Abstract
Measurements of 3-pentanone fluorescence quantum yield (FQY) over a wide range of temperatures and pressures in air and nitrogen bath gases are reported and a comprehensive FQY model in support of quantitative planar laser-induced fluorescence diagnostics at elevated pressures and temperatures is presented. Measurements were made of the FQY for 20 mbar of 3-pentanone in nitrogen and air for pressures between 1 and 25 bar in a high-pressure and high-temperature cell for excitation wavelengths of 248, 266, 277, and 308 nm. The measurements were performed in nitrogen from 298 to 745 K and in air from 298 to 567 K. The 3-pentanone FQY data were used to optimize FQY model parameters, including the oxygen and nitrogen quenching rates and vibrational relaxation cascade parameters for nitrogen and oxygen. This work introduces vibrational energy dependence for cascade parameters, as well as a nitrogen quenching rate. The new 3-pentanone FQY model agrees with the measurements within 10%, as well as with fluorescence signal measurements from optical internal combustion engines at pressures and temperatures up to 28 bar and 1100 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tracer-based planar laser-induced fluorescence (PLIF) is a powerful diagnostic that allows for measurements of useful parameters in various flow fields [1]. The diagnostic relies on the introduction of the tracer species into the flow field, the exposure of the flow field to laser light, and the collection of the resulting fluorescence signal. Based on the variations in fluorescence signal intensity, information about the flow field can be extracted. Ketones, such as acetone and 3-pentanone, have been particularly popular tracers for this diagnostic [2–4]. 3-pentanone tracer-based PLIF is commonly used as a diagnostic for internal combustion engine (ICE) studies and has been used to spatially measure temperature and fuel distributions at different times in the pre-ignition phase of the engine cycle [1, 5–8].
Obtaining information in this and related applications requires knowledge of photophysical parameters, particularly the fluorescence quantum yield (FQY), over a wide range of temperatures, excitation wavelengths, pressures, and compositions, including combined high-pressure and high-temperature conditions corresponding to top dead center in an ICE. FQY measurements over a range of temperatures and pressures have been made by previous workers in optical cells, but, with the exception of recent measurements by Braeuer et al., investigated temperatures have been limited to 600 K [9–12]. Previous work in optical ICEs was able to achieve simultaneous high-temperature and high-pressure conditions, but the range of temperatures and pressures cannot be easily varied independently [13–16]. In order to maximize the utility of 3-pentanone PLIF, there is a need to extend the range of temperatures and excitation wavelengths for which the pressure variation of 3-pentanone FQY is known.
To facilitate the application of PLIF diagnostics, a simple step-ladder kinetic model of FQY has been proposed for acetone [3], which was then adapted for use with 3-pentanone [17]. However, subsequent work has found that the 3-pentanone FQY model does not reproduce the FQY at engine conditions [6, 13]. A companion work utilizing FQY measurements in 3-pentanone vapor at low pressures to optimize certain parameters in the FQY model has improved the model’s ability to predict FQY for 3-pentanone vapor across a wide range of temperatures [18]. However, application of the model to diagnostics in ICEs or other practical flow fields will require a model capable of predicting FQY in nitrogen and air across a wide range of pressure and temperature conditions.
2 Photophysics background
The linear fluorescence equation, shown below, describes the emitted fluorescence signal and is necessary to the extraction of quantitative information from the diagnostic.

In this equation, E is the incident laser energy fluence, h is Planck’s constant, c is the speed of light in vacuum, λ is the laser wavelength, n is the number density of the fluorescing tracer, L is the length of the illuminated volume, Ω is the detector collection angle, and η is the detector collection efficiency. The absorption cross section σ and the FQY ϕ are photophysical parameters that describe the propensity for a molecule to absorb and emit photons, respectively, when excited by a laser of wavelength λ. As the equation shows, the dependence of the fluorescence signal on the flow field properties of temperature T, pressure p,3-pentanone mole fraction X 3p, and mole fraction of other bath gases X M is defined by the number density and photophysical parameters.
Number density can be easily determined using the ideal gas equation. The absorption cross section is also well characterized from previous measurements of the 3-pentanone absorption spectrum at various temperatures that resulted in an explicit, Gaussian expression for calculating the absorption cross section [19]. Thus, it is the lack of knowledge and modeling of the FQY with temperature and pressure in nitrogen or air that currently limits application of 3-pentanone PLIF to practical flow fields.
Previous work has shown that a FQY model based on the simplified photophysics behind ketone fluorescence can be used successfully to model the FQY of tracers like acetone and 3-pentanone. The energy diagram on which the FQY step-ladder model is based is shown in Fig. 1. The unexcited molecule resides in the ground rovibronic singlet state (S 0) at the average vibrational energy E thermal. Upon absorption of a photon, the molecule enters the excited singlet state (S 1) with vibrational energy given by
where λ is the wavelength of the photon and E 0−0 is the zero-point energy difference between the excited and ground singlet states, which is 30770 cm−1 for 3-pentanone [20]. E thermal is the thermal vibrational energy calculated by summing the contributions of the 42 vibrational modes of 3-pentanone at the local temperature [13].
From this initial vibrational energy level E 1, the molecule can relax through a sequence of vibrational energy levels E n . This sequence due to relaxation is described by
where α is the species-specific vibrational relaxation cascade parameter and n is the nth step in the vibrational energy cascade. For mixtures of collision partners, the overall cascade parameter is calculated by the collision frequency-weighted average of the species-specific cascade parameters. In the absence of fluorescence or transition to another electronic state, the relaxation continues until the molecule reaches the Nth vibrational energy level in the cascade, with energy E thermal.
