Abstract
3-Pentanone photophysics measurements and subsequent fluorescence quantum yield (FQY) model development are presented. A heated, flowing optical cell and laser excitation at 248, 266, 277, and 308 nm were utilized, allowing investigation of FQY and absorption cross-section values for 3-pentanone vapor from 298 to 690 K and 10 to 30 mbar. Measurements of FQY were also made for 20 mbar of 3-pentanone at 1.3 bar total pressure in nitrogen from 298 to 530 K and in air from 298 to 487 K. Absolute FQY was determined by calibration to Rayleigh scattering of nitrogen gas. Based on these FQY measurements and fluorescence lifetime data from previous work, the fluorescence rate k f was determined to be 3.70×105 s−1. The current work extends knowledge of the non-radiative rate k nr to vibrational energies of 15000 cm−1, and the expression for k nr was optimized to include a fit to these new data points. Finally, variation of FQY with 3-pentanone vapor pressure was used to optimize the vibrational relaxation cascade parameter α 3p . The updated FQY model for 3-pentanone vapor shows agreement within 8% to the current FQY data across the investigated range of temperatures and pressures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tracer-based planar laser-induced fluorescence (PLIF) is a powerful spatially resolved, temporally resolved, and species-specific diagnostic for the measurement of flow field properties [1]. Various classes of hydrocarbons have been investigated for use in PLIF diagnostics, and ketones, such as acetone and 3-pentanone, have become commonly used tracers for the application of PLIF diagnostics to laboratory flow fields and energy conversion devices.
This work focuses on 3-pentanone, a ketone tracer popular for measurements in optically accessible internal combustion engines (ICEs) owing to its desirable properties. It is relatively easy to handle due to its relatively low toxicity and low cost and it has a similar boiling point as iso-octane, a surrogate fuel often used in ICE studies. Photophysically, 3-pentanone has an ultraviolet (UV) broadband absorption spectrum that extends from 220 to 350 nm with a peak at around 280 nm, allowing excitation using commercially available high-powered UV pulsed lasers. The fluorescence spectrum spans 320 to 600 nm and peaks around 410 nm, so fluorescence signal is easily captured using charge-coupled-device (CCD) cameras.
Due to these favorable properties, 3-pentanone has been employed in numerous ICE studies. 3-pentanone PLIF has been utilized to study various aspects of engine operation, such as temperature distribution and exhaust gas residual concentration [1–4]. However, the accuracy of these measurements is heavily dependent on knowledge of the absorption cross section and fluorescence quantum yield (FQY), also known as photophysical parameters. In particular, measurements and modeling of the FQY across a wide range of conditions are necessary for quantitative PLIF imaging of systems like ICEs.
2 FQY model background
Quantitative PLIF imaging is based on the linear fluorescence equation, which describes the emitted fluorescence signal:

In this equation, E is the incident laser energy fluence, h is Planck’s constant, c is the speed of light in vacuum, λ is the laser wavelength, n is the number density of fluorescing tracer, L is the length of the illuminated volume, Ω is the detector collection angle, and η is the detector collection efficiency. The absorption cross section σ and the FQY ϕ are photophysical parameters that describe the propensity for a molecular to absorb and emit photons, respectively, when excited by a laser of wavelength λ. As the equation shows, the dependence of the fluorescence signal on the flow field properties of temperature T, pressure p,3-pentanone mole fraction X 3p, and mole fraction of other bath gases X M , is defined by the number density and photophysical parameters.
Because the pressure, temperature, and compositional dependencies of number density are easily predicted using the ideal gas equation, the key to predicting temperature, pressure, and compositional dependencies of fluorescence signal is knowledge of the photophysical parameters. The temperature dependence of the absorption spectrum and cross section has been studied up to 1000 K, and the absorption spectrum has been found to be well-predicted using a Gaussian fit [5]. There has also been experimental work in optical cells measuring the temperature and pressure dependencies of FQY [6–11]. However, most studies utilize a single laser wavelength and are limited to temperatures below 600 K. A step-ladder FQY model was proposed that was optimized from this limited set FQY data [12], but this model has been found to be inadequate at the combined high-pressure and high-temperature conditions produced in ICE studies [13], which also limits its potential application in other energy conversion devices, such as gas turbine combustors.
The energy diagram on which the step-ladder model is based is shown in Fig. 1. The unexcited molecule resides in the ground rovibronic singlet state (S 0) at the total vibrational energy E thermal. Upon absorption of a photon, the molecule enters the excited singlet state (S 1) with vibrational energy given by
where λ is the wavelength of the photon in nanometers and \(E_{0\mbox{--}0}\) is the zero-point energy difference between the excited and ground singlet states, which is 30770 cm−1 for 3-pentanone [9]. E thermal is the thermal vibrational energy calculated by summing the contributions of the 42 vibrational modes of 3-pentanone at the local temperature [13]; energy units are cm−1.
