Abstract
The present work shows the dielectric properties of (100-X)Ba2TiSi2O8–(X)TiO2 composites (X in wt%) at radio frequency (RF) region (< 300 MHz). X-ray diffraction and Rietveld’s refinement showed the presence of Ba2(Ti7/9Si2/9)9O20 (BTSO) phase in the studied composites. To analyze the relationship between the electrical properties and the microstructure of the matrix and composites, Impedance spectroscopy (IS) was used. The applied model that best describes the behavior found in the samples is the Havriliak–Negami model. From the IS it was observed that the dielectric relative permittivity presented high values in BTS10 (X = 10%) and BTS20 (X = 20%) which allows classifying them as Colossal permittivity materials. This is an important result due to the potential for applications in new storage systems, capacitive devices and microelectronics. Nyquist diagrams were employed to evaluate the contribution of the grain and grain boundary in the electrical response of the samples and fitted employing an equivalent circuit with two associations of R-CPE (constant phase element). Temperature coefficient of capacitance (TCC) was also obtained and it was possible to observe that there was an increase in the TCC value for the compounds in all frequencies. In addition, IS demonstrated that the activation energy decreasing with TiO2 addition indicating a decrease in the resistive character of the materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dielectric ceramics have been extensively studied due to their physical properties [1] which allow these materials to be used for different applications [1,2,3]. These materials, when endowed with a low dielectric loss combined with high values of permittivity and quality factor, are generally used in microwave circuits [1,2,3], while ceramics with low permittivity can be used in communication technology of fifth-generation (5G) [4]. In this technology, factors such as transmission speed and frequency are essential, which can be optimized by small values of the dielectric constant [4].
On the other hand, materials that have a colossal dielectric constant (CDC), that is, exhibit permittivities higher than 103, are fundamental for the miniaturization of electronic devices and allow for vast technological applications [5,6,7,8,9,10]. However, the electrical properties of a material depend on imperfections in its crystal lattice, porosity, particle size, etc. [1]. So, to characterize the electrical properties of ceramic materials, it is common to use impedance spectroscopy (IS) [11], which will be applied in this work focusing on the ceramic matrix Ba2TiSi2O8 (BTS).
BTS is a rare silicate mineral that was discovered in Fresno County (USA) and its first reports date back to 1960 [1], it belongs to the space group P4bm [12,13,14,15], and has a non-center-symmetric tetragonal structure [16,17,18], due to its physical properties such as ferroelectricity [12, 15,16,17], pyroelectricity [12, 17, 19, 20], and piezoelectricity [19, 20] have attracted numerous studies on its structure, electrical properties, and possible technological applications.
This work sought to investigate the effect of the additions of 10%, 20%, 30%, and 40% by mass of TiO2 in the pure phase of Ba2TiSi2O8 on the electrical properties of BTS in the radiofrequency region through Spectroscopy Impedance (IS). It is noteworthy that the choice of TiO2 as an addition to the BTS phase is due to its technological applications in the radiofrequency (RF) region [20]. The structural characterization of the samples was carried out via X-ray diffraction (XRD), and the Rietveld refinement was used to confirm the phases. IS was used to study electrical properties varying the temperature from 380º to 460 ºC and using a frequency spectrum of 1 Hz – 1 MHz. In this work, the following electrical parameters were studied: complex dielectric permittivity, capacitance temperature coefficient (TCC), conductivity and activation energy (Ea). The Nyquist diagram was used to identify and differentiate intrinsic effects (grains, grain boundaries) from extrinsic effects (electrode and interfacial effects).
2 Experimental procedure
For the preparation of the BTS matrix, the solid-state reaction was used following the stoichiometric proportions. In the synthesis, the following solid precursors were employed: BaCO3 (99.99%, Vetec), TiO2 anatase (99.8%, Aldrich), and SiO2 (99.9%, Aldrich). The matrix was obtained according to the chemical equation (Eq. 1):
The high-energy mill with a rotation speed of 360 rpm for 4 h was used to grind the material. The resulting powder was calcined at 1100 °C for 6 h in a conventional oven. Subsequently, 10%, 20%, 30%, and 40% by mass of TiO2 was added and mixed thoroughly to the BTS matrix using mortar, resulting in composites that were called BTS, BTS10, BTS20, BTS30, and BTS40, respectively. The prepared series are (100-X)Ba2TiSi2O8–(X)TiO2 composites (X in mass units). To produce the cylindrical disks the samples were compacted via uniaxial pressure of 15.43 MPa. Finally, the pieces were sintered at 1200 °C for 4 h.