This model considers two types of processes that depopulate the excited singlet: collisionless and collisional processes. Collisionless processes do not depend on collisions between molecules, and such processes include fluorescence, represented by the fluorescence rate k f, as well as intersystem crossing (ISC) and internal conversion (IC), which are represented together by the non-radiative rate k nr. Collisional processes that result from molecular collisions between the excited molecules are oxygen and nitrogen quenching, represented by the rates \(k_{\mathrm{O}_{2}}\) and \(k_{\mathrm{N}_{2}}\). The rate of vibrational relaxation, which is also a collisional process, but does not de-excite the singlet, is represented by the rate k vib.
The overall FQY can then be calculated using the equation
In this equation, the contribution to FQY ϕ n from each step n in the vibrational energy cascade is weighted by the probability of relaxing to step n from step 1. This probability is computed by the multiplication of the probability of relaxation from step j(P j ) over the vibrational energy cascade.
The contribution to FQY from a given vibrational level n with energy E n is
For ϕ N , where the molecule is at the Nth step of the vibrational energy cascade with energy E thermal,k vib is set to zero because the molecule can no longer relax. The probability that the molecule at vibrational level n successfully relaxes to level n+1 is given by
The original model by Koch approximated k vib as the collision frequency [17], calculated using the Lennard–Jones formulation [21]. Subsequent work utilized FQY measurements of 3-pentanone vapor at low pressures to optimize the collisionless rates k f and k nr, as well as the cascade parameter for 3-pentanone, α 3p, since a sensitivity analysis showed that these parameters are most sensitive to FQY measurements at low pressures [18]. The current work seeks to optimize the remaining rates representing collisional quenching by oxygen (\(k_{\mathrm{O}_{2}}\)) and nitrogen (\(k_{\mathrm{N}_{2}}\)) and the vibrational cascade parameters for nitrogen (\(\alpha_{\mathrm{N}_{2}}\)) and oxygen (\(\alpha_{\mathrm{O}_{2}}\)).
Model optimization is performed with measurements of 3-pentanone FQY across a range of excitation wavelengths, temperatures, and pressures, and these data are utilized to optimize the FQY model. Measurements were made in nitrogen for temperatures from 295 to 750 K and for pressures from 1 to 25 bar. Data were also acquired in air from 295 to 580 K and from 1 to 25 bar. Laser excitation wavelengths of 248, 266, 277, and 308 nm were utilized. The result of this work is a comprehensive database of FQY measurements and an FQY photophysical model that is applicable to diagnostic applications over a wide range of temperatures and pressures, including ICE conditions.
3 Experimental setup
3.1 High temperature and pressure optical cell design
In order to access a wide range of temperature and pressure conditions, we used a custom-built optical cell designed to operate at pressures between 1 and 60 bar at temperatures of up to 1000 K. Because the cell was designed to be held at high temperatures as fluorescence data are collected, the cell body is made from Inconel 625 for increased strength and creep resistance at high temperature. Four ports were machined into the corners of the cell to allow gas flow in and out of the cell, and two ports were machined on the top of the cell to allow for thermocouple mounting.
The cell viewport design is based on another high-pressure cell design [22], and exploded and cutaway views of the windows are shown in Fig. 2. The window holder is made from Inconel. In addition to its high-strength and low-creep properties, Inconel possesses a lower thermal expansion coefficient than stainless steel, which facilitates the sealing of the window in the holder since window materials also tend to have low thermal expansion coefficients. The window shape is a conical frustrum tapered such that the gas pressure in the cell will force the window against the holder. The windows utilized in the cell are custom made from UV-grade sapphire. The crystal axis is aligned with the center axis of the frustrum, which is also the optical axis of the window. A conical copper gasket is sandwiched between the window and the window holder, and the window is secured into the viewport using a custom-machined stainless steel nut with a hexagonal aperture. A copper washer cushions the window from the nut, and Belleville washers are placed between the copper washer and nut to maintain pressure on the window as the Inconel window holder expands with temperature. The assembled viewports are held in the cell body using gland nuts. Once installed in the window holder, the viewing diameter is 12.7 mm, and the beam path length between the windows is 61.7 mm.
The cell temperature was varied by heating the cell inside a furnace with viewports to allow for optical access to the furnace interior. To monitor the gas temperature, a K-type thermocouple was mounted to the top of the cell. In addition, the cell’s external surface temperature was monitored using a second K-type thermocouple. To ensure temperature uniformity within the cell, fluorescence data were taken only when these two thermocouples read within 5 K of each other or less.
For this study, a flowing gas stream of 20 mbar of 3-pentanone with excess nitrogen or air at total pressures varying from 1 to 25 bar was introduced into the cell via the system shown in Fig. 3. The system introduced bottled nitrogen or air using a mass flow controller. Degassed 3-pentanone liquid was introduced into the gas flow using a metering pump, which drew liquid 3-pentanone from a sealed glass flask and injected the liquid into a ‘T’ junction heated to about 150∘C. The 3-pentanone vaporized on injection and was entrained by the bath gas flow. Downstream of this junction, a heated mixing chamber filled with stainless steel mesh baffling ensured complete mixing of the 3-pentanone with the bath gas. To vary the pressure, the flow rates of nitrogen and air ranged from 2.5 SLPM at 1 bar to 30 SLPM at 25 bar. Pressure could also be adjusted as necessary using the needle valve on the gas exhaust circuit. The pump output was adjusted to achieve the desired 3-pentanone partial pressure, and the liquid flow rate ranged from about 0.1 to 0.2 mL/min of 3-pentanone liquid. With these flow rates, the residence time of the gas in the optical cell was about 0.2 s at 2.5 SLPM. A Setra 0–50 psia pressure transducer was used to monitor pressure up to 3 bar, and a Kistler 1–100 bar pressure transducer measured pressures above 3 bar.