From this initial vibrational energy level E 1, the molecule can relax through a sequence of vibrational energy levels E n . This sequence due to relaxation is described by
where α is the species-specific vibrational relaxation cascade parameter and n is the nth step in the vibrational energy cascade. For mixtures of collision partners, the overall cascade parameter is calculated by the collision frequency-weighted average of the species-specific cascade parameters. In the absence of fluorescence or transition to another electronic state, the relaxation continues until the molecule reaches the Nth vibrational energy level in the cascade, with energy E thermal.
The excited singlet can be depopulated via collisionless or collisional processes. Collisionless processes do not depend on collisions between molecules, and such processes include fluorescence, represented by the fluorescence rate k f , as well as intersystem crossing (ISC) and internal conversion (IC), which are represented together by the non-radiative rate k nr . Collisional processes that result from molecular collisions between the excited molecule and bath gases can also de-excite the excited molecule. Previous work by Koch considered oxygen quenching, represented by the rate k O2 [12]. One can also consider quenching by nitrogen, represented by the rate k N2. The quenching rates of other molecules, such as carbon dioxide and water, were not considered in the model because they are often present in significantly lower concentrations than oxygen or nitrogen in many 3-pentanone PLIF applications. The rate of vibrational relaxation, which is also a collisional process, but does not de-excite the singlet, is represented by the rate k vib.
The overall FQY can then be calculated using the equation
In this equation, the contribution to FQY ϕ n from each step n in the vibrational energy cascade is weighted by the probability of relaxing to step n from step 1. This probability is computed by the multiplication of the probability of relaxation from step j(P J ) over the vibrational energy cascade.
The contribution to FQY from a given vibrational level n with energy E n is
For ϕ N , where the molecule is at the Nth step of the vibrational energy cascade with energy E thermal,k vib is set to zero because the molecule can no longer relax. The probability that the molecule at vibrational level n successfully relaxes to level n+1 is given by
The optimization of these model parameters by Koch primarily used FQY data at pressure conditions of 1 bar or greater [12]. However, at these pressures, collisional and collisionless effects are both significant, and insight into any particular process is difficult. A sensitivity analysis reveals that the collisionless non-radiative rate k nr is most sensitive to low-pressure FQY data. The sensitivity of the FQY to k nr is calculated by
Figure 2 shows the calculated sensitivity of the FQY to k nr for excitation wavelengths of 248 and 308 nm for 20 mbar of 3-pentanone in nitrogen. The FQY is most sensitive to k nr with no added nitrogen for both excitation wavelengths, and the sensitivity drops significantly with the addition of nitrogen. At low pressures, the FQY is dominated by k nr because it is unaffected by collisions, but as nitrogen is added, collisional processes begin affecting the FQY, reducing sensitivity.
Sensitivity of 3-pentanone FQY to non-radiative rate k nr at initial vibrational energy E 1 for (a) 248 nm excitation and (b) 308 nm excitation for a range of temperatures and nitrogen pressures calculated with the Koch model [12]. The partial pressure of 3-pentanone is 20 mbar. The FQY is most sensitive to k nr with no added nitrogen
The use of low-pressure data greatly simplifies the fluorescence model. The collisional rates k O2, k N2, and k vib in the FQY model are small compared to the non-radiative rate k nr . Because vibrational relaxation is also considered insignificant at low pressures, there is no cascade of vibrational energy, and k nr only needs to be considered at the initial energy E 1, calculated from (2). Previous work has also shown the fluorescence rate k f is small compared to the k nr [6, 12]. The FQY can then be calculated by
This simple expression facilitates determination of the fluorescence rate, as well as the vibrational energy dependence of the non-radiative rate, from low-pressure FQY data. Unfortunately, the availability of FQY data low pressures is limited. Koch made measurements at room temperature and low pressure in a static optical cell, and used these data to optimize k f and k nr in his model [12]. Some additional room-temperature, low-pressure data are also available [6]. This work looks to provide a comprehensive set of low-pressure 3-pentanone FQY data over a wide range of temperatures and excitation wavelengths. The measurements will be used to further knowledge of the fluorescence rate k f and the non-radiative rate k nr at higher vibrational energies. In addition, the vibrational relaxation cascade parameter for 3-pentanone collisions α 3p was determined using FQY variation with 3-pentanone pressure, and a model predicting FQY for 3-pentanone vapor is presented.
3 Measurement methodology
3.1 Absorption cross-section measurement methodology
The absorption cross section was determined using the Beer–Lambert equation:
where I 0 is the incident beam intensity, I is the transmitted beam intensity, n is the 3-pentanone number density, L is the path length, and σ is the absorption cross section. In this study, variations in incident and transmitted beam energy were monitored using fast photodiodes. The transmission can be determined by comparing the ratio of the two photodiode measurements with and without 3-pentanone in the cell. The absorption cross section can be calculated using
where I 2 is the transmitted beam energy measurement, I 1 is the split beam measurement, and the ‘0’ subscript denotes a measurement with no 3-pentanone in the cell.
