Abstract
Ce3+ doped ABaPO4 (A=Li, Na, K) phosphors were prepared by conventional high temperature solid-state reaction. The phosphors were investigated by XRD, photoluminescence excitation and emission spectra, and luminescence decay curves. The five 5d levels corresponding to the 4f1→4f05d1 transition of Ce3+ ions were identified. The spectroscopic parameters, e.g., the 5d barycenter, the crystal-field splitting, and the Stokes shift, were discussed. LiBaPO4:Ce3+ phosphor could be efficiently excited by the near-UV lights (330–420 nm) and showed a broad emission band in the range of 430–620 nm with the maximum wavelength at 468 nm. In contrast, Ce3+-doped NaBaPO4 and KBaPO4 showed only excitation bands in a limited UV region (230–370 nm) and have blue emission at 385 nm and 416 nm, respectively. The temperature quenching of luminescence and the chromaticity coordinates were reported. The luminescence properties were discussed by analyzing the crystal structure and the local surroundings of Ce3+ ions on the Ba2+ sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rare-earth (RE) activated inorganic materials have been established as useful luminescent materials in fabricating the optoelectronic devices. Nowadays, more and more interest has been focused on the synthesis and luminescence properties of the new RE-doped phosphors due to the theory researches and the potential applications [1–5].
The 5d-level spectroscopy of Ce3+ ion is simple, which has only one electron in the 4f shell and exhibits strong absorption and efficient fluorescence from the allowed inter-configurational 4f1–4f05d1 transition. The spectroscopic parameters of Ce3+, e.g., the positions of 5d level, can be used to predict the 5d energies of other lanthanides, since the influence of the crystal field and covalence of the host lattices on the 4f5d levels are approximately equal for all the RE ions [6, 7]. Efficient energy transfer from the Ce3+ ions (as an energy donor) to acceptors (RE or transition metal ions), for example, Ce3+ to Tb3+ ions [8], Ce3+ to Nd3+ [9], Ce3+ to Mn2+ [10], Ce3+ to Eu3+ ions [11], and Ce3+ to Eu2+ ions [12], can be realized in many inorganic materials excited by the VUV–UV. Consequentially, Ce3+-doped materials are attractive for numerous applications, such as white light emitting diodes (W-LEDs) [13, 14], and fast scintillators [15], etc. The luminescence spectra of the doped Ce3+ ion have been investigated in many hosts [16, 17].
The phosphates with ABPO4 formula (A and B are mono- and divalent cations, respectively) are in a family of mono-phosphates with the different structure types strictly depending on the relative size of the A and B ions [18]. These compounds have been considered to be efficient luminescent hosts due to the excellent thermal and hydrolytic stability [19–21]. The variety in these structures makes it possible to tailor the physical and chemical properties of ABPO4 hosts. It has been confirmed that LiBaPO4 is suitable for second harmonic generating (SHG) responses and shows larger SHG effects relative to quartz [22]. Upadeo and Moharil [23] investigated radiation-induced valence changes (Eu3+→Eu2+ conversion) in Eu-doped NaBaPO4. Waite et al. [24] firstly report the Eu2+ luminescence doped in LiBaPO4.
Recently, Eu2+-doped ABPO4 phosphates have received much attention for the potential applications as new white light emission diodes (W-LEDs) phosphors [19]. Wu et al. have reported that LiBaPO4:Eu2+ is a good candidate phosphor applied in W-LEDs and exhibits higher thermal stability than that of commercially available Y3Al5O12:Ce3+ [25]. ABaPO4:Eu2+ (A=Na, K) phosphors were reported to emit a strong blue light under UV excitation [26]. NaBaPO4:Eu3+ [27] has been reported to be potential red-emitting phosphor. KBaPO4:Eu2+ show an emission band with a maximum at 420 nm [28]. Lin et al. [29] reported the full-color band phosphors, Eu2+, Tb3+, and Sm3+ doped KBaPO4, which could generate blue, green, and orange-red light, respectively. The thermal stabilities of KBaPO4:Ln (Ln=Eu2+, Tb3+, Sm3+) are higher than the commercially available YAG:Ce3+. KBaPO4:Eu2+ coated by silica on the phosphor surface is very potent as a blue-emitting phosphor for PDPs (plasma display panels), CCFLs (cold cathode fluorescence lamp), and phosphor-converted (pc) W-LEDs based on near ultraviolet LED [30]. However, there are few reports on the luminescence properties of Ce3+ ions doped into ABaPO4:Eu2+ (A=Li, Na, K).