3.2 Laser and collection optics setup
The optical setup for this work is shown in Fig. 4. Four different laser excitation wavelengths were employed in this study: 248, 266, 277, and 308 nm. The 248-nm beam was provided by a krypton fluoride excimer laser. The output from this excimer laser was also used to generate the 277-nm beam using a Raman shifter filled with 19 bar of hydrogen gas. The 277-nm beam was selected from the output of the Raman cell using a Pellin–Broca prism. The 266-nm beam was provided by a frequency-quadrupled Nd:YAG laser, and the 532-nm output of the laser was also used to pump a pulsed dye laser circulating rhodamine 640 laser dye. The dye laser was tuned to output a 616-nm laser beam, which was frequency doubled to 308 nm using a BBO crystal.
The laser beams were routed to the optical cell using wavelength-appropriate high-efficiency reflectors and were aligned to pass through the center of the optical cell’s windows. Each beam first passed through an iris to reduce it to the aperture of the following optics. The beam then passed through an MgF2 Rochon polarizer with a 14.5-mm-diameter aperture. The polarizer was aligned to allow the vertical polarization of the lasers to pass undeviated and deflect the horizontal polarization, which is blocked. The vertically polarized beam was passed through a 1000 mm focal length cylindrical lens to collimate the beam in the vertical direction, since the laser beams exhibited a slight divergence in that plane. The beam was then passed through an iris to reduce the collimated beam to 2-mm diameter. For the 248- and 266-nm beams, the beam energy was about 0.4 mJ/pulse (12.7 mJ/cm2); for the 277- and 308-nm beams, the beam energy was about 0.1 mJ/pulse (3.2 mJ/cm2).
To monitor the laser beam energy, about 10% of the beam energy was diverted using a 3∘ fused silica wedge to the beam energy detector. The beam was focused into a custom-made aluminum integrating sphere with an internal diameter of 25.4 mm using a 100 mm focal length fused silica lens. A high-speed photodiode monitored the output from the integrating sphere, and the photodiode signal was sent to an oscilloscope, corrected for detector dark current, and integrated to determine laser energy. The laser beam exiting the optical cell was also sent to an identical integrating sphere, photodiode, and oscilloscope setup to monitor output beam energy. The energy measurements were used to normalize the fluorescence signal for laser energy variations.
Fluorescence signal was collected using a 105-mm UV lens and imaged onto the slit of a spectrograph. A 325-nm-long-pass optical filter was placed in front of the collection lens to filter the Rayleigh scattering of the laser beam. The spectrograph grating is blazed at 300 nm with 300 grooves/mm with a spectral resolution of about 4.0 nm/pixel. The output of the spectrograph was imaged using 512 ×512 pixel intensified charge coupled device (ICCD) Princeton Instruments PI-MAX camera. The spectrograph was centered at 440 nm to collect the fluorescence spectrum.
3.3 Experimental setup spectral characterization
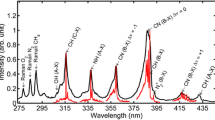
In order to correctly measure the shape of the fluorescence spectrum, the spectrograph and ICCD camera need to be calibrated for wavelength. In addition, raw spectral measurements need to be corrected for the relative spectral responsivity (RSR) of the collection optics, spectrograph, and ICCD camera. The RSR describes the camera signal in counts per fluorescence photon emitted from the gas as a function of photon wavelength, but is unitless because it is normalized. Calibration lamps, placed as shown in Fig. 4, were used to calibrate the spectrograph wavelength and determine the RSR of the optical cell and collection optics. For the calibration, only the viewport between the laser beam and the collection optics was installed in the cell body.
The wavelength calibration of the spectrograph was performed with five lines from a mercury calibration lamp. The RSR of the collection optics was determined using two calibration lamps. A deuterium lamp was used to calibrate the RSR of the system from 318 to 370 nm, and a tungsten lamp was used to calibrate the RSR above 350 nm. The output from the spectrograph was normalized to the manufacturer-provided calibration lamp spectrum to determine the RSR, shown in Fig. 5. The RSR is highest at 319 nm and decreases with increasing wavelength to about 10% of that value at 563 nm.
4 Fluorescence measurement results
In this work, the cell is heated to a set temperature, and the fluorescence signal is measured as total pressure is varied. Fluorescence signal was measured by imaging the spectrograph output using the ICCD camera, which accumulated signal over a minimum of 400 laser pulses to improve the signal to noise ratio. At higher temperatures, up to 1000 images were accumulated due to low signal levels. The image was corrected with a background image taken with only bath gas flowing through the cell. The fluorescence spectrum was then determined by summing the middle 100 rows of the background-corrected image and normalizing for the RSR.
4.1 Fluorescence spectrum
Previous work with acetone has found that its fluorescence spectrum is invariant with temperature [3], and this assumption had been carried over to 3-pentanone [4]. While measurements have shown that this assumption is true for 3-pentanone fluorescence of pure vapor up to 642 K [18], in the presence of nitrogen there is clear variation with temperature. Figure 6 shows fluorescence spectra for 248- and 308-nm excitation at 1.3 and 25 bar in nitrogen with a 3-pentanone partial pressure of 20 mbar for temperatures between 298 and 745 K. For 308-nm excitation, the peak shifts slightly to the blue, and the spectrum to the left of the peak widens by about 20 nm between 298 and 745 K, while the spectrum remains nearly unchanged to the right of the peak. This blue shift may be the result of fluorescence from higher vibrational energy levels in the upper electronic state. In addition, at a fixed temperature, the addition of nitrogen pressure has no effect on the spectrum, showing that vibrational relaxation has little effect on the upper state population at this excitation wavelength.