3.2 FQY measurement using Rayleigh scattering
As the linear fluorescence equation indicates, calculating FQY from collected fluorescence signal requires knowledge of the collection angle and efficiencies, both of which are difficult to quantify accurately. As a result, absolute FQY measurements are often determined via calibration of the signal to other known radiative processes to account for these parameters. This work uses Rayleigh scattering as the primary reference source, but due to limitations of the experimental setup at higher temperatures that will be discussed later, room-temperature 3-pentanone FQY is also used as a reference.
When using another fluorescent species as a reference, the FQY can be calculated using
where S LIF is the fluorescence signal, σ is the absorption cross section, and n is the tracer number density. The ‘0’ subscript refers to the quantity measured for the reference species. Hansen and Lee used this method for calibrating 3-pentanone FQY using acetone at 296 K and 313 nm excitation as a Ref. [6]. This method of calibration for measurements of FQY is used in the current work over a limited range of temperatures where the Rayleigh scattering method is not possible due to experimental setup limitations.
Absolute FQY measurements can also be calibrated to Rayleigh scattering of nitrogen, a technique adapted from previous fluorescence saturation concentration measurements [14] and utilized in previous 3-pentanone FQY measurements [9]. Nitrogen was chosen as the scatterer because a significant amount of theory and literature is available to describe the Rayleigh scattering from diatomic molecules [15]. The Rayleigh signal collected orthogonal to the plane defined by the polarization vector and the propagation vector of the laser beam can be described by the following equation:
where E is the incident laser energy fluence, h is Planck’s constant, c is the speed of light in vacuum, λ is the laser wavelength, n Ray is the number density of the scatterer, L is the length of the illuminated volume, (∂σ/∂Ω) is the differential Rayleigh cross section, Ω is the detector collection angle, and η Ray is the collection efficiency. Normalizing the fluorescence signal to Rayleigh signal accounts for collection angle and collection efficiency terms from the calculation of FQY.
However, because 3-pentanone is a broadband emitter and the collection efficiency varies significantly over the fluorescence spectrum, it is necessary to consider the spectral responsivity R(λ). Fortunately, because both the fluorescence and Rayleigh signal will be normalized, the relative spectral responsivity (RSR) can be used instead of absolute responsivity. The RSR is defined as
where λ ref is a reference wavelength. The FQY can then be calculated by

Most parameters in (14) are determined from measurements. However, the differential Rayleigh cross section is determined using literature values. Miles provides a review of Rayleigh scattering theory [15], which states that the differential cross section for diatomic molecules is determined by
where λ is the laser wavelength in nm, F K is the King factor, and (r−1) is the simple index of refraction at the laser wavelength.
The equation is based on electric dipole theory for a simple atomic spherical scatterer, such as argon; the King factor is an anisotropic factor for polyatomic molecules. For this study, the King factor for nitrogen is determined using [16]:
The ratio of the refractive index (r−1) with number density n is proportional to the polarizability of the species. This work utilizes an expression based on a fit to experimental measurements of the refractive index of nitrogen in the UV [17]:
These refractive index measurements were made at 273 K and 1.013 bar, so the number density n is 2.686×1019 cm−3. For (16) and (17), the unit of wavelength λ is nm.
The use of Rayleigh scattering as a reference for absolute FQY measurement requires consideration of polarization for both the laser beam and collected signals. Rayleigh scattering depends on the laser beam polarization, and (14) only applies to a vertically polarized laser beam. Moreover, the polarization of the fluorescence and Rayleigh signals must also be considered. Fluorescence signal is effectively depolarized, but Rayleigh scattering for diatomics like nitrogen is largely, but not completely, polarized. Thus, to obtain an accurate count of total fluorescence or Rayleigh photons, the detector setup must be polarization insensitive. These considerations influence the design of the optical cell system and collection optics described in the following section.
4 Experimental setup
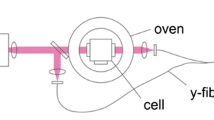
4.1 Optical cell design
The overall experimental setup is shown in Fig. 3. The optical cell body is a commercially available “cube” fitting with high-vacuum copper flange seals. The windows along the axis of the collection optics are UV-grade sapphire windows. For measurements taken between 300 and 560 K, the laser beam passed through UV-grade fused silica viewports; the laser beam path length between these windows is 88.3 mm. These viewports are limited to temperatures of 560 K because the brazing holding the window to the flange melts at around that temperature. For temperatures higher than 560 K, the fused silica viewports were replaced with sapphire viewports due to the higher melting point (720 K) of the brazing. The beam path length between the sapphire ports is 73.8 mm. Fused silica windows were used for most of the measurements because they are amorphic, ensuring low birefringence and maintenance of a laser beam polarization. However, above temperatures of 560 K, Rayleigh scattering can no longer be used as a reference emission due to the use of sapphire viewports, which are crystalline and birefringent. To measure the FQY at these temperatures above 560 K, the fluorescence signal was scaled according to (11), with fluorescence from 3-pentanone at 296 K in nitrogen with a total pressure of 1.3 bar used as the reference.