In this study, Ce3+-doped ABaPO4 (A=Li, Na, K) phosphor were prepared by conventional high temperature solid-state reaction. The structures were confirmed by XRD measurements. The photoluminescence excitation and emission spectra, and luminescence decay curves, the temperature quenching and chromaticity coordinates of luminescence were investigated. The luminescence properties of the Ce3+ ion were investigated by spectroscopic parameters such as emission bands, 5d components, Stokes shift, barycenter of Ce3+ ions doped in ABaPO4 (A=Li, Na, K) host. This research can be referential for the luminescence investigation of other RE ions doped into these phosphates.
2 Experimental
The preparation of Ce3+ ions doped ABaPO4 (A=Li, Na, K) were carried out by conventional solid state synthesis. The raw materials were high-purity (4N) A 2CO3 (A=Li, Na, K), BaCO3, NH4H2PO4 and CeO2. The starting materials with stoichiometric amounts were ground together in an agate mortar. The mixture was firstly heated up to 350 ∘C and kept at this temperature for 5 h. After a second homogenization in the mortar, the samples were heated up to 750 ∘C and kept at this temperature for 10 h. After that, the samples were mixed and finally heated at 1000 ∘C for 10 h in crucibles along with the reducing agent (active carbon).
The X-ray diffraction (XRD) patterns were collected on a Rigaku D/Max-2000 diffractometer operating at 40 kV, 30 mA with Bragg–Brentano geometry by using Cu Kα radiation (λ=1.5418 Å) and analyzed using Jade-5.0 software. The optical excitation and emission spectra were recorded by a Perkin–Elmer LS-50B luminescence spectrometer and a Hitachi F-4500 fluorescence spectrophotometer. The luminescence decay was measured using the fourth harmonic of a Nd:YAG laser (266 nm). To study thermal quenching of the phosphors between 25 to 150 ∘C the same spectrofluorimeter was equipped with a homemade heating cell under the excitation of a 266 nm Nd:YAG laser.
3 Results and discussion
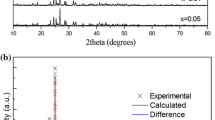
Figure 1 show the XRD patterns of LiBaPO4:Ce3+, NaBaPO4:Ce3+ and KBaPO4:Ce3+. The peak positions and the relative intensities of the samples are the same as the PDF2 standard cards in the International Centre for Diffraction Data (ICDD) database: No. 14-0270 for LiBaPO4, No. 33-1210 for NaBaPO4 and No. 33-0996 for KBaPO4. No impurity lines were observed. The sharp peaks in each XRD pattern indicate the good crystallizability of the samples.
Inset in Fig. 1 are the corresponding schematic views of the structures. This structure of LiBaPO4 (Fig. 1a) involves the existence of a 1:1 ordering between the cross-linked LiO4 and PO4 tetrahedra on the basis of an isotopic with the β-SiO2 trydimite [31]. The hexagonal phase structure shows mixed rings with alternately “up” and “down” tetrahedral of LiO4 and PO4.
NaBaPO4 has a structure (Fig. 1b) isotypic with that of glaserite with space group\(P\overline{3}m1\) [32, 33]. The network is built up with PO4 tetrahedra, NaO6 octahedra, BaO12, and YO10 polyhedra (Y=Na or Ba). It contains chains of polyhedra parallel to c (Fig. 1b). These alternately involved NaO6 and BaO12 oxygen atoms. The remaining cations (Y=Na or Ba) lie at sites with 10 neighboring atoms in another B chain [32, 33].
KaBaPO4 have the β-K2SO4 type structure (Fig. 1c), which is made up of two types of string along the a-axis [18]. One string consists of alternating [PO4] tetrahedra and alkaline atoms. Another is made up of only alkaline earth atoms parallel to the a-axis. K+ lies in a site with 10-fold coordination, much like the Y site in NaBaPO4, and Ba2+ in a 9-fold coordinated site [34]. The difference between β-K2SO4-type KBaPO4 and NaBaPO4 structure is that the Ba2+ ions have the 9-fold coordinated sites in the former and have an equal number of 10- and 12-fold coordinated sites in the latter.