For 248-nm excitation, the same blue shift in the peak occurs as for 308-nm excitation. Unlike 308-nm excitation, though, the fluorescence spectrum widens on both sides of the peak. In addition, the curvature of the spectrum to the left of the peak changes with increased temperature. The change in fluorescence spectrum shape may be due to the rapid increase in the collisionless non-radiative rate k nr at high vibrational energies [18]. Increasing the nitrogen pressure significantly narrows the fluorescence spectrum and restores the original spectral curvature, illustrating the significant effect of vibrational relaxation. These results show that temperature and nitrogen pressure have an effect on the shape of the fluorescence spectrum, contrary to previous assumptions. Measurements also show that the replacement of nitrogen with air has no effect on the fluorescence spectrum, reaffirming that oxygen has little effect on the fluorescence spectrum, in accordance with previous work [4].
4.2 FQY measurements
To determine the total fluorescence signal, the measured spectrum was integrated across the spectrograph range and corrected for laser energy and absorption of the beam energy as it traverses the cell. The absolute value of FQY can be determined by calibrating the total signal normalized for tracer number density and the laser energy to the known FQY at a reference condition. Because of the availability of absolute FQY measurements for 20 mbar of 3-pentanone in nitrogen up to 650 K and air up to 490 K at 1.3-bar total pressure [18], the 1.3-bar pressure condition was chosen as the reference for those temperatures. For temperatures higher than 650 K, the reference condition was chosen to be 1.3 bar and 295 K. In this case, it is also necessary to normalize the energy and number density normalized fluorescence signal for absorption cross section, which was determined using a published expression [19].
Measurements of 3-pentanone FQY were made for 20 mbar of 3-pentanone in nitrogen from 295 to 750 K for pressures of 1 to 25 bar, and in air from 295 to 570 K for pressures of 1 to 25 bar, shown in Figs. 7 and 8, respectively. The primary sources of uncertainty are the value of the absolute FQY at the reference condition and the measurement of fluorescence signal normalized for laser energy, resulting in an overall uncertainty of about ±7%.
There are previous measurements of relative FQY variation with pressure across a range of temperatures and pressures for 248-, 266-, 277-, and 308-nm excitation in nitrogen and air to which the current measurements can be compared [9–12, 17]. For comparison purposes, these previous measurements are scaled to absolute FQY in the same manner as the data in the current work. Figure 7 shows a comparison of this work with previous data in a nitrogen bath gas. Measurements of relative FQY variation with pressure up to 6 bar are available for room temperature and 248-, 266-, and 308-nm excitation, and these are in excellent agreement with the current work [17]. In addition, data are available at higher temperatures for 266-nm excitation from Modica et al. [11], though these show a significantly larger increase in FQY with pressure than the current work. The discrepancy may be due to the high laser pulse energy (28 mJ) utilized by Modica et al. This previous work also presents measurements in air that are inconsistent with the current work and other previous work, as the following comparison shows.
Figure 8 shows a comparison of the current measurements to previous work in an air bath gas and, generally, the current work is consistent with previous work. The largest inconsistency is seen with Modica et al. [11]. As with the measurements in nitrogen, previous measurements in air for 266-nm excitation show a significantly larger increase in FQY with pressure than the current work. However, the current work agrees well with previous measurements by Fujikawa et al. for 266- and 248-nm excitation [9], as well as for previous data up to 6 bar at room temperature by Koch [17]. At higher temperatures, work by Braeuer et al. at 423 K is 15% higher than the current data, while measurements at 573 K are consistent with measurements at 562 K by Braeuer et al. [12]; the source of this discrepancy is not immediately apparent. Finally, measurements by Grossman et al. using 248- and 277-nm excitation are in good agreement up to 473 K, but show a 10% decrease in signal with pressure at 523 K [10]. This discrepancy may be due to oxidation of 3-pentanone, which may occur at this temperature since the gases were held at elevated temperatures in a static optical cell in the previous work.
5 Model development
In addition to the FQY measurements described in the previous section, quantitative 3-pentanone PLIF diagnostics require the development of a FQY model. In a companion effort, low-pressure FQY data were used to optimize the fluorescence rate (k f) and non-radiative rate (k nr), which are associated with collisionless processes [18]. The vibrational relaxation cascade parameter for 3-pentanone self-collisions (α 3p) was also optimized. The remaining parameters relating to collisional processes are nitrogen quenching rate (\(k_{\mathrm{N}_{2}}\)), oxygen quenching rate (\(k_{\mathrm{O}_{2}}\)), and the vibrational relaxation cascade parameters for nitrogen and oxygen collisions (\(\alpha_{\mathrm{N}_{2}}\) and \(\alpha_{\mathrm{O}_{2}}\)). The current work provides 3-pentanone FQY data across a wide range of pressure, temperatures, and excitation wavelengths in nitrogen and air, allowing for optimization of these collisional parameters.
The optimization of the model parameters relating to collisional quenching and vibrational relaxation is complicated by the fact that the FQY can be simultaneously affected by these processes. Thus, determination of these parameters involves a strategy, determined by sensitivity analysis, to best isolate temperature, pressure, and excitation-wavelength regimes where one process dominates the others.
The behavior of the 3-pentanone FQY in nitrogen was considered first because only one bath gas molecule is present. As the variation of FQY with pressure may depend on both vibrational relaxation and collisional quenching, a sensitivity analysis of the FQY to \(\alpha_{\mathrm{N}_{2}}\) was performed over a range of pressures, temperatures, and excitation wavelengths to determine under what conditions vibrational relaxation is most significant. The sensitivity is given by
For this analysis, the FQY and \(\alpha_{\mathrm{N}_{2}}\) were calculated using Koch’s model [17]. The derivative was approximated using a central difference method. The results of this analysis for 248- and 308-nm excitation are shown in Fig. 9.