Through X-ray diffraction (XRD) the structures of the samples were analyzed. The Rigaku model D/max-B diffractometer with Cu cathode (Cu-Kα), 40 kV, 30 mA, and λ = 1.5404 Å, with an angular range in (2θ) of 20º–80º was used in this analysis. Using the Rietveld method and with the aid of the GSAS software, the phases obtained were confirmed and compared with the crystallographic sheets Inorganic Crystal Structure Database (ICSD) and Open Database of Crystallography (COD).
For the analysis of the electrical properties of the samples in RF, the ceramic discs had their faces covered with conductive silver paint to produce a capacitor. In this study, the conductivity and permittivity behavior are analyzed as a function of frequency with temperature variation to relate their macro and microscopic structures [21]. The temperature range used was 380º–460º C, with a frequency range of 1 Hz—1 MHz using the Impedance Analyzer (Solartron model 1260).
3 Results and discussion
3.1 X-ray diffraction
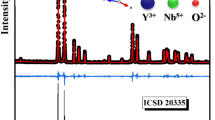
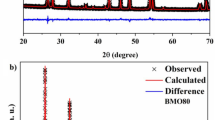
Figure 1a, b presents the Rietveld refinement of the powder XRD patterns of the BTS and BTS40 samples. Diffractogram obtained from BTS sample Fig. 1a demonstrate that peaks are well indexed to the tetragonal structure belonging to the P4bm space group (ICSD No. 15715) demonstrated that a single-phase was obtained.
In Fig. 1b, it is observed a significant change in the diffraction pattern of BTS40, where the presence of new diffraction peaks is observed, indicating that in addition to BTS and TiO2, there is a new crystalline phase. Rietveld refinement confirmed the presence of the Ba2(Ti7/9Si2/9)9O20 (BTSO) phase in all composites and demonstrates that there was a reaction between BTS and TiO2. The formation reaction of the BTSO phase is shown in Eq. (2):
Statistical parameters and mass fractions obtained from Rietveld refinement for all materials are presented in the Table 1. As observed in the BTS10 and BTS20 composites, all TiO2 added was reacted completely forming the BTSO phase, whereas a certain percentage of TiO2 remained in the compositions BTS30 and BTS40.
3.2 RF analysis
Impedance spectroscopy is an extremely powerful technique that allows analyzing the electrical behavior of materials and obtaining important information about the structure of the material, its electrical nature, conduction mechanisms, etc. [10, 22, 23]. To analyze the electrical and dielectric properties, BTS and composites impedance spectroscopy is utilized from the frequency spectrum of 1 Hz–1 MHz and varying the temperature from 380º to 460º C. The complex dielectric permittivity can be written mathematically according to Eq. (3):
where the real component of dielectric permittivity (ε’r) represents energy storage, whereas the imaginary one (ε”r) represents all losses [21, 24]. The model that showed the best compatibility to analyze the effects of increasing frequency with temperature variation on the behavior of ε’r was the Havriliak–Negami model [21, 22, 25], described in Eq. (5).
εrs relative static permittivity, εr∞ relative permittivity for high frequencies (ω → ∞), ω is the natural frequency of angular oscillation, and τ it's relaxation time. The values of α and β belong to the intervals 0 < α < 1e 0 < β < 1.
In Fig. 2, the ε’r curves of all samples at room temperature are presented, through which it can be seen that samples BTS10 and BTS20 exhibit values well above 103, which demonstrate that these samples have colossal permittivities [23, 24]. Since BTS has a low permittivity value at room temperature [28, 29], the elevated values obtained for these composites could be explained due to presence to the BTSO phase indicating that this phase has colossal permittivity. These results obtained are interesting since they would allow to reduce the geometric measurements of capacitive components, applications such as random access memories, energy storage, multifunctional electronic devices, and sensors [5,6,7,8,9, 26]. The effect of temperature variation on permittivity in the range of 380–460 ºC is presented in the Supplementary Material.
Figure 3 a–e shows the imaginary impedance spectra (Zʹʹ) by frequency at different temperatures. It is possible to observe that the Zʹʹ peak moves to high frequencies with increasing temperature, this fact indicates a thermally activated process [30,31,32]. The asymmetry of the peaks and their broadening indicates the non-Debye nature of the samples this fact agrees with Eq. (4) [31, 33]. The influence of the temperature variation on real impedance (Zʹ) is showed in the Supplementary Material.
The effect of conductivity at different temperatures is shown in Fig. 4a, b, whereas it is observed that conductivity increases with temperature variation, confirming a thermally activated process [30, 36]. The universal power law, Eq. (7), describes the behavior of the samples [38,39,40].