Figure 2 shows the normalized excitation and emission spectra of ABaPO4:Ce3+ (A=Li, Na, K) phosphors. The three distinct excitation spectra were taken by monitoring emission at 468 nm for LiBaPO4:Ce3+, 385 nm for NaBaPO4:Ce3+, and 416 nm for KBaPO4:Ce3+. Each spectrum contains five excitation bands which are characteristic absorption transitions of direct 4f1(2F5/2)→4f05d1 of Ce3+ ions. It is well known that the 4f configuration of Ce3+ ion has only one electron, and a UV photon can excite the 4f electron into the 5d orbital, leaving the 4f shell empty. So the excitation spectrum of Ce3+ shows the direct splitting information of 5d orbital in the crystal field [35].
The each broad excitation band in Fig. 2 actually is composed of several sub-bands, which can be assigned to five transitions of 4f15d1(2F5/2)→4f05d1 of Ce3+. It indicates that the mean energy of the 5d configuration shift downward relative to the free ion value and the 5d levels split into the five levels 5d(1), 5d(2), 5d(3), 5d(4), and 5d(5), which could be roughly estimated from the excitation spectra as shown in Table 1. The 5d barycenter values in LiBaPO4:Ce3+, NaBaPO4:Ce3+, and KBaPO4:Ce3+ are 34,157, 37,130, and 34,690 cm−1, respectively.
The excitation bands show a red shift in the order of NaBaPO4:Ce3+ (maximum wavelength position 324 nm) < KBaPO4:Ce3+ (340 nm) < LiBaPO4:Ce3+ (390 nm). The results show that LiBaPO4:Ce3+ could be efficiently excited by the incident lights of 330–430 nm, which well matched with the emissions wavelength of near-UV LEDs (360–400 nm). This is necessary for the potential application in the W-LEDs fabricated with near-UV chips. However, the application as a potential LED phosphor is limited for NaBaPO4:Ce3+ and KBaPO4:Ce3+.
Generally, the emission spectra of Ce3+ ions have a doublet character due to the spin–orbit splitting of ground state (2F5/2 and 2F7/2). The emission spectra were recorded under the excitation of 380 nm (LiBaPO4:Ce3+), 324 nm (NaBaPO4:Ce3+), and 363 nm (KBaPO4:Ce3+), which corresponds to excitation into the lowest energy d-level of Ce3+. The two 5d(1)→2F J (J=5/2, 7/2) sub-bands can be resolved by using the deconvolution method based on their curve shapes shown in Fig. 2b. The Gaussian peak-fittings suggest that the L–S splitting value of Ce3+ 4f level is 1,900 cm−1 for LiBaPO4:Ce3+, 2,125 cm−1 for NaBaPO4:Ce3+, and 1,900 cm−1 for KBaPO4:Ce3+. These values are in agreement with the normal ground-state splitting of Ce3+ ions (2,000 cm−1) [6, 7].
The CIE (Commission Internationale de l’Eclairage) coordinates were calculated from emission spectra shown in Fig. 3. Three phosphors show different luminescence colors, which are consistent with the emission spectra. LiBaPO4:Ce3+ shows bright blue-green color with CIE coordinates (0.142, 0.298). The CIE chromaticity coordinates of NaBaPO4:Ce3+ (0.134, 0.08) and KBaPO4:Ce3+ (0.135, 0.141) are in the blue region.
The CIE chromaticity coordinates of LiBaPO4:Ce3+, NaBaPO4:Ce3+ and KBaPO4:Ce3+ calculated from the emission in Fig. 2(b)
The fluorescence decay curves for the Ce3+ emission under excitation of 266 nm pulsed YAG laser are shown in Fig. 4. The curves can be fitted into a single exponential function: I=I 0exp[−t/τ], where I 0 is the initial emission intensity for t=0, τ is lifetime. The lifetime of LiBaPO4:Ce3+ (468 nm), NaBaPO4:Ce3+ (385 nm) and KBaPO4:Ce3+ (416 nm) are calculated to be 41.4 ns, 49.8 ns, and 51.8 ns, respectively, characteristic of the allowed Ce3+5d1→4f1 transition.
The approximate positions of Ce3+5d sublevels, emission bands and Stokes shift values are reported in Table 1. The Stokes shift is increased in the order of KBaPO4: Ce3+ (2,920 cm−1) < NaBaPO4:Ce3+ (4,330 cm−1) < LiBaPO4:Ce3+ (4,810 cm−1).