Sensitivity of 3-pentanone FQY to relaxation parameter α for (a) 248-nm excitation and (b) 308-nm excitation for a range of temperatures and nitrogen pressures. The partial pressure of 3-pentanone is 20 mbar. Note that the sensitivity for 248-nm excitation is significantly higher than for 308-nm excitation
One result from this sensitivity analysis is that the sensitivity of the FQY to \(\alpha_{\mathrm{N}_{2}}\) is over an order of magnitude smaller for 308-nm excitation than for 248-nm excitation, showing that vibrational relaxation has almost no effect on the FQY for 308-nm excitation. This result is expected at this wavelength because the molecule is excited to a vibrational energy only slightly higher than the thermal energy level E thermal and is essentially fully relaxed. The sensitivity analysis indicates that an effective way to investigate the collisional quenching rate \(k_{\mathrm{N}_{2}}\) without interference from vibrational relaxation is to use 308-nm excitation data in nitrogen.
The sensitivity analysis also indicates that the FQY is more sensitive to \(\alpha_{\mathrm{N}_{2}}\) at higher temperatures and pressures. For 248-nm excitation, the sensitivity begins to increase around 400 K, corresponding to an E 1 of 11400 cm−1 per (2), and the sensitivity is largest at around 800 K and 15 bar. As the molecule relaxes to lower vibrational levels, the likelihood of the molecule fluorescing increases due to a declining value of k nr with lower vibrational energy. Larger decreases in k nr with lower vibrational energy result in a greater increase in FQY from vibrational relaxation. k nr begins increasing rapidly around 11000 cm−1, corresponding to the increase in sensitivity at 400 K [18]. This analysis thus indicates that FQY data at high temperature and short excitation wavelength conditions, corresponding to a higher vibrational energy E 1, are more sensitive to vibrational relaxation and should be used to optimize \(\alpha_{\mathrm{N}_{2}}\).
Once \(k_{\mathrm{N}_{2}}\) and \(\alpha_{\mathrm{N}_{2}}\) have been determined, FQY data in air can be used to determine \(\alpha_{\mathrm{O}_{2}}\) and \(k_{\mathrm{O}_{2}}\). A sensitivity analysis of the FQY in air to \(\alpha_{\mathrm{O}_{2}}\) yields similar results as for \(\alpha_{\mathrm{N}_{2}}\) described previously. In addition, because \(k_{\mathrm{O}_{2}}\) can be represented using a simple bimolecular rate expression [17], optimization is best performed with long excitation wavelength data, and \(\alpha_{\mathrm{O}_{2}}\) optimization with high-temperature, short excitation wavelength data, as well as high-pressure data in air.
In summary, the sensitivity analysis provides a plan for optimizing the model parameters related to collisional processes. To minimize the effect of vibrational relaxation, the collision quenching parameters \(k_{\mathrm{N}_{2}}\) and \(k_{\mathrm{O}_{2}}\) were optimized first using long excitation wavelength FQY data in nitrogen and air, respectively. Further \(k_{\mathrm{N}_{2}}\) optimization utilized atmospheric pressure data in nitrogen, another condition where vibrational relaxation has a lesser effect, while \(k_{\mathrm{O}_{2}}\) optimization also utilized high-pressure data in air. Short excitation wavelength FQY data, especially at higher temperatures, were then utilized for the optimization of \(\alpha_{\mathrm{N}_{2}}\) and \(\alpha_{\mathrm{O}_{2}}\).
5.1 Nitrogen quenching
Following the optimization strategy, the current work investigates nitrogen quenching using FQY data in nitrogen with 308-nm excitation over a wide range of temperatures. An examination of FQY data in nitrogen for 308 nm plotted in Fig. 7 shows that it remains approximately constant with nitrogen pressure for temperatures from 295 to 750 K. A comparison of 1.3-bar FQY measurements with data taken at high pressures and a range of pressures in an optical engine further illustrates the constancy of the FQY with nitrogen pressure [15]. These data are plotted versus E 1 in Fig. 10 and show that, at a given vibrational energy, the FQY is nearly constant with pressure. The optimization of \(k_{\mathrm{N}_{2}}\) can thus utilize 308-nm excitation data in nitrogen at any pressure, with only consideration of E 1 being necessary. Moreover, the data confirm that vibrational relaxation has no significant effect on the FQY.
3-pentanone absolute FQY for pure vapor and in nitrogen at various pressures as a function of initial vibrational energy E 1. Comparison with engine FQY data at high pressures shows little pressure dependence for 308-nm excitation [14, 15]. Quenching is apparent from 7000 to 12500 cm−1. Addition of an energy-dependent nitrogen quenching rate \(k_{\mathrm{N}_{2}}\) improves model performance
If vibrational relaxation has little effect on the FQY at 308 nm, and no collisional quenching occurs, then one would expect that the FQY for 308-nm excitation in a nitrogen bath gas at a given pressure would be identical to the FQY for pure 3-pentanone at low pressures, where collisional effects are insignificant. Figure 10 also shows a comparison between the 308-nm data in nitrogen and the pure vapor FQY data from [18]. Up to a vibrational energy of about 7000 cm−1, the data agree well, confirming that no collision quenching is occurring. However, between 7000 and 12500 cm−1, the FQY in nitrogen decreases below the low-pressure data, confirming that introduction of nitrogen has a quenching effect.