σDC is the DC conductivity; A it is thermally activated quantity; n is an exponent with variation 0 < n < 1.
Moreover, it is possible to observe that the increase in conductivity as a function of frequency occurs exponentially, obeying the power law, σ (ω) α ωn mentioned previously. In this way, the value of n expresses the physical meaning of the type of jump manifested by the charge carriers. That is, when 0 < n < 1 it means that the carriers present a translational jump movement with a sudden jump, while when n > 1 it means that the movement of the charge carriers involves a localized jump without them leaving the neighborhood [30, 33, 41]. Table 2 presents the values of σDC, A, n, and R2. Observing Figure and the results obtained in table, it is valid to infer for all samples that the movement of charge carriers involves translational jump movement with a sudden jump.
In Eq. (8) and (9) the Arrhenius relation was used to calculate the activation energy of the samples, whereas was employed values of σ’ and Z”.
σ0 and f0 are the constant pre-exponential factor; fmax is the maximum frequency of Zʹʹ; Ea is the activation energy of the mobile charge carriers; k is the Boltzmann constant; T the temperature in Kelvin.
In the Fig. 5a–e and Table 3, it is possible to notice that the values of activation energy calculated by Zʹʹ and σ’ are close indicating that the same charge carriers are involved in the conduction process. Moreover, Ea decrease with the increase of TiO2 addition, with BTS demonstrating the highest value of activation energy, whereas BTS40 presents the lowest value. This fact could be explained due to the presence of the BTSO and TiO2 phases in the composites, it is emphasized that the TiO2 has a lower activation energy than the BTS phase [42].
4 Nyquist analysis
To study the effects of grains, grain boundaries, and electrode effects, the Nyquist diagram was used. Thus, for modeling the equivalent circuits in each sample, the R (resistor) and CPE (constant phase element) elements were used.
The CPE can be used in different ways within an electrical circuit assuming inductor, resistor or capacitor functions depending on the values of n (n = – 1, 0, 1), respectively [34]. All curves were plotted using an equivalent circuit composed of two parallel R-CPE associations (see Fig. 6a) that are related to grain and grain boundary effects. Equation (10) allows calculating the impedance of the CPE,
where q is the proportionality factor.
The equivalent impedance of each loop (R-CPE) can be calculated according to Eq. (11),
The equivalent impedance will be the sum of the impedances calculated according to Eq. (8) [35]. According to Fig. 6a–e it is possible to observe that the increase in temperature causes the attenuation of the curves—Zʹʹ (Ω) × Zʹ (Ω). Table 4 presents the data obtained in the adjustments, whereas Rg, Pg, Rgb and Pgb refer to grain resistance, grain capacitance, grain boundary resistance and grain boundary resistance capacitance, respectively.
According to Table 4, it was possible to observe that the value of n in the grains, in all samples was approximately between 0.8 ≤ n < 1, and in this case the CPE represents a capacitance distortion caused by the accumulation /distribution of charge carriers or by the roughness of the electrode surface [35]. Regarding the grain boundary, the BTS sample showed the behavior indicated above for temperatures 380º–400 ºC, while at temperatures 420º–460 ºC, the contribution of the grain boundary was in the range of 0.4 < n ≤ 0.6, and in this range the CPE indicates diffusion effects with deviations from Fick's second law [35]. All composites presented grain boundary values of 0.4 < n ≤ 0.6 in the range of 380º–460 ºC, and again the CPE is related to the previously mentioned diffusion effects [35].
Finally, it should be noted that the increase in temperature causes a decrease in the values of the grain boundary resistances in all the samples studied, and this effect favors the conduction process, as it facilitates the movement of charge carriers within the samples, which behavior is related to potential barrier lowering [30].
4.1 TCC (temperature coefficient of capacitance)
With the objective of evaluating the behavior of the capacitance variation of the material from the increase in temperature, the study of the temperature coefficient of capacitance (TCC) was used [30]. It is worth mentioning that the TCC close to zero is a relevant factor for the miniaturization of capacitive electronic devices [3, 26].
Table 5 shows the TCC results of BTS and composites, calculated from 10 Hz to 1 MHz. It is possible to see that the TiO2 additions cause an increase in the thermal stability in the BTS30 and BTS40 samples since TCC values close to zero were obtained. For BTS30 and BTS40, it is expected to obtain TCC = 0 in the range between 100 kHz and 1 MHz. These values are important in the miniaturization of electronic devices, and therefore, it can be inferred that the addition of TiO2 caused an increase in the thermal stability of the composites.