The lowest 5d energy level of Ce3+ in a specific compound, the 5d energy levels of other rare earth ions in the same compound can be predicted. Dorenbos [6, 7] proposed that the crystal-field depression D(Ln,A) of the energy of lowest. The 4fn−15d levels of a lanthanide ion in compound “A” relative to the level energies in the free ion is almost independent of the type of lanthanide ion:
where E(Ln,free) is the energy of the first f–d transition of Ln3+(Ce3+) in a free ion and E(Ln,A) the energy of 4fn−15d-levels of a lanthanide ion in compound A. The d level energy of free Ce3+ is 49,340 cm−1 [36]. It could be calculated that the f–d transition energy of Ce3+ in LiBaPO4 D(Ce3+, LiBaPO4) is depressed by 7,840 cm−1, in NaBaPO4 D(Ce3+, NaBaPO4) is depressed by 6,790 cm−1, in KBaPO4 D(Ce3+, KBaPO4) is depressed by 6,970 cm−1 (Table 1).
The 5d manifold Ce3+ ions doped in a crystal field undergoes an energetic depression on the basis of the nephelauxetic effect and a splitting due to the crystal field. According to the nephelauxetic effect, the stronger the covalency is, the smaller the energy difference between the ground state 4f1 and activated band 5d of the Ce3+ ions is. The nephelauxetic effect shifts the center of gravity of 5d bands to lower energy. From the calculations of the crystal-field depression effects, it can be suggested that the covalency effects are stronger in LiBaPO4:Ce3+ than those in NaBaPO4:Ce3+ and KBaPO4:Ce3+.
In Fig. 2b it is obvious that the emission wavelength increases from 385 nm in NaBaPO4:Ce3+, to 416 nm in KBaPO4:Ce3+, to 468 nm in LiBaPO4:Ce3+. The observed variation of emission center should also result from the position of the 5d band, which can be shifted by the crystal field. ABaPO4 (A=Li, Na, K) exhibit similar stoichiometries, but the crystal structures are different. However, the Ce3+ 5d1 crystal-field splitting should be dependent upon both the Ce3+–O2− bond length and the distortion. Figure 5 illustrates the comparison of the coordination geometries around the Ba in LiBaPO4:Ce3+, NaBaPO4:Ce3+, and KBaPO4:Ce3+. In LiBaPO4, Ba is surrounded by the cross-linked LiO4 and PO4 tetrahedra with an average Ba–O (BaO9) distance of 2.74 Å [31]. The average Ba–O distances in NaBaPO4 (BaO12) and KBaPO4 (BaO9) are 3.018 [32, 33] and 2.82 Å [18], respectively. For LiBaPO4:Ce3+, the smaller Ba(Ce)–O bond length and the larger distortion should lead to a larger crystal-field splitting. This indicates that the crystal-field strength of LiBaPO4 is stronger than that of KBaPO4:Ce3+ and NaBaPO4:Ce3+. Consequently, the emission peak of LiBaPO4:Ce3+ should shift to a long wavelength band.
The concentration dependence of the emission intensity is also investigated. The strongest emission intensity has been obtained for three samples with about 3 % Ce3+ doping (figure omitted). The position of the emission bands does not change with increasing Ce3+ concentration in NaBaPO4:Ce3+ and KBaPO4:Ce3+, however, a red shift in the emission spectrum and its maximum is observed in LiBaPO4:Ce3+ as shown in Fig. 6. The red shift is about 15 nm. The red shift may be mainly related to the crystal-field effects upon the 5d levels of the Ce3+ ions due to the modification of the crystal field as well as the structural relaxation. This trend is in good accordance with the degree of crystalline volume constriction mentioned above. No significant emission band shifts in NaBaPO4:Ce3+ and KBaPO4:Ce3+ might be caused by a weak shrinkage of the unit cell volume due to the bigger Ba2+ sites accommodating Ce3+ ions.