Examination of the data in Fig. 10 allows for the development of an overall rate expression for \(k_{\mathrm{N}_{2}}\). It is apparent that \(k_{\mathrm{N}_{2}}\) has vibrational energy dependence, but apparently has very little dependence on nitrogen number density due to the constancy of the FQY with pressure for 308-nm excitation. The vibrational energy dependence is best fitted using a double Gaussian function, and a weak pressure dependence term was added to maintain the model fit to high-pressure FQY data in nitrogen for 308-nm excitation. Additional examination of model agreement to FQY data at 1.3 bar in nitrogen motivates the inclusion of a temperature-dependent term. The final expression for the quenching rate is shown in Table 1. The Koch model did not have a term for nitrogen collisional quenching (i.e. \(k_{\mathrm{N}_{2}}= 0\)). Figure 10 compares the model with and without \(k_{\mathrm{N}_{2}}\) nitrogen quenching to FQY data; the addition of k N2 greatly improves the fit of the model to the data.
The developed \(k_{\mathrm{N}_{2}}\) expression differs significantly from the expression previously used for the oxygen quenching rate \(k_{\mathrm{O}_{2}}\) [17]. Quenching is hypothesized to occur via the formation of an intermediate complex between the collider and the excited molecule. The rate \(k_{\mathrm{O}_{2}}\) for ketones is modeled as proportional to the collision frequency and, thereby, the number density of oxygen due to the stability of the oxygen-excited ketone complex [23, 24]. However, for other molecules, such as derivatives of anthracene, the quenching rate has been found to be non-linear with oxygen partial pressure [25]. Thus, because the expression for \(k_{\mathrm{N}_{2}}\) is not bimolecular, it is likely the nitrogen-excited 3-pentanone complex is less stable than that for oxygen. In addition, other quenching mechanisms, such as static quenching, may also play a role in the unusual nitrogen pressure dependence [26]. Further investigation of the quenching mechanism for nitrogen may yield a more physical expression for the nitrogen quenching rate, instead of the empirical expression used in this work.
5.2 Oxygen quenching
For ketones and other organic molecules, oxygen quenching is a well-documented effect [23]. Oxygen quenching is effective in ketones because of interaction between the electronic levels of the oxygen molecule and the ketone molecule. The quenching rate \(k_{\mathrm{O}_{2}}\) has been previously modeled using a bimolecular collision rate [17], given by
where \(q_{\mathrm{O}_{2}}\) is the vibrational energy-dependent quenching efficiency, \(Z_{3\mathrm{p}\mbox{\scriptsize-}\mathrm{O}_{2}}\) is the collision frequency calculated using the Lennard–Jones formulation, and \(n_{\mathrm{O}_{2}}\) is the oxygen number density.
The sensitivity analysis indicated that the oxygen quenching rate is best optimized utilizing FQY data with 308-nm excitation in air, as well as data at high air pressures. The optimized expression for \(q_{\mathrm{O}_{2}}\) from the current work and that from previous work [17] are shown in Table 2. The current work found that the exponential expression used by the Koch model, with some adjustment, allows the model to reproduce the FQY data in air. However, the Koch model predicted a step increase in \(q_{\mathrm{O}_{2}}\) at 11000 cm−1, while the current work uses a purely exponential form, as shown in Fig. 11. Since the previous work also used FQY data in air to optimize \(q_{\mathrm{O}_{2}}\) at high vibrational energies, the step increase in \(q_{\mathrm{O}_{2}}\) may be the result of the assumption that nitrogen quenching does not occur.
Variation of \(q_{\mathrm{O}_{2}}\) with vibrational energy. Comparison to previous work, which utilized a step increase in \(q_{\mathrm{O}_{2}}\) for vibrational energies greater than 11000 cm−1, is shown [17]
5.3 Vibrational relaxation
Per the sensitivity analysis, expressions for \(\alpha_{\mathrm{N}_{2}}\) and \(\alpha_{\mathrm{O}_{2}}\) were determined by optimization with FQY data in nitrogen and air, respectively, at 248-nm excitation, as well as data at high temperatures and high pressures. Previous iterations of the 3-pentanone FQY model have utilized temperature-dependent expressions for \(\alpha_{\mathrm{N}_{2}}\) and \(\alpha_{\mathrm{O}_{2}}\) [11–13, 17], which is supported by previous relaxation studies in nitrogen. Unlike the previous 3-pentanone fluorescence models, however, this work will also consider vibrational energy dependence of α. Previous work with toluene, azulene, and pyrazine has found that the rate of energy loss changes as the vibrational energy approaches the thermal energy level [27–29]. The availability of FQY data in air and nitrogen will allow for examination of both the temperature and energy dependences of \(\alpha_{\mathrm{N}_{2}}\) and \(\alpha_{\mathrm{O}_{2}}\).
To examine the temperature dependence, FQY data in nitrogen and air at shorter wavelengths (248 nm) and lower temperatures up to 450 K, as well as longer wavelengths (266 and 277 nm) and temperatures up to 650 K, were used in the optimization. These data were chosen to examine a wide range of temperatures, but avoid E 1 greater than about 11000 cm−1. At each temperature, optimum values of \(\alpha_{\mathrm{N}_{2}}\) and \(\alpha_{\mathrm{O}_{2}}\) were determined, and these optimum values were fitted using an exponential function.
