. Becucci, G. Pietraperzia, J. Mol. Struct. 924, 457 (2009)
H. Hippler, J. Troe, H. Wendelken, J. Chem. Phys. 78, 6709 (1983)
N. Pasquier, B. Lecordier, M. Trinité, A. Cessou, Proc. Combust. Inst. 31, 1567 (2007)
W. Robertson, J. Seriff, F. Matsen, J. Am. Chem. Soc. 72, 1539 (1950)
W. Koban, J. Koch, R. Hanson, C. Schulz, Phys. Chem. Chem. Phys. 6, 2940 (2004)
S. Fally, M. Carleer, A. Vandaele, J. Quant. Spectrosc. Radiat. Transf. 110, 766 (2009)
M. Orain, P. Baranger, B. Rossow, E. Grisch, Appl. Phys. B 100, 945 (2010)
R. Matsumoto, K. Sakeda, Y. Matsushita, T. Suzuki, T. Ichimura, J. Mol. Struct. 735, 153 (2005)
M. Luong, W. Koban, C. Schulz, J. Phys. 45, 133 (2006)
M. Luong, R. Zhang, C. Schulz, V. Sick, Appl. Phys. B 91, 669 (2008)
Acknowledgments
M. Tran was supported by a joint PhD grant from the French Ministry of Research. This work was partly funded by the FUI (French Fond Unique Interministériel) in the framework of the MODELESSAIS Pôles de Compétitivité MOVE’O project, (www.pole-moveo.org) project number 07 2 90 6147.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tran, K.H., Morin, C., Kühni, M. et al. Fluorescence spectroscopy of anisole at elevated temperatures and pressures. Appl. Phys. B 115, 461–470 (2014). https://doi.org/10.1007/s00340-013-5626-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-013-5626-8