The temperature-dependent luminescence of Ce3+-doped ABaPO4 (A=Li, Na, K) phosphors were investigated. With the increasing of temperature the thermal quenching can be observed in three phosphors. Figure 7 represents the temperature dependence of the integrated emission intensities normalized at the at 25 ∘C values. With the increasing of temperature up to 150 ∘C, the normalized emission intensity is decreased to 49 % for LiBaPO4:Ce3+, 56 % for NaBaPO4:Ce3+ and 62 % for KBaPO4:Ce3+. The thermal quenching of Ce3+-doped phosphors is usually affected by many factors, such as the rigidity of the crystal structure, crystallinity, defect levels, Stokes shift, etc. [37]. This stronger thermal quenching in LiBaPO4:Ce3+ might be due to the lower 5d energy position and the relatively large Stokes shift.
4 Conclusions
The luminescence properties of Ce3+ are reported in three monophosphates ABaPO4 (A=Li, Na, K) with similar stoichiometries, but different structures. The spectroscopic parameters and luminescence properties are different.
(1) The crystal-field splitting of Ce3+ ions increases going from about 11,690 cm−1 for NaBaPO4:Ce3+ to about 14,820 cm−1 for KBaPO4:Ce3+ and about 15,185 cm−1 for LiBaPO4:Ce3+; The Ce3+ 5d center of gravity decreases to lower energy with increase in covalency (nephelauxetic effect) in the series: NaBaPO4:Ce3+ (37,130 cm−1) > KBaPO4:Ce3+ (34,690 cm−1) > LiBaPO4:Ce3+ (34,157 cm−1); The Stokes shift decreases in the order: LiBaPO4:Ce3+ (4,810 cm−1) > NaBaPO4:Ce3+ (4,330 cm−1) > KBaPO4:Ce3+ (2,920 cm−1).
(2) LiBaPO4:Ce3+ shows a bright blue–green color (468 nm) with CIE coordinates (0.142, 0.298). The CIE chromaticity coordinates of NaBaPO4:Ce3+ (385 nm) (0.134, 0.08) and KBaPO4:Ce3+ (416 nm) (0.135, 0.141) are in the blue region. The position of the emission bands does not change with increasing Ce3+ concentration in NaBaPO4:Ce3+ and KBaPO4:Ce3+, however, a significant red shift in the emission spectrum and its maximum is observed in LiBaPO4:Ce3+.
(3) A large Stokes shift causes quenching at relatively low temperatures. With the increasing of temperature up to 150 ∘C, the normalized emission intensity is decreased to 49 % for LiBaPO4:Ce3+, 56 % for NaBaPO4:Ce3+, and 62 % for KBaPO4:Ce3+.
(4) The efforts for the further exploration of the applications in Ce3+ doped ABaPO4 (A=Li, Na, K) can be suggested. An intense absorption and excitation band in the near-UV range (300–420 nm) in LiBaPO4:Ce3+ is a hint that this material is promising as regards conversion phosphors for white-light LEDs. However, the application as a potential LED phosphor is limited for NaBaPO4:Ce3+ and KBaPO4:Ce3+ because the phosphors only show blue emission and the absorption of Ce3+ is limited in UV region. Taking into account of the emission properties (in the purplish blue region) and the possible energy transfer from Ce3+ ions in NaBaPO4 and KBaPO4 hosts, high efficient phosphors could be obtained by co-doping Ce3+ ions with other ions, for example, co-doping of Tb3+, Eu3+,2+, Mn2+ ions, to get green, red and deep red phosphors, respectively.