5 Conclusion
This work presents a study of the electrical properties of the ceramic matrix Ba2TiSi2O8 with additions of TiO2 (100-X)Ba2TiSi2O8 – (X)TiO2 composites (X in mass units) in the radiofrequency region. X-ray diffraction and Rietveld’s refinement demonstrated the formation of Ba2(Ti7/9Si2/9)9O20 (BTSO) phase in the composites. The study of the electrical properties of the samples was carried out using impedance spectroscopy, where it was observed that the dielectric relative permittivity (εʹr) presented colossal values for samples BTS10 (X = 10%) and BTS20 (X = 20%) and the relaxation phenomenon is consistent with the model of Havriliak–Negami. Through the analysis of the dielectric permittivity and impedances, it was possible to perceive that the relaxation effects observed in the samples may have been caused Maxwell–Wagner mechanism. The study of the temperature coefficient of capacitance (TCC) was carried out and the sample of BTS30 presented the value closest to the range for applications in capacitive components. Regarding the activation energy, a decrease in its value was noticed with the increase of TiO2 additions.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
M.T. Sebastian, Dielectric Materials for Wireless Communication (Elsevier Science, 2008)
L.F. Chen, C.K. Ong, C.P. Neo, V.V. Varadan, V.K. Varadan, Microwave Electronics: Measurement and Materials Characterization (Wiley, 2004)
A.J. Moulson, J.M. Herbert, Electroceramics: Materials, Properties, Applications (Wiley, Chichester, 2003)
C. Yin, Z. Yu, L. Shu, L. Liu, Y. Chen, C. Li, J. Adv. Ceram. 10, 108 (2021)
J. Sun, R. Ahmed, G.J. Wang, S.T. Wang, J. Wang, S.A. Suhaib, Y.M. Xie, H. Bi, C.C. Wang, J. Mater. Sci. 54, 6323 (2019)
N. Humera, S. Riaz, N. Ahmad, F. Arshad, R. Zafar, S. Ali, S. Idrees, H. Noor, S. Atiq, and S. Naseem, J. Mater. Sci. Mater. Electron. (2020).
Y. Wang, W. Jie, C. Yang, X. Wei, J. Hao, Adv. Funct. Mater., p. 1808118 (2019).
J. Zheng, Q. Fu, X. Chen, C. Chakrabarti, P. Wang, H. Yin, C. Li, Y. Qiu, S. Yuan, J. Mater. Sci. Mater. Electron. 32, 290 (2021)
Z. Wang, P. Peng, L. Zhang, N. Wang, B. Tang, B. Cui, J. Liu, D. Xu, J. Mater. Sci. Mater. Electron. 33, 6283 (2022)
Y. Song, P. Liu, B. Guo, X. Cui, W. Yang, J. Am. Ceram. Soc. 103, 4313 (2020)
E. Barsoukov, J.R. Macdonald, Impedance Spectroscopy, 3rd edn. (Wiley, Hoboken, 2018)
Q. Shi, T.-J. Park, J. Schliesser, A. Navrotsky, B.F. Woodfield, J. Chem. Thermodyn.Thermodyn. 72, 77 (2014)
S. Cao, B. Jiang, Y. Zheng, X. Tu, K. Xiong, P. Gao, E. Shi, J. Cryst. GrowthCryst. Growth 451, 207 (2016)
C. Shen, H. Zhang, D. Wang, J. Wang, R.I. Boughton, Crystals (2017)
M. Sahu, S. Mukherjee, M. Keskar, K. Krishnan, S. Dash, B.S. Tomar, Thermochim. Acta. Acta 663, 215 (2018)
E.E. Abbott, M. Mann, J.W. Kolis, J. Solid State Chem. 184, 1257 (2011)
S.K. Barbar, M. Roy, J. Mol. Struct.Struct. 1024, 132 (2012)
C. Jiang, F. Chen, F. Yu, S. Tian, X. Cheng, S. Zhang, X. Zhao, Crystals 9, 11 (2019)
S. Weber, A. Lebedinskaya, D. Rudsky, Y. Kabirov, A. Rudskaya, M. Kupriyanov, J. Adv. Dielectr. (2020).
J.A.R. Lopez, L.M.A. Silva, H. Leon-Leon, M. Valverde-Alva, L. Korotkov, E. Rysiakiewicz-Pasek, N. Emelianov, AIP Conf. Proc., p. 050002 (2020)
A.J. Vadim, F. LvovichWiley, Impedance Spectroscopy: Applications to Electrochemical and Dielectric Phenomena (Wiley, 2012)
J. Shukla, P. Saxena, P. Joshi, P. Joshi, A. Mishra, Appl. Phys. A 129, 731 (2023)
R.K. Veena, A. Anand, M. Manjuladevi, V.S. Veena, J. Cyriac, N. Kalarikkal, S. Sagar, Appl. Phys. A. Phys. A 129, 598 (2023)
K.C. Kao, Dielectric Phenomena in Solids (Elsevier Science, 2004)
S. Havriliak, S. Negami, Polymer (Guildf). 8, 161 (1967)
P. Lunkenheimer, V. Bobnar, V. Bobnar, A.V. Pronin, A. V. Pronin, A.I. Ritus, A.A. Volkov, A. Loidl, Phys. Rev. B-Condens. Matter Mater. Phys. (2002).