Gas was introduced into the cell via a flowing system shown in Fig. 3, which was chosen over static systems used in other low-pressure measurements [6, 9] to avoid tracer adsorption on the cell walls, to reduce the risk of pyrolysis effects by decreasing residence in the cell, and to allow for collection of fluorescence signal from multiple laser pulses without photolysis effects. The system was able to introduce both 3-pentanone mixed with nitrogen or air, as well as pure vapor, into the optical cell.
For 3-pentanone and nitrogen/air mixtures, the system introduced bottled nitrogen/air using a mass flow controller. Degassed 3-pentanone liquid was introduced into the gas flow using a metering pump. The pump drew liquid 3-pentanone from a sealed glass flask and injected the liquid into a “T” junction heated to about 150°C. The 3-pentanone vaporized on injection and was entrained by the bath gas flow. Downstream of this junction, a heated mixing chamber filled with stainless steel mesh baffling ensured complete mixing of the 3-pentanone with the bath gas. The mass flow controller provided 2.5 SLPM of nitrogen or air, and the pump provided 0.185 mL/min of 3-pentanone liquid. The residence time of the gas in the optical cell was about 0.2 s. The stream passed through the cell and exhausted into ambient air.
The system was also able to provide a stream of pure 3-pentanone vapor. A 3-way valve directed the exhaust to a mechanical pump instead of ambient air, which drew vapor from the flask of liquid 3-pentanone and through the optical cell. A mass flow controller was used to regulate the flow of the 3-pentanone vapor, and the flow rate was kept constant at 23 SCCM throughout the study.
The gas stream was fed into the top of the optical cell and vented out of the bottom, then exhausted either to the pump or atmosphere. The pressure in the cell was changed using the needle valve at the exhaust. The total pressures of 3-pentanone and nitrogen/air mixtures were measured with a Setra 0–50 psia pressure transducer tapped into the exhaust line. 3-pentanone vapor pressures were measured with a 1000 Torr Baratron pressure transducer directly connected to the optical cell.
The temperature of the cell was varied using a viewport-equipped furnace. The interior gas temperature and the external cell surface temperature were monitored using K-type thermocouples. To ensure temperature uniformity within the cell, the external and internal temperature difference was maintained within 5 K. A computer data acquisition system was used to monitor outputs from the various thermocouples and pressure transducers.
4.2 Laser and collection optics setup
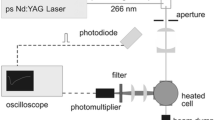
Figure 4 shows the layout of the laser excitation and optical signal collection systems. Four different laser excitation wavelengths were employed in this study: 248, 266, 277, and 308 nm. The 248 nm beam was provided by a krypton fluoride excimer laser. The output from the excimer laser was also used to generate the 277 nm beam using a Raman shifter filled with 19 bar of hydrogen gas. The 277 nm beam was selected from the output of the Raman cell using a Pellin-Broca prism. The 266 nm beam was provided by a frequency-quadrupled Nd:YAG laser, and the 532 nm output of the laser was also used to pump a pulsed dye laser circulating Rhodamine 640 laser dye. The dye laser was tuned to output a 616 nm laser beam, which was frequency doubled to 308 nm using a BBO crystal.
The laser beams were routed to the optical cell using wavelength-appropriate high-efficiency reflectors and were aligned to pass through the center of the optical cell’s windows. Each beam first passed through an iris to reduce it to the aperture of the following optics. The beam then passed through an MgF2 Rochon polarizer with a 14.5 mm diameter aperture. The polarizer was aligned to allow the vertical polarization of the lasers to pass undeviated and deflect the horizontal polarization, which is blocked. The vertically polarized beam was passed through a 1000 mm focal length cylindrical lens to collimate the beam in the vertical direction. The beam was then passed through an iris to reduce the beam diameter to 2 mm diameter. For 248 nm and 266 nm beams, the beam energy was about 0.4 mJ/pulse; for 277 and 308 nm beams, the beam energy was about 0.1 mJ/pulse.
To monitor the laser beam energy, about 10% of the beam energy was diverted using a 3° fused silica wedge to the beam energy detector. The beam was focused into a custom-made aluminum integrating sphere with an internal diameter of 25.4 mm using a 100 mm focal length fused silica lens. A high-speed photodiode monitored the output from the integrating sphere, and the photodiode signal was sent to an oscilloscope, corrected for detector dark current, and integrated to determine laser energy. The laser beam exiting the optical cell was also sent to an identical integrating sphere, photodiode, and oscilloscope setup to monitor output beam energy. The energy measurements were used to normalize the fluorescence signal for laser energy variations and to measure absorption.
Fluorescence signal was collected using a 105 mm UV lens and imaged onto the slit of a spectrograph. The spectrograph grating is blazed at 300 nm with 300 grooves/mm with a spectral resolution of about 4.0 nm/pixel. The output of the spectrograph was imaged using 512×512 pixel intensified charge-coupled-device (ICCD) Princeton Instruments PI-MAX camera. Due to the low density of grooves, the grating is polarization insensitive.