References
A.A. Setlur, E.V. Radkov, C.S. Henderson, J.H. Her, A.M. Srivastava, N. Karkada, M.S. Kishore, N.P. Kumar, D. Aesram, A. Deshpande, B. Kolodin, L.S. Grigorov, U. Happek, Chem. Mater. 22, 4076 (2010)
U. Happek, A.A. Setlur, J.J. Shiang, J. Lumin. 129, 1459 (2009)
A.M. Srivastava, H.A. Comanzo, S. Camardello, S.B. Chaney, M. Aycibin, U. Happek, J. Lumin. 129, 919 (2009)
A.M. Srivastava, A.A. Setlur, H.A. Comanzo, M.E. Hannah, P.A. Schmidt, U. Happek, J. Lumin. 129, 126 (2009)
X.M. Zhang, W.L. Li, L. Shi, X.B. Qiao, H.J. Seo, Appl. Phys. B, Lasers Opt. 99, 279 (2010)
P. Dorenbos, J. Lumin. 91, 91 (2000)
P. Dorenbos, J. Lumin. 91, 155 (2000)
N. Hashimoto, Y. Takada, K. Sato, S. Ibuki, J. Lumin. 48–49, 893 (1991)
R.M. Brewer, M. Nicol, J. Lumin. 21, 367 (1980)
U.G. Caldiño, J. Phys., Condens. Matter 15, 3821 (2003)
M. Nazarov, D.Y. Noh, J. Sohn, C. Yoon, Opt. Mater. 30, 1387 (2008)
K.Y. Jung, J.H. Kim, J. Lumin. 128, 2004 (2008)
A.A. Setlur, W.J. Heward, M.E. Hannah, U. Happek, Chem. Mater. 20, 6277 (2008)
V. Bachmann, C. Ronda, A. Meijerink, Chem. Mater. 21, 2077 (2009)
C. Michail, A. Toutountzis, S. David, N. Kalyvas, I. Valais, I. Kandarakis, G.S. Panayiotakis, Appl. Phys. B, Lasers Opt. 95, 131 (2009)
A.A. Setlur, U. Happek, J. Solid State Chem. 183, 1127 (2010)
A.M. Srivastava, S.J. Camardello, H.A. Comanzo, M. Aycibin, U. Happek, Opt. Mater. 32, 936 (2010)
L. Elammari, M. El Koumiri, I. Zschokke-Gränacher, B. Elouadi, Ferroelectrics 158, 19 (1994)
C.C. Lin, Z.R. Xiao, G.Y. Guo, T.S. Chan, R.S. Liu, J. Am. Chem. Soc. 132, 3020 (2010)
S. Zhang, Y. Nakai, T. Tsuboi, Y. Huang, H.J. Seo, Inorg. Chem. 50, 2897 (2011)
S. Zhang, Y. Nakai, T. Tsuboi, Y. Huang, H.J. Seo, Chem. Mater. 23, 1216 (2011)
B. Liu, J. Barbier, J. Solid State Chem. 102, 115 (1993)
S.V. Upadeo, S.V. Moharil, J. Phys., Condens. Matter 9, 735 (1997)
M.S. Waite, J. Electrochem. Soc. 121, 1122 (1974)
Z.C. Wu, J. Liu, M.L. Gong, Q. Su, J. Electrochem. Soc. 156, 153 (2009)
W.J. Tang, D.H. Chen, J. Am. Ceram. Soc. 92, 1059 (2009)
X. Li, G. Li, X.N. Li, J.W. Wen, Z.P. Yang, Powder Technol. 200, 12 (2010)
S.H.M. Poort, W. Janssen, G. Blasse, J. Alloys Compd. 260, 93 (1997)
C.C. Lin, Y.S. Tang, S.F. Hu, R.S. Liu, J. Lumin. 129, 1682 (2009)
W.B. Im, H.S. Yoo, S. Vaidyanathan, K.H. Kwon, H.J. Park, Y.I. Kim, D.Y. Jeon, Mater. Chem. Phys. 115, 161 (2009)
P. Boutinaud, C. Parent, G. Le Flem, B. Moine, C. Pedrini, J. Mater. Chem. 6, 381 (1996)
C. Calvo, R. Faggiani, Can. J. Chem. 53, 1849 (1975)
A.W. Kolsi, M. Quarton, W. Freundlich, J. Solid State Chem. 36, 107 (1981)
C.W. Struck, J.G. White, Acta Crystallogr. 15, 290 (1962)
P. Dorenbos, J. Phys., Condens. Matter 15, 8417 (2003)
P. Dorenbos, J. Lumin. 87–89, 970 (2000)
H.L. Li, G.H. Zhou, R.J. Xie, N. Hirosaki, X.J. Wang, Z. Sunm, J. Solid State Chem. 184, 1036 (2011)
Acknowledgements
This work was supported by Mid-career Researcher Program through National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science and Technology (MEST) (Project No. 2009-0078682), and by the Industrial Strategic technology development program (Project No: 10037416, Establishment of infrastructure for LED-marine convergence technology support and technology development for commercialization) funded by the Ministry of Knowledge Economy (MKE, Korea), and by A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, D., Huang, Y., Zhang, S. et al. Luminescence spectroscopy of Ce3+-doped ABaPO4 (A=Li, Na, K) phosphors. Appl. Phys. B 108, 447–453 (2012). https://doi.org/10.1007/s00340-012-4969-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-4969-x