Vibrational energy dependences of \(\alpha_{\mathrm{N}_{2}}\) and \(\alpha_{\mathrm{O}_{2}}\) were considered using short-wavelength, high-temperature FQY data. To illustrate the importance of including energy dependence, Fig. 12 shows FQY variation with pressure in nitrogen for a temperature of 745 K and various excitation wavelengths. The plot also shows the model with the temperature-dependent exponential function for \(\alpha_{\mathrm{N}_{2}}\) and \(\alpha_{\mathrm{O}_{2}}\). As the excitation wavelength decreases, the model with only temperature dependence begins to underpredict the increase in FQY with pressure, indicating that at higher vibrational energies, relaxation occurs more efficiently. To account for this effect, an exponential term with a near-unity value at lower vibrational energies, but with increasing effect at higher vibrational energies, was optimized using 248-nm data at higher temperatures and multiplied to the temperature-dependent term. Optimized expressions for \(\alpha_{\mathrm{N}_{2}}\) and \(\alpha_{\mathrm{O}_{2}}\) are shown in Table 3, along with those from previous work [17], and the expressions for \(\alpha_{\mathrm{N}_{2}}\) are plotted as a function of temperature for varying wavelengths in Fig. 13. The figure shows that, for 300–550 K, \(\alpha_{\mathrm{N}_{2}}\) decreases with temperature for all wavelengths but, for higher temperatures, \(\alpha_{\mathrm{N}_{2}}\) increases with temperature. This increase is higher for shorter laser wavelengths, reflecting the effect of the vibrational energy dependence. Similar trends are observed for \(\alpha_{\mathrm{O}_{2}}\). In the previous work, \(\alpha_{\mathrm{N}_{2}}\) decreased with temperature and was independent of wavelength. As Fig. 12 shows, the inclusion of vibrational energy dependence allows for a better fit between the model and the data.
Plot of the expression for \(\alpha_{\mathrm{N}_{2}}\) as a function of temperature for various laser excitation wavelengths. The expression from the previous work is plotted for comparison [17]
5.4 Model performance with cell data
The updated parameters for the FQY model are summarized in Table 4, and these parameters are applied to the model structure as described in Sect. 2. The non-radiative rate k nr, fluorescence rate k f, and vibrational relaxation cascade parameter α 3p for 3-pentanone self-collisions are taken from low-pressure work described in [18]. The performance of the 3-pentanone FQY model using updated collisional parameters can be compared to FQY data taken over a range of pressures, temperatures, and excitation wavelengths in air and nitrogen as shown in Figs. 7 and 8, respectively. The updated model provides excellent agreement with the FQY measurements, and agrees within 10% across the indicated conditions. The model shows the weakest agreement with FQY data taken with 248-nm excitation at 540 K in nitrogen, 248-nm excitation at 430 K in air, and 266-nm excitation at 480 K in air. These discrepancies are likely due to the representation of \(k_{\mathrm{N}_{2}}\) as a complex empirical fit. A further understanding of the nitrogen quenching process would likely yield improved model agreement with the FQY data at these conditions.
The agreement obtained with the current model is a significant improvement over that found with the Koch model, which is also plotted in the figures for comparison. In nitrogen, the Koch model significantly overpredicts the increase in FQY with pressure at temperatures above room temperature, which may be the result of the exclusion of nitrogen quenching. In air, the Koch model predicts a greater decrease in FQY with pressure, especially for the 248- and 266-nm excitation, which is likely the result of the step increase of \(q_{\mathrm{O}_{2}}\).
5.5 Model performance with optical ICE data
In addition to optical cell data, additional relative fluorescence signal per molecule (S* LIF) data is available from optical ICE studies for comparison to the FQY model. Optical engines are able to access simultaneous high-pressure and high-temperature conditions beyond those accessed here with an optical cell. Furthermore, this comparison can confirm the applicability of the model to optical ICE diagnostics. To compare the model to optical ICE data, the FQY determined from the model is multiplied with the absorption cross section calculated using the expression in [19] and normalized to a reference condition to determine relative S* LIF.
The current model performs significantly better than the Koch model over ICE conditions. Figure 14 shows a comparison of the two models with measurements made in an optical ICE for 277- and 308-nm excitation over a temperature range from 400 to 810 K and pressures from 1.3 to 16.4 bar [6, 8, 13]. As the figure shows, the current model reproduces the measured S* LIF within 10% for all temperatures, even predicting the decrease in signal for 277-nm excitation beginning at 500 K. The Koch model greatly overpredicts the fluorescence signal temperatures beyond 650 K, in contrast with the current model, thus demonstrating the advances in predicting fluorescence at top dead center conditions from the current work.
Similar measurements for 277- and 308-nm excitation were performed at even higher pressures and temperatures in another engine study [14, 15], shown in Fig. 15. The pressure and temperature ranges for these measurements are 560 to 1120 K and 4.5 to 28.1 bar, respectively. The Koch model poorly predicts the S* LIF variation, especially at temperatures greater than 700 K. The current model improves upon this performance and is able to reproduce the variation of signal within 10% up to 1100 K. At temperatures higher than 1100 K, there is a slight decline in measured fluorescence signal as compared to the model, which may be the result of 3-pentanone pyrolysis or oxidation. Nonetheless, the current work extends the model to temperature and pressure conditions significantly beyond the previous work, providing useful support for ICE diagnostics.
Comparison of 3-pentanone S* LIF for 277- and 308-nm excitation from an optical ICE [15] to values predicted by the FQY model from the current work show excellent agreement up to 28 bar and 1100 K. The bath gas is (a) nitrogen and (b) air. Comparison to the previous model is also shown [17]. Error bars are equivalent to 10%
6 Conclusion
FQY measurements were made for 20 mbar of 3-pentanone in nitrogen for temperatures from 298 to 745 K and pressures of 1 to 25 bar and in air for temperatures from 298 to 570 K and pressures from 1 to 25 bar. 248-, 266-, 277-, and 308-nm excitation wavelengths were utilized in the current work. Absolute FQY values were determined by scaling to Rayleigh scattering calibrated measurements made at atmospheric pressure. The data are consistent with previous measurements from previous work up to 600 K. The current work extends the range of temperature and pressure for which FQY data are available beyond that of previous work, allowing for quantitative PLIF diagnostics for a variety of flow fields, including ICEs.