4.3 Experimental setup spectral characterization
The spectrograph wavelength calibration and the RSR of the optical cell and collection optics characteristics are characterized using calibration lamps that are placed as indicated in Fig. 4. For the calibration, only the viewport between the laser beam and the collection optics was installed.
In order to capture the entire 3-pentanone fluorescence and the Rayleigh scattering of the laser beams used in this study, the spectrograph must be used from about 240 to 570 nm, requiring the rotation of the grating turret, and the spectral characterization needs to take this into account. The spectrograph grating was rotated to a center wavelength of 440 nm to measure the fluorescence signal and 340 nm to measure Rayleigh signal. Thus, it is necessary to characterize the detector system for the spectrograph set at 340 and 440 nm center wavelengths.
Calibration of the spectrograph for wavelength was performed with lines from a mercury calibration lamp. With the spectrograph calibrated for wavelength, the RSR of the collection optics was determined using two calibration lamps. A deuterium lamp was used to calibrate the RSR of the system from 220 to 370 nm, and a tungsten lamp was used above 350 nm. The lamp spectra were collected by the ICCD, and the rotation of the spectrograph grating does not influence the collected spectrum, so the system can be characterized by a single RSR curve. To determine the RSR, the lamp spectra were compared to the manufacturer provided spectral photon flux calibration, and arbitrarily normalized to the value at 350 nm. The resulting RSR curve, shown in Fig. 5, peaks at about 270 nm and falls to about 10% of the peak value at 562 nm. The RSR measured by the two calibration lamps agrees well within the overlapping region of 350 to 370 nm.
5 Measurement results
5.1 Absorption cross section
3-Pentanone absorption cross section was determined using (10), and in this calculation, the absorbance is normalized by the 3-pentanone number density. To determine this quantity, measurements of transmission, averaged over 500 laser pulses, were taken at 3-pentanone pressures from 10 to 30 mbar, and the slope of the absorbance plotted against number density was used to calculate absorption cross section.
Figure 6 shows the absorption cross-section measurements for the 248, 266, 277, and 308 nm laser wavelengths at various temperatures. The estimated uncertainty in these experiments is about ±4%, primarily the result of uncertainties in the temperature and beam transmission measurements. These measurements are compared to laser absorption cross-section measurements made in an optical cell [8], absorption spectra measurements made in a shock tube [5], and room-temperature measurements made in a spectrophotometer [18]. Measurements are also compared to a Gaussian fit of the absorption spectrum [5]. There is excellent agreement with previous cell measurements for 248 and 308 nm, but for 266 nm excitation, Koch measured an absorption cross section about 10% lower, which may be the result of local saturation effects due to the non-uniformity of the laser beam used in his work [12]. However, the current work also shows excellent agreement with shock tube and spectrophotometer data, as well as the Gaussian absorption spectral fit, for all wavelengths. These values of absorption cross section will be used to determine the FQY according to (14).
5.2 Fluorescence spectrum
Fluorescence signal was measured by imaging the output from the spectrograph. 500 images were accumulated on the ICCD to improve the SNR, except for measurements at temperatures at 650 K for 277 and 308 nm excitation, where 1000 images were accumulated due to low levels of signal. The acquired image was corrected for background using an image taken with the cell under vacuum. The fluorescence spectrum was calculated from the corrected images by summing the central 100 rows of pixels and correcting for the RSR.
Previous work on ketone photophysics has either found, for acetone, or assumed, for 3-pentanone, that the fluorescence spectrum remained constant with temperature [8, 19]. This work allows investigation of that assumption for several 3-pentanone and nitrogen compositions, and the results show that this assumption applies in certain situations, but is invalid in others. Figure 7 shows fluorescence spectra for several gas compositions and temperatures for 248 and 308 nm excitation. In pure 3-pentanone vapor, the fluorescence spectrum does remain effectively constant with temperature, although a slight blue shift is observed. Moreover, the spectrum is unaffected by increasing 3-pentanone vapor pressure. Thus, for 3-pentanone vapor, the assumption of constant fluorescence spectrum is effectively valid up to 642 K.
However, as Fig. 7 shows, the addition of a nitrogen bath gas renders this assumption incorrect. At 298 K, nitrogen causes a narrowing of the fluorescence spectrum to the left of the peak, and the magnitude of this shift is larger for 248 nm excitation than for 308 nm excitation. At 642 K, however, there is almost no narrowing with the addition of nitrogen for either 248 or 308 nm excitation. Thus, in nitrogen, the shape of the fluorescence does have dependence on temperature, rendering the assumption of constant spectral shape incorrect in this situation.
5.3 Absolute FQY
The total signal was determined by integrating the fluorescence spectrum from 329 nm to 562 nm to determine total fluorescence signal. The total signal was further corrected for losses in laser energy due to 3-pentanone absorption and variations in laser pulse energy using simultaneous measurements of energy from the photodiodes and normalized for the number laser pulses accumulated on the ICCD. The Rayleigh line from the laser beam was collected by accumulating 50 to 100 laser pulses on the ICCD. The total Rayleigh signal was determined by correcting the line measurement for the RSR of the system, integrating the line ±14.3 mm from the laser wavelength, and normalizing for laser energy. To normalize the Rayleigh signal for scatterer number density, the slope of the signal with nitrogen number density for a pressure range from 0 to 2 bar was calculated. The FQY was calculated using these quantities according to (14).