M. Idrees, M. Nadeem, M. Atif, M. Siddique, M. Mehmood, M.M. Hassan, Acta Mater. (2011).
R.F. Abreu, T.O. Abreu, D.M. da Colares, S.O. Saturno, J.P.C. do Nascimento, F.A.C. Nobrega, A. Ghosh, S.J.T. Vasconcelos, J.C. Sales, H.D. de Andrade, I.S.Q. Júnior, A.S.B. Sombra, J. Mater. Sci. Mater. Electron.. Mater. Sci. Mater. Electron. 32, 7034 (2021)
J. Shen, J. Zhou, X. Cui, L. Li, J. Electroceramics 21, 565 (2008)
B. Behera, P. Nayak, R.N.P. Choudhary, J. Alloys Compd. 436, 226 (2007)
K.C. VaradaRajulu, B. Tilak, K. Sambasiva Rao, Appl. Phys. A 106, 533 (2012)
B.C. Sutar, R.N.P. Choudhary, P.R. Das, Ceram. Int. 40, 7791 (2014)
D.K. Kushvaha, B. Tiwari, S.K. Rout, J. Alloys Compd. 829, 154573 (2020)
A. Lasia, Electrochemical Impedance Spectroscopy and Its Applications (2014)
X.-Z. Yuan, C. Song, H. Wang, J. Zhang, Electrochemical Impedance Spectroscopy in PEM Fuel Cells (Springer London, London, 2010)
R.F. Abreu, F.A.C. Nobrega, D.M. da Colares, S.O. Saturno, J.P.C. do Nascimento, T.O. Abreu, A. Ghosh, F.F. Carmo, M.A.S. Silva, A.J.M. Sales, R.S. Silva, A.S.B. Sombra, J. Mater. Sci. Mater. Electron.. Mater. Sci. Mater. Electron. 34, 457 (2023)
C. Karthik, K.B.R. Varma, J. Phys. Chem. Solids 67, 2437 (2006)
A.K. Jonscher, J. Phys. D Appl. Phys. 32, R57 (1999)
T. Acharya, R.N.P. Choudhary, J. Electron. Mater. (2015).
T. Acharya, R.N.P. Choudhary, Phys. Lett. A 380, 2437 (2016)
K. Funke, Prog. Solid State Chem.. Solid State Chem. 22, 111 (1993)
M.A.S. Silva, R.G.M. Oliveira, A.S.B. Sombra, Ceram. Int. 45, 20446 (2019)
Acknowledgements
This work was partly sponsored by the Brazilian Research Agencies CNPq—Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant INCT NANO(BIO)SIMES), CAPES—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (grant Project PNPD), FUNCAP—Fundação Cearense de Apoio ao Desenvolvimento Cientifico e Tecnológico, FINEP—Financiadora de Estudos e Projetos (grants INFRAPESQ-11 and INFRAPESQ-12) and the U. S. Air Force Office of Scientific Research (AFOSR) (FA9550-16-1-0127).
Author information
Authors and Affiliations
Contributions
All the authors participated in the work, however some dedicated more time to some specific activities, which we describe below: conceptualization—RFA, JPCdN. Methodology—Software—DdMC, CS, FFdC. Validation—MASS, FFdC. Formal analysis—FACN, TOA. Investigation—SJTV, RFA. Resources—ASBS. Data curation—DZ, AG. Writing—original draft preparation—RFA, FEAN. Writing—review and editing—MASS, SOS, JPCdN. Visualization—FEN, DBdF. Supervision—SVT, MASS. Project administration—RSS, ASBS. Funding acquisition—ASBS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abreu, R.F., Saturno, S.O., Nobrega, F.A.C. et al. Study of electrical properties with temperature variation by complex impedance spectroscopy (CIS) and effects on the Ba2TiSi2O8–TiO2 matrix. Appl. Phys. A 130, 138 (2024). https://doi.org/10.1007/s00339-024-07295-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07295-z