Collisional parameters in the FQY model were optimized using data from the current work according to a strategy developed through sensitivity analysis. In particular, the nitrogen and oxygen quenching rates \(k_{\mathrm{N}_{2}}\) and \(k_{\mathrm{O}_{2}}\), as well as the vibrational relaxation cascade parameters \(\alpha_{\mathrm{N}_{2}}\) and \(\alpha_{\mathrm{O}_{2}}\), were updated. The current work shows that nitrogen quenching, previously assumed to be non-existent, does occur for 3-pentanone. Moreover, \(\alpha_{\mathrm{N}_{2}}\) and \(\alpha_{\mathrm{O}_{2}}\) were found to have vibrational energy dependence in addition to temperature dependence. This new model agrees with measurements from the current work within 10% across all temperature, pressure, and excitation-wavelength conditions. This performance is an improvement over that of the Koch model, especially at combined high-temperature and high-pressure conditions.
The model also shows good agreement with measurements of fluorescence signal in optical ICEs. For engine measurements with 277- and 308-nm excitation up to 1100 K, agreement is within 10%, a significant improvement over the Koch model. These comparisons establish that the new model is valid for the high-temperature and high-pressure conditions found in ICEs and a useful improvement over previous models.
One needed area of future work is to extend the temperatures at which FQY measurements in air and oxygen are available. The current work was limited to 570 K in air due to evidence that oxidation was occurring at temperatures above 600 K, even with a residence time of 0.2 s, and no measurements were made in oxygen. Another needed area is the investigation of the nitrogen quenching mechanism, which does not appear to follow the simple bimolecular collision behavior of oxygen quenching. An improved quenching mechanism would assist in improving the performance of the FQY model.
References
C. Schulz, V. Sick, Prog. Energy Combust. Sci. 31, 75 (2005)
A. Lozano, B. Yip, R. Hanson, Exp. Fluids 13, 369 (1992)
M. Thurber, F. Grisch, B. Kirby, M. Votsmeier, R. Hanson, Appl. Opt. 37, 4963 (1998)
J.D. Koch, R.K. Hanson, Appl. Phys. B 76, 319 (2003)
S. Einecke, C. Schulz, V. Sick, Appl. Phys. B 71, 717 (2000)
D. Rothamer, J. Snyder, R. Hanson, R. Steeper, SAE Int. J. Fuels Lubr. 1, 520 (2009)
J.A. Snyder, R.K. Hanson, R.P. Fitzgerald, R.R. Steeper, SAE Int. J. Engines 2, 460 (2009)
D. Rothamer, J. Snyder, R. Hanson, R. Steeper, Appl. Phys. B, Lasers Opt. 99, 371 (2010)
T. Fujikawa, Y. Hattori, K. Akihama, SAE Tech. Pap. Ser. 41, 972944 (1997)
F. Grossman, P.B. Monkhouse, M. Ridder, V. Sick, J. Wolfrum, Appl. Phys. B 62, 249 (1996)
V. Modica, C. Morin, P. Guibert, Appl. Phys. B 87, 193 (2007)
A. Braeuer, F. Beyrau, A. Leipertz, Appl. Opt. 45, 4982 (2006)
D.A. Rothamer, Ph.D. thesis, Stanford University, 2007
J.A. Snyder, Appl. Opt. (2012), in progress
J.A. Snyder, Ph.D. thesis, Stanford University, 2011
W. Koban, J. Koch, V. Sick, N. Wermuth, R.K. Hanson, C. Schulz, Proc. Combust. Inst. 30, 1545 (2005)
J.D. Koch, Ph.D. thesis, Stanford University, 2005
B.H. Cheung, R.K. Hanson, Appl. Phys. B (2011). doi:10.1007/s00340-011-4817-4
J. Koch, J. Gronki, R.K. Hanson, J. Quant. Spectrosc. Radiat. Transf. 109, 2037 (2008)
J.D. Koch, R.K. Hanson, W. Koban, C. Schulz, Appl. Opt. 43, 5901 (2004)
J. Troe, J. Chem. Phys. 66, 4758 (1977)
G.B. Rieker, X. Liu, H. Li, J.B. Jeffries, R.K. Hanson, Appl. Phys. B 87, 169 (2006)
W.M. Nau, J.C. Scaiano, J. Phys. Chem. 100, 11360 (1996)
R.G. Brown, D. Phillips, J. Chem. Soc. Faraday Trans. II 70, 630 (1974)
G. Zalesskaya, A. Kuchinskii, J. Appl. Spectrosc. 75, 36 (2008)
J.R. Lakowicz, B.R. Masters, J. Biomed. Opt. 13, 029901 (2008)
H. Hippler, J. Troe, H.J. Wendelken, J. Chem. Phys. 78, 6709 (1983)
H. Hippler, B. Otto, J. Troe, Ber. Bunsenges. Phys. Chem. 93, 428 (1989)
L.A. Miller, J.R. Barker, J. Chem. Phys. 105, 1383 (1996)
Acknowledgements
This work was funded by the Air Force Office of Scientific Research (Aerospace, Chemical, and Material Sciences Directorate), with Dr. Julian Tishkoff as the technical monitor.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheung, B.H., Hanson, R.K. 3-pentanone fluorescence yield measurements and modeling at elevated temperatures and pressures. Appl. Phys. B 106, 755–768 (2012). https://doi.org/10.1007/s00340-012-4901-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-4901-4