Figure 8 shows the measured absolute FQY for 3-pentanone vapor from about 10 to 30 mbar pressure and 248, 266, 277, and 308 nm excitation for a range of temperatures. The FQY generally decreases with increasing temperature for all four excitation wavelengths, but it remains essentially constant with pressure except at 295 K for 248 and 266 nm excitation, where increased FQY is observed with additional pressure. Moreover, for a given temperature, the 3-pentanone FQY increases with increasing excitation wavelength.
Absolute FQY data for 10 to 30 mbar of 3-pentanone and (a) 248, (b) 266, (c) 277 and (d) 308 nm excitation. Comparison to current and previous models [12] is also shown in the figure. Error bars are equivalent to 6%
Additional measurements of absolute FQY for 20 mbar of 3-pentanone in air and nitrogen at a total pressure of 1.3 bar over a range of temperatures is shown in Fig. 9. The FQY is identical in air and nitrogen, consistent with previous measurements at these conditions [8, 10]. The FQY in the bath gases at 1.3 bar total pressure is also higher than for 3-pentanone vapor at the same temperature, although this effect lessens with increasing wavelength and temperature. The uncertainty in the absolute FQY measurements is primarily due to the uncertainties in the absorption cross-section measurement, the RSR measurement, and the collected signal from the ICCD, resulting in an overall estimated uncertainty of about 6%.
Absolute FQY variation for 248, 266, and 308 nm excitation in nitrogen or air at 1.3 bar total pressure. Current measurements are made with 3-pentanone partial pressure of 20 mbar. The current work follows similar trends as previous work [8]. Error bars represent 6% uncertainty
The current work can be compared to previous measurements of relative FQY [8], scaled to absolute FQY using the room-temperature value measured in this work, shown in Fig. 9. Generally, the current work is consistent with trends observed in the previous work for all excitation wavelengths. For 308 nm excitation, the current work shows good agreement with Koch and Hanson, but, for 248 and 266 nm excitation, the FQY in the current work is lower by about 10%. One contributing factor is uncertainty in the composition of the cell mixture. Koch and Hanson utilized a bubbler system to seed their optical cell and assumed the vapor was saturated with 3-pentanone to infer tracer number density. The current work, however, meters the 3-pentanone and nitrogen gas mixture resulting in direct knowledge of the composition. Moreover, the difference in 266 nm absorption cross section discussed in section 5.1 contributes to the discrepancy. Nonetheless, both studies show similar trends in FQY with temperature.
Figure 10 compares room-temperature 3-pentanone absolute FQY measurements from the current work to previous measurements at low 3-pentanone pressures [6, 9]. The current work is consistent with previous work by Koch et al. for 248 and 266 nm excitation. For 308 nm excitation, the current work is about 10% lower, but still within the 15% error of the previous work, as shown in the figure. However, the current measurements are approximately 3 times smaller than the FQY measured by Hansen and Lee, and this discrepancy may be due to uncertainty in the absolute FQY of the reference species. Measurements from Hansen and Lee were calibrated to acetone fluorescence at 313 nm excitation, and the acetone FQY was taken from the literature [20]. However, a survey shows that there is poor agreement among different measurements of acetone FQY at this excitation wavelength [9, 21, 22], and this uncertainty in the reference value may be the root cause of the differences in measured 3-pentanone FQY.
6 Model development
The vibrational energy dependence of the fluorescence rate k f and non-radiative de-excitation rate k nr were investigated using the absolute FQY data from Fig. 8 for 3-pentanone vapor at the lowest available pressures, around 10 mbar. These data are shown in Fig. 11 as a function of the initial vibrational energy E 1, calculated using (2).
Variation of absolute FQY for 3-pentanone vapor for pressures around 10 mbar with initial vibrational energy E 1. Previous measurements are shown for comparison [9]. Error bars correspond to 6% uncertainty in FQY measurements
6.1 Determination of fluorescence rate
According to (8), k f can be determined with low-pressure FQY data and knowledge of k nr . Fortunately, k nr can be determined from the observed fluorescence lifetime τ f [s] at low pressures by
Hansen and Lee made measurements of τ f at room temperature over a range of wavelengths, and Ossler and Alden made measurements of τ f at 266 nm excitation over a range of temperatures [6, 23]. With this knowledge of k nr and with the low-pressure FQY data, k f can be calculated using (8). Figure 12 shows k f as a function of vibrational energies calculated from the current FQY data, as well as calculated with low-pressure FQY measurements from [9]. The error bars (17%) on k f measurements are a combination of the uncertainty in the τ f measurement (15% for both studies) and the uncertainty in the FQY measurements (6%). From 2000 to 13000 cm−1,k f is constant within the indicated error, and the current work agrees with previous measurements at the coincident vibrational energies [9].
The fluorescence rate k f has previously been considered to be a constant with vibrational energy [24], and previous models of acetone and 3-pentanone FQY made this assumption [12, 19]. The constancy of the fluorescence rate can be examined using the Strickler–Berg equation for radiative lifetime [25], which is analogous to the relationship between the Einstein A and B coefficients for atomic transitions. The Strickler-Berg equation shows that the fluorescence rate is dependent on the cube of the average fluorescence frequency. The fluorescence spectrum for pure 3-pentanone vapor remains constant for all excitation wavelengths over a range of temperatures, so the average fluorescence frequency also remains constant. Thus, k f should also be a constant, which is confirmed in the current work for the vibrational energy range from 2000 to 13000 cm−1.
An overall rate was determined by averaging the data points in Fig. 12 and assumed to be valid across all vibrational energies. The average is shown in Table 1 along with the rate from Koch et al., which is about 11% higher [12]. The rate determined by Hansen and Lee is significantly higher due to the discrepancy in FQY values previously described. The k f value from the current work is consistent with previous measurements for several other ketones, including acetone, and aldeydes, including formaldehyde, which range from 105–106 s−1 [24]. Thus, the current measurement of k f is regarded as consistent with previous work.
6.2 Determination of non-radiative rate k nr
Once the value of k f has been determined, k nr can be calculated from the FQY data over a range of vibrational energies using (8). Figure 13 shows k nr determined from the FQY data as a function of vibrational energy compared to k nr determined from lifetime data. The current work shows good agreement with the previous work. However, the current work shows a slight dip in k nr at 10500 cm−1 compared to Koch’s model and extends knowledge of k nr to 15100 cm−1, a higher vibrational energy level than in previous work [6, 23]. The current work updates the formula fit of k nr as a function of vibrational energy using the measurements of k nr .
The expression is shown in Table 2, as well as the previous fit from [12], and both expressions are plotted in Fig. 13. The current fit shows good agreement with k nr data across all vibrational energies, while the Koch expression underpredicts k nr above 12700 cm−1. This difference is because Koch used FQY data in 1 bar of nitrogen with 248 nm excitation to fit his k nr expression at higher energy levels [12], but as the current work shows, the addition of nitrogen at 1 bar increases the FQY due to vibrational relaxation, resulting in the calculation of a lower k nr value.
6.3 3-Pentanone self-collision vibrational relaxation
By measuring the FQY as a function of 3-pentanone vapor pressure, insight can be gained into the vibrational relaxation characteristics of tracer to tracer collisions. The vibrational relaxation energy cascade is defined by the cascade parameter α, which has a dependence on the identity of the collider and the temperature. Previous work has considered the vibrational relaxation cascade parameter for 3-pentanone collisions, α 3p, to be a constant with temperature [12]. However, as the measurements show, the FQY remains constant with pressure at higher temperatures, indicating that no vibrational relaxation is occurring. Thus, the current work shows that α 3p should decrease with increasing temperature, contrary to what previous work had assumed. A new expression for α 3p was optimized using 3-pentanone FQY data from 10 to 30 mbar and updated k f and k nr expressions from the current work, and the updated α 3p expression is shown in Table 3.
6.4 Model performance
A comparison of the updated model to the FQY measurements for 3-pentanone vapor in the current work is shown in Fig. 8. A comparison is also shown to the previous model by Koch [12]. The updated model shows agreement with the low-pressure FQY data within 8% across all conditions. For 248 nm excitation, the model shows excellent agreement with the FQY data across the temperature and pressure range, and significantly improves on the performance of the Koch model. For 266 and 277 nm excitation, the model shows excellent agreement with the data, except at 650 K, where the model differs from the data by up to 8%. These conditions correspond to vibrational energies of about 11000 cm−1, where k nr begins to rapidly rise with increasing vibrational energy. This rise has been hypothesized to be the result of an increase in IC rate contribution to k nr [26], but further work clarifying the ISC and IC rates would assist in improving the model agreement at these wavelength and temperature conditions. This performance is an improvement over the performance of the Koch model, which generally performs similarly at lower temperatures, but breaks down at higher temperatures. For 308 nm excitation, both the model in the current work and the Koch model show similar performance and differ from the FQY data by about 5%. This discrepancy could be also be improved by further measurements characterizing ISC and IC. Thus, the model optimized using low-pressure FQY data in the current work shows significant improvement over the previous model.
7 Conclusions
Absolute FQY measurements were made for 3-pentanone at temperatures from 295 to 690 K, and at pressures of 10 to 30 mbar, for excitation wavelengths of 248, 266, 277, and 308 nm. In addition, absolute FQY measurements were also made for this temperature range for 20 mbar of 3-pentanone in nitrogen and for 20 mbar of 3-pentanone in air for temperatures from 295 to 485 K at 1.3 bar of total pressure. Fluorescence signals were calibrated for the efficiencies of the collection optics, spectrograph, and ICCD camera using nitrogen Rayleigh scattering. The FQY measurements are in excellent agreement with previous work utilizing a similar calibration method. While there is significant disagreement with previous measurements calibrated using acetone fluorescence, the large variation in published FQY values for acetone introduces significant uncertainty in that previous work.
Measurements of absorption cross section were also made for excitation wavelengths of 248, 266, 277, and 308 nm from 295 to 690 K. The data are in excellent agreement with previous work, and agree well with a previously published Gaussian fit of the absorption spectrum.
The low-pressure data were used to optimize a previously proposed FQY model and allows for insight into the fluorescence rate k f and the intramolecular non-radiative deactivation rate k nr . The data were also used to optimize the vibrational relaxation cascade parameter α 3p. The updated model shows good agreement with 3-pentanone FQY data for pressures of 10 to 30 mbar and temperatures of 295 to 690 K.
The current work combines ISC and IC rates into a single rate, but future work could investigate these rates separately. Insight into the ISC rate could be derived from an examination of the triplet population via the yield of photolysis products like CO, since photolysis of 3-pentanone has been postulated to occur from the triplet state. Phosphorescence may be another candidate process for monitoring the triplet population. Improved understanding of these processes would be beneficial to improving model performance. Another area for future work is creating a similar database of low-pressure fluorescence lifetimes over a wide range of temperature and excitation wavelengths. As the current work demonstrates, fluorescence lifetime can provide insight into the non-radiative de-excitation of the first excited singlet. An improved fluorescence lifetime database coupled with an FQY database at low pressures and a wide range of temperatures and excitation wavelengths would allow, thus, for significant insight into the collisionless processes of the excited singlet.
References
C. Schulz, V. Sick, Prog. Energy Combust. Sci. 31, 75 (2005)
S. Einecke, C. Schulz, V. Sick, Appl. Phys. B 71, 717 (2000)
D.A. Rothamer, J.A. Snyder, R.K. Hanson, R.R. Steeper, SAE Int. J. Fuels Lubr. 1, 520 (2009)
J.A. Snyder, R.K. Hanson, R.P. Fitzgerald, R.R. Steeper, SAE Int. J. Eng. 2, 460 (2009)
J. Koch, J. Gronki, R.K. Hanson, J. Quant. Spectrosc. Radiat. Transf. 109, 2037 (2008)
D.A. Hansen, E.K.C. Lee, J. Chem. Phys. 62, 183 (1975)
F. Grossman, P.B. Monkhouse, M. Ridder, V. Sick, J. Wolfrum, Appl. Phys. B 62, 249 (1996)
J.D. Koch, R.K. Hanson, Appl. Phys. B 76, 319 (2003)
J.D. Koch, R.K. Hanson, W. Koban, C. Schulz, Appl. Opt. 43, 5901 (2004)
A. Braeuer, F. Beyrau, A. Leipertz, Appl. Opt. 45, 4982 (2006)
V. Modica, C. Morin, P. Guibert, Appl. Phys. B 87, 193 (2007)
Koch, J. D., Ph.D. thesis, Stanford University (2005)
Rothamer, D. A., Ph.D. thesis, Stanford University (2007)
J.T. Salmon, N.M. Laurendeau, Appl. Opt. 24, 65 (1985)
R.B. Miles, W.R. Lempert, J.N. Forkey, Meas. Sci. Technol. 12, 33 (2001)
H. Naus, W. Ubachs, Opt. Lett. 25, 347 (2000)
U. Griesmann, J.H. Burnett, Opt. Lett. 24, 1699 (1999)
R. Martinez, A. Buitrago, N. Howell, C. Hearn, J. Joens, Atmos. Environ., A Gen. Topics 26, 785 (1992)
M. Thurber, F. Grisch, B. Kirby, M. Votsmeier, R. Hanson, Appl. Opt. 37, 4963 (1998)
J. Heicklen, W.A. Noyes, J. Am. Chem. Soc. 81, 3858 (1959)
A. Halpern, W. Ware, J. Chem. Phys. 54, 1271 (1971)
G. Luckey, W. Noyes, J. Chem. Phys. 19, 227 (1951)
F. Ossler, M. Alden, Appl. Phys. B 64, 493 (1997)
E.K.C. Lee, R.S. Lee, Photochemistry of Simple Aldehydes and Ketones in the Gas Phase (Wiley, Hoboken, 1980)
S.J. Strickler, R.A. Berg, J. Chem. Phys. 37, 814 (1962)
G.D. Gillispie, E.C. Lim, Chem. Phys. Lett. 34, 513 (1975)
Acknowledgements
This work was funded by the Air Force Office of Scientific Research (Aerospace, Chemical, and Material Sciences Directorate), with Dr. Julian Tishkoff as the technical monitor.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheung, B.H., Hanson, R.K. Determination of fluorescence and non-radiative de-excitation rates of excited 3-pentanone at low pressures. Appl. Phys. B 106, 741–753 (2012). https://doi.org/10.1007/s00340-011-4817-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-011-